Abstract
Objectives. Joint pain comorbidity (JPC) is common in individuals with knee OA. This study investigates the longitudinal association between JPC and health-related quality of life (HRQoL) and physical activity levels in individuals with knee OA.
Methods. Data from the progression cohort of the Osteoarthritis Initiative (n = 1233; age 61 years and 58% females) were analysed. JPC was considered present if individuals reported pain in three or more joint groups, including the knee joints. HRQoL was assessed using the Knee Injury and Osteoarthritis Outcome Score (KOOS) Quality of Life subscale, and self-reported physical activity was determined using the Physical Activity Scale for the Elderly (PASE). Generalized estimating equation (GEE) analyses were performed, adjusted for age, sex, duration of complaints, medical comorbidity, and physical and mental functioning.
Results. Over the 4-year period, 32% of participants never reported JPC, whereas 12% always reported JPC. GEE modelling demonstrated that having JPC was negatively associated with HRQoL [regression coefficient β (95% CI) −3.57 (−4.69, −2.44)] and not associated with physical activity [−1.32 (−6.61, 3.98)].
Conclusion. Considering the impact of JPC on the HRQoL of individuals with knee OA, the assessment of JPC in individuals with knee OA might be a daily routine.
Keywords: joint pain comorbidity, osteoarthritis, knee, cohort study, health-related quality of life, physical activity
Introduction
Chronic widespread pain is common in the general population (ranging between 11% and 24%) [1]. In persons with knee OA, joint pain comorbidity (JPC) is considered even more common (prevalence >50%) [2–5] and burdensome [4, 5]. Recent studies have demonstrated that JPC is associated with lower levels of physical and psychological health [4, 5] than in people without JPC. However, due to the cross-sectional nature of these data, it remains unclear whether the association between health-related quality of life (HRQoL) and JPC is longitudinally consistent over time. If this relationship is indeed apparent, it could be valuable to advise clinicians to address JPC to optimize treatment outcomes in this group of patients.
Hoogeboom et al. [5] demonstrated that individuals reporting JPC had twice the odds of having other medical comorbidities, such as cardiovascular and respiratory diseases. One of the most important therapy strategies to prevent these comorbid diseases is the promotion of physical activity. We hypothesized that individuals with JPC, because of the widespread pain, might be specifically prone to physical inactivity [6–8].
Therefore the first aim of this study was to confirm the longitudinal association between JPC and HRQoL and the second aim was to study the longitudinal association between JPC and physical activity levels in individuals with established knee OA.
Patients and methods
The Osteoarthritis Initiative (OAI) is a publicly and privately funded prospective 4-year longitudinal cohort study (available for public access at http://www.oai.ucsf.edu) [9]. We included data from the progression subcohort. The progression subcohort comprises 1390 persons with symptomatic knee OA in one or both knees. Exclusion criteria for the OAI population included self-reported RA, SLE, PsA, AS or another inflammantory arthritis (defined as self-report of a physician diagnosis and ever use of specific prescription medications), MRI contraindication, (plans for) bilateral total knee joint replacement and comorbid conditions that could interfere with the ability to participate in a 4-year study [9]. To ensure a homogeneous OA cohort, we also excluded participants who self-reported other forms of inflammatory arthritis (n = 157), resulting in a total of 1233 cases.
Baseline data on age, sex, marital status, number of medical comorbidities and duration of the disease were acquired. Data on JPC, HRQoL and physical activity were acquired at five yearly time points: at baseline: and at 1-, 2-, 3- and 4-year follow-up (OAI databases v0.2.2, v1.2.1, v3.2.1, v5.2.1 and v6.2.1, respectively). Joint pain was considered present when a participant reported pain, aching or stiffness in a joint (i.e. neck, thoracic spine, lower back, shoulder, elbow, wrist, hand, hip, knee, ankle and foot) for more than half of the days in the past 30 days [4] when answering the following question: during the past 30 days, which of these joints have had pain, aching or stiffness on most days? Self-reported HRQoL was measured using the Quality of Life subscale of the Knee Osteoarthritis Outcome Score (KOOS) [10]. Self-reported activity levels were measured using the Physical Activity Scale for the Elderly (PASE) [11].
Participants were stratified into two categories: having JPC (at least three affected joint groups) or having no JPC (two or fewer affected joint groups). This classification was used to establish a clear threshold; one often used to define widespread pain or generalized OA [1, 12]. Consequently participants were pragmatically categorized into the following four groups: (i) never JPC, (ii) sometimes JPC (JPC at one or two time points), (iii) often JPC (JPC at three or four time points) and (iv) always JPC (JPC at all five time points), to allow us to study the impact of persistence of JPC and to study a possible dose–response relationship. Descriptive statistics were used to describe (i) the different groups, (ii) the dropout rate and (iii) the number of missing values. Missing data mechanisms were studied by the use of indicator variables [13]. As the data were considered at least missing at random, missing data were imputed by the use of multiple imputations by chained equations to increase power, enable more efficient analyses and reduce bias [14]. All analyses were performed on 10 multiple imputed data sets [15] and combined using Rubin’s rules [16]. HRQoL and physical activity data were plotted over time for each of the four groups [mean (95% CI)]. By use of generalized estimating equation (GEE) modelling, we studied the longitudinal association between the presence of JPC (yes/no) and HRQoL/physical activity. To do so, we first built a base model comprising JPC, HRQoL/physical activity and time. Thus we built controlled models, i.e. base models adjusted for age, sex, number of medical comorbidities, duration of complaints and knee pain (WOMAC subscale pain) [13]. Quasi-likelihood under the independence model criterion was used to find an acceptable working correlation structure for the models [17]. Sensitivity analyses were performed on the complete case data (i.e. non-imputed data set). All statistical analyses were carried out using the statistical package Stata/IC 12 (Statacorp, College Station, TX, USA).
Results
Of all 1233 participants [mean (s.d.) age 61 (9) years and 58% females], 44% reported three or more painful joint groups at baseline. At 1 year 99 (8%) people were lost to follow-up, and after 2, 3 and 4 years this was, respectively, 148 (12%), 170 (14%) and 174 (14%). The number of missing values for the KOOS Quality of Life subscale was negligible (<1%) and ranged from 3% to 8% for the PASE variable. In Table 1, baseline characteristics of the four groups are presented. Over the 4-year period, 32% of participants never reported JPC, whereas 12% reported JPC at each of the measurement points.
Table 1.
Baseline characteristics of the four groups of individuals with or without JPC
| Study group characteristics | Never group | Sometimes group | Often group | Always group | P-value |
|---|---|---|---|---|---|
| n (%) | 315 (32) | 331 (33) | 234 (23) | 119 (12) | |
| Age, mean (s.d.), years | 62 (9) | 62 (9) | 62 (9) | 60 (9) | 0.39 |
| Sex, female, % | 43 | 55 | 64 | 71 | <0.01 |
| Complaints duration >5 years, % | 38 | 48 | 52 | 64 | <0.01 |
| Medical comorbidities > 1, % | 4 | 7 | 10 | 10 | <0.01 |
| WOMAC stiffness, mean (s.d.) | 2.38 (1.6) | 2.88 (1.7) | 3.42 (1.6) | 3.49 (1.5) | <0.01 |
| WOMAC pain, mean (s.d.) | 4.03 (3.2) | 5.21 (3.7) | 6.57 (3.8) | 7.24 (3.9) | <0.01 |
| WOMAC activities, mean (s.d.) | 12.4 (10.8) | 16.8 (12.1) | 20.1 (12.2) | 23.6 (12.6) | <0.01 |
In Fig. 1, we show as a graph the relationship between the persistence of JPC and HRQoL. A dose–response relationship is visible, i.e. the participants in the never JPC group reported better HRQoL scores than the sometimes JPC group, who reported better HRQoL scores than the often JPC group. Individuals who reported JPC at each of the measurement moments had the worst HRQoL scores (Fig. 1). The base model demonstrated that the presence of JPC was statistically significantly negatively associated with HRQoL [regression coefficient β (95% CI) −14.50 (−16.14, −12.87)], indicating that individuals with JPC had a lower score of 14.5 points on the KOOS HRQoL than those without JPC. The corrected model demonstrated oriented point estimates for having JPC and the influence of time on JPC similar to the base [−3.57 (−4.69, −2.44)]. Time was a positive factor for HRQoL in both models, indicating an increase in HRQoL over time: base model 1.25 (0.94, 1.55) and corrected model 0.79 (0.54, 1.03). Complete case analysis produced a similar plot with broader and overlapping CIs (supplementary Fig. 1, available as supplementary data at Rheumatology Online). In addition, GEE modelling on complete case data produced similar statistically significant associations with smaller β-values.
Fig. 1.
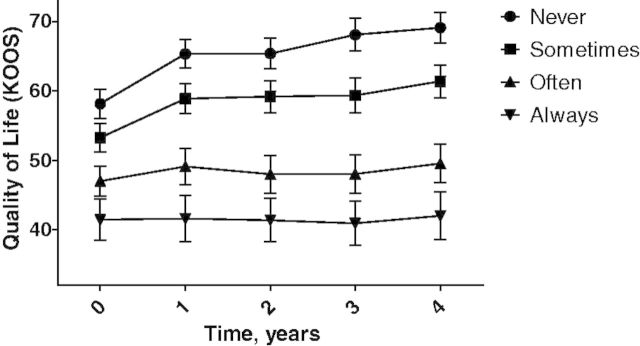
HRQoL scores plotted over time for the four JPC groups.
Physical activity decreased over time, with similar slopes for each of the groups and CIs overlapping, with the exception of the never and always groups. GEE analyses revealed in the base model a negative, longitudinal association between physical activity levels and having JPC [−9.88 (−16.00, −3.76)]. However, the corrected model demonstrated that having JPC was not associated with physical activity levels [−1.32 (−6.61, 3.98)]. In both the base and corrected model, time was a negative factor [−4.64 (−5.85, −3.44) and −4.79 (−6.01, −3.57)]. Visual and statistical analyses on complete case data yielded similar results to the multiple imputed data (see also supplementary Fig. 2, available as supplementary data at Rheumatology Online).
Discussion
Our results confirm that JPC is highly prevalent in individuals with knee OA. Moreover, in line with our hypothesis we found that JPC was negatively associated with HRQoL, whereas our hypothesis that JPC was associated with physical activity could not be confirmed. The unfavourable association between health-related outcomes and JPC in individuals with OA has already been established cross-sectionally [4, 5]. However, we are the first to demonstrate this relationship longitudinally, thus providing a more precise estimate of the strength of this relationship.
Our findings may have some implications for clinical care in knee OA. We propose that the assessment of JPC should be part of routine (clinical and research) practice, and that health care providers should be aware of the association between JPC and HRQoL when treating individuals with knee OA. In case JPC is present, a clinician or researcher should be aware that this person could be clinically and prognostically different from a patient without JPC. Perhaps therapy goals need to be adjusted accordingly, as focusing merely on the most affected joint might result in disappointing therapy results. The latter needs to be confirmed in intervention studies [18]. Unfortunately research on the management of JPC in individuals with OA is still scarce [19] and requires further study [12].
We are the first to study the association between having JPC and physical activity. Our results indicate that physical activity decreased markedly over a 4-year period [∼11% (P < 0.01) for the whole group (data not shown)]. However, we could not confirm our hypothesis that having JPC contributed to an extra decline in physical activity while accounting for socio-demographics, medical comorbidities and knee pain. On the other hand, it should be noted that the validity of the PASE questionnaire is questioned [20], necessitating further study on JPC in OA and objectively measured physical activity.
Although the strengths of this study include the large, representative sample of individuals from a variety of cultural backgrounds, this study also has some limitations. Patient-reported outcomes are prone to a phenomenon called response shift: the potential of subjects’ views, values or expectations to change over the course of a study, thereby adding an additional factor of change to results [21]. Response shift might explain why HRQoL improved over time in our analyses. Another limitation is the lack of insight into the nature of the joint pain, as only global questions were asked about the chronicity of JPC, while acute joint pain information was not collected.
In conclusion, JPC has a significant impact on the HRQoL of individuals with knee OA. Therefore both researchers and clinicians should assess JPC in their daily practice when working with individuals with knee OA. In addition, the general trend towards physical inactivity is reason for concern.

Supplementary Material
Acknowledgements
The OAI is a public–private partnership comprising five contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261 and N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI study investigators. Private funding partners include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH or the private funding partners.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25:173–83. doi: 10.1016/j.berh.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Forestier R, Francon A, Briole V, Genty C, Chevalier X, Richette P. Prevalence of generalized osteoarthritis in a population with knee osteoarthritis. Joint Bone Spine. 2011;78:275–8. doi: 10.1016/j.jbspin.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Gunther KP, Sturmer T, Sauerland S, et al. Prevalence of generalised osteoarthritis in patients with advanced hip and knee osteoarthritis: the Ulm Osteoarthritis Study. Ann Rheum Dis. 1998;57:717–23. doi: 10.1136/ard.57.12.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suri P, Morgenroth DC, Kwoh CK, Bean JF, Kalichman L, Hunter DJ. Low back pain and other musculoskeletal pain comorbidities in individuals with symptomatic osteoarthritis of the knee: data from the osteoarthritis initiative. Arthritis Care Res. 2010;62:1715–23. doi: 10.1002/acr.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoogeboom TJ, den Broeder AA, Swierstra BA, de Bie RA, van den Ende CH. Joint-pain comorbidity, health status, and medication use in hip and knee osteoarthritis: a cross-sectional study. Arthritis Care Res. 2012;64:54–8. doi: 10.1002/acr.20647. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Prevalence and impact of chronic joint symptoms–seven states, 1996. JAMA. 1998;279:1940–1. [PubMed] [Google Scholar]
- 7.Feinglass J, Nelson C, Lawther T, Chang RW. Chronic joint symptoms and prior arthritis diagnosis in community surveys: implications for arthritis prevalence estimates. Public Health Rep. 2003;118:230–9. doi: 10.1093/phr/118.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busija L, Buchbinder R, Osborne RH. Quantifying the impact of transient joint symptoms, chronic joint symptoms, and arthritis: a population-based approach. Arthritis Rheum. 2009;61:1312–21. doi: 10.1002/art.24508. [DOI] [PubMed] [Google Scholar]
- 9.Nevitt M, Felson D, Lester G. The Osteoarthritis Initiative: protocol for the cohort study. 2006 http://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf (3 November 2012, date last accessed) [Google Scholar]
- 10.Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17. doi: 10.1186/1477-7525-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39:336–40. [PubMed] [Google Scholar]
- 12.Hoogeboom TJ, Stukstette MJ, de Bie RA, Cornelissen J, den Broeder AA, van den Ende CH. Non-pharmacological care for patients with generalized osteoarthritis: design of a randomized clinical trial. BMC Musculoskelet Disord. 2010;11:142. doi: 10.1186/1471-2474-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twisk JWR. a practical guide. New York: Cambridge University Press; 2003. Applied longitudinal data analysis for epidemiology. [Google Scholar]
- 14.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royston P. Multiple imputation of missing values: update of ice. Stata J. 2005;5:527–36. [Google Scholar]
- 16.Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. doi: 10.1186/1471-2288-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui J. QIC program and model selection in GEE analyses. Stata J. 2007;7:12. [Google Scholar]
- 18.Miles CL, Pincus T, Carnes D, et al. Can we identify how programmes aimed at promoting self-management in musculoskeletal pain work and who benefits? A systematic review of sub-group analysis within RCTs. Eur J Pain. 2011;15:775 e1–11. doi: 10.1016/j.ejpain.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Conaghan PG, Birrell F, Burke M, et al. Osteoarthritis: national clinical guideline for care and management in adults. 2008 http://publications.nice.org.uk/osteoarthritis-cg59/introduction (3 November 2012, date last accessed) [PubMed] [Google Scholar]
- 20.Svege I, Kolle E, Risberg MA. Reliability and validity of the Physical Activity Scale for the Elderly (PASE) in patients with hip osteoarthritis. BMC Musculoskelet Disord. 2012;13:26. doi: 10.1186/1471-2474-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robling M, Hood K. Response shift, responsiveness or recall bias? Br J Gen Pract. 2002;52:585. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


