Abstract
Objectives:
To determine the incidence and prevalence of treated epilepsy in an adult Medicaid population.
Methods:
We performed a retrospective, dynamic cohort analysis using Ohio Medicaid claims data between 1992 and 2006. Individuals aged 18–64 years were identified as prevalent cases if they had ≥2 claims of epilepsy (ICD-9-CM: 345.xx) or ≥3 claims of convulsion (ICD-9-CM: 780.3 or 780.39) and ≥2 claims of antiepileptic drugs. Incident cases were required to have no epilepsy or convulsion claims for ≥5 years before epilepsy diagnosis. Subjects were determined as having preexisting disability and/or comorbid conditions, including brain tumor, depression, developmental disorders, migraine, schizophrenia, stroke, and traumatic brain injury, when at least one of these conditions occurred before epilepsy onset.
Results:
There were 9,056 prevalent cases of treated epilepsy in 1992–2006 and 1,608 incident cases in 1997–2006. The prevalence was 13.2/1,000 (95% confidence interval, 13.0–13.5/1,000). The incidence was 362/100,000 person-years (95% confidence interval, 344–379/100,000 person-years). The incidence and prevalence were significantly higher in men, in older people, in blacks, and in people with preexisting disability and/or comorbid conditions. The most common preexisting conditions in epilepsy subjects were depression, developmental disorders, and stroke, whereas people with brain tumor, traumatic brain injury, and stroke had the higher risk of developing epilepsy.
Conclusions:
The Medicaid population has a high incidence and prevalence of epilepsy, in an order of magnitude greater than that reported in the US general population. This indigent population carries a disproportionate amount of the epilepsy burden and deserves more attention for its health care needs and support services.
Medicaid, financed jointly by the US federal and state governments, provides health care coverage for low-income families and for individuals with disabilities. Medicaid is the single largest health insurer in the United States with an average monthly enrollment of nearly 60 million beneficiaries.1 In general, subjects enrolled in Medicaid are educationally and socioeconomically disadvantaged and a large number of them have disabling physical and/or mental ailments, several of which are also risk factors for epilepsy. However, no study, to our knowledge, has specifically examined the incidence and/or prevalence of epilepsy in this subgroup of the US population. Both incidence and prevalence data on Medicaid beneficiaries are crucial for the complete understanding of the burden of epilepsy in the United States. Moreover, this information is necessary for planning appropriate actions or interventions to provide needed health care and support services for this highly vulnerable subgroup of the population. This study was designed to estimate the incidence and prevalence of epilepsy in the Medicaid population. We hypothesized that Medicaid beneficiaries had high incidence and prevalence of epilepsy, likely greater than the US general population.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Institutional Review Boards at Case Western Reserve University and by the Ohio Department of Job and Family Services.
Data source.
We obtained Ohio Medicaid claims data between 1992 and 2006 from the Ohio Department of Job and Family Services. Medicaid claims data consist of enrollment and claims files. For each enrollee, the enrollment files carry data on demographic characteristics, duration of enrollment, and eligibility category. The claims files encompass claims for care received in the physician's office, inpatient/outpatient hospital settings, including emergency departments, as well as prescription drugs, and include the dates of service, diagnosis, and procedure codes. Prescription drug claims are for filled prescriptions only; they carry the date when the prescription was filled, drug name, dose, and number of days supplied. The diagnoses are coded using the ICD-9-CM. The procedure codes are in ICD-9 in the inpatient claims, but in Current Procedural Terminology, 4th edition, or in the Healthcare Common Procedures Coding System in the outpatient claims.
Study population.
We performed a retrospective, dynamic cohort analysis, which means that the population at risk changes over time as people enter (or leave) the cohort throughout the study period. Subjects entered our cohort if they were between 18 and 64 years of age at the time of enrollment. We created a subcohort for incidence estimation in which we selected only those subjects who had enrolled in Medicaid for ≥5 years. Individuals who had enrollment gaps between the first and the last dates of enrollment of >20%, those who enrolled in managed care programs, the Medicare program, or spend-down program were excluded from analyses because of potentially incomplete claims data.
Epilepsy case ascertainment.
We used standard criteria for epilepsy case ascertainment consistent with previous studies that used claims data.2–4 Individuals were identified as having epilepsy if they met the following criteria (figure e-1 on the Neurology® Web site at www.neurology.org):
At least 1 visit with an epilepsy diagnosis (ICD-9-CM: 345.xx); or ≥2 visits, on different dates, with a diagnosis of nonfebrile convulsions (ICD-9-CM: 780.3 or 780.39). The epilepsy onset or epilepsy index date was determined as the date of the first diagnosis of epilepsy or the second diagnosis of a nonfebrile convulsion.
At a minimum of 30 days after the epilepsy index date, there was ≥1 visit related to epilepsy or nonfebrile convulsions (ICD-9-CM: 345.xx, 780.3, or 780.39).
A minimum of 2 pharmacy dispensing claims, ≥30 days apart subsequent to the epilepsy index date, for any of the following antiepilepsy drugs: carbamazepine, ethosuximide, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, phenobarbital, phenytoin, pregabalin, primidone, tiagabine, topiramate, valproic acid, or zonisamide.
Definition of prevalent and incident cases.
Cohort members meeting the epilepsy case definition were identified as prevalent cases. Individuals were considered incident cases if they met the epilepsy case definition for the first time and had no epilepsy or seizure claims for ≥5 years before epilepsy onset (epilepsy-free interval) (figure e-1). To allow for all subjects to have ≥5 years of an epilepsy-free interval before epilepsy onset, incident cases were identified between 1997 and 2006.
Identification of comorbid conditions.
Because of the overrepresentation of disabling physical and/or mental ailments in the Medicaid population,5 several of which are risk factors for epilepsy, subjects were determined as having preexisting disability and/or comorbid conditions, including brain tumor, depression, developmental disorders, migraine, schizophrenia, stroke, and traumatic brain injury (TBI), when at least one of these conditions occurred before the epilepsy index date (figure e-1). The preexisting disability was determined by the presence of disability status in the enrollment files. The preexisting comorbid conditions were identified by the presence of ≥2 ICD-9-CM codes of the conditions of interest (table e-1) that occurred ≥30 days apart.
Other variables of interest.
Age at the time of epilepsy onset was determined by using the epilepsy index date and was categorized as 18–24, 25–34, 35–44, 45–54, and 55–64 years. For subjects without epilepsy, age was determined at the time of cohort entry. Race was categorized as black, white, and other.
Analysis.
The prevalence of epilepsy was estimated by dividing the number of persons with epilepsy (PWE) by the total number of cohort members. The incidence rate (IR) of epilepsy was calculated by dividing the number of incident cases of epilepsy by the number of accrued person-years across the study period between 1997 and 2006. We used log-binomial Poisson regression analysis to estimate prevalence ratio (PR) and log-linear Poisson regression to estimate IR ratio (IRR), controlling for sex, age, race, preexisting disability, and comorbid conditions. In addition, we estimated attributable risk for each comorbid condition using the equation (IRexposed − IRunexposed)/IRexposed, where IRexposed represented the incidence of epilepsy among subjects with preexisting comorbid condition of interest and IRunexposed represented the incidence of epilepsy among subjects without preexisting comorbid condition of interest. Because of a small number of new cases among subjects with preexisting disability and/or comorbid conditions, the incidence estimates by sex, race, and age of epilepsy onset were unstable and therefore were not presented. All analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC). The GENMOD procedure was used for Poisson regression analysis. All p values were 2-sided and values <0.05 were considered statistically significant.
RESULTS
Prevalence sample.
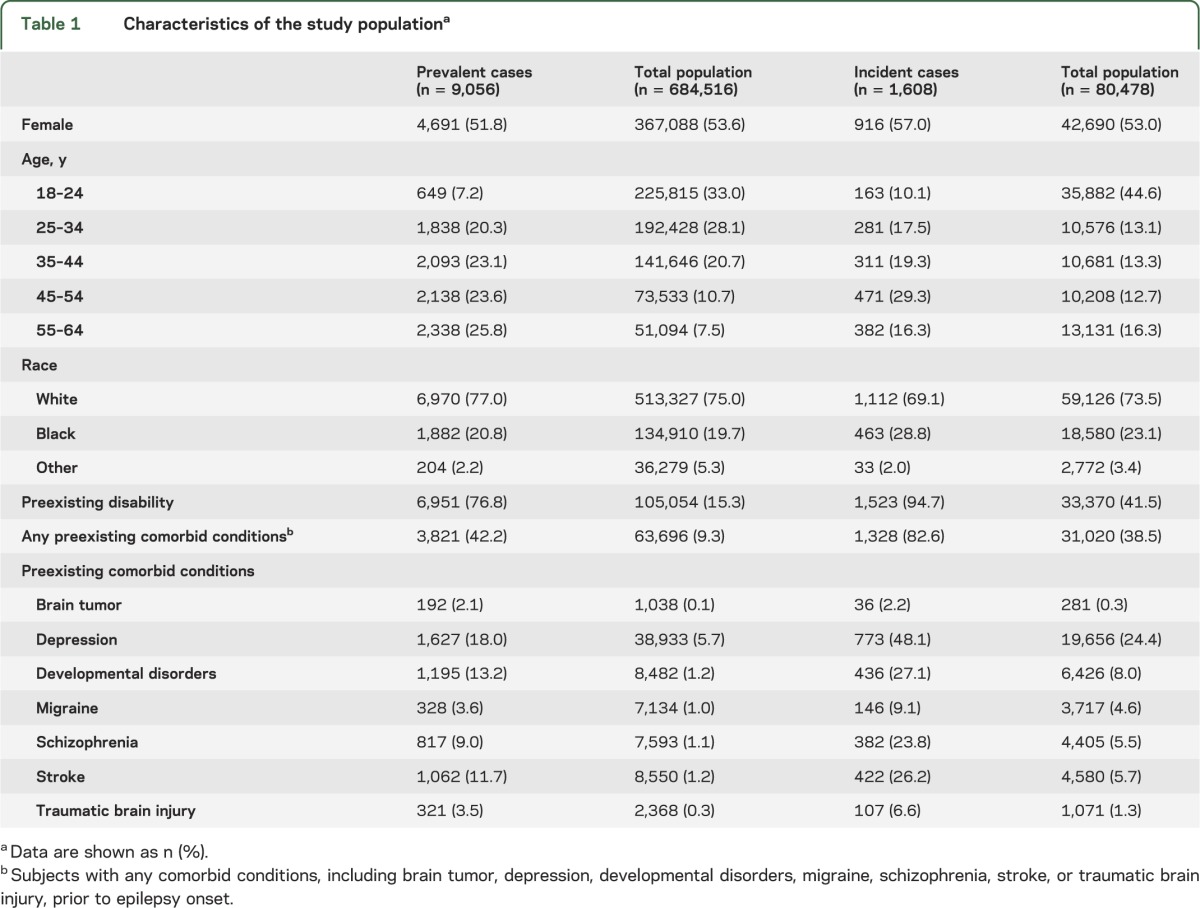
Between 1992 and 2006, there were 684,516 Medicaid enrollees who met the inclusion criteria (table 1). The mean age was 31.2 ± 11.7 years (range, 18–64 years). The majority of subjects (58.1%) were young adults (aged 18–34 years); 75.0% were white; and approximately 15% of them had preexisting disability based on the eligibility for Medicaid enrollment. Less than 10% of the entire study population had preexisting comorbid conditions of interest. During the study period, 9,056 individuals met the epilepsy case definition. PWE were slightly older than subjects without epilepsy and approximately 26% of them were older than 55 years. The mean age was 36.9 ± 12.5 years (range, 18–64 years). The mean observation period from the first to the last day of Medicaid enrollment was 8.1 ± 5.1 years (range, 1–15 years). Racial distribution was similar between subjects with and without epilepsy. Nearly 80% of PWE had preexisting disability and approximately 40% had at least one comorbid condition. The most common preexisting comorbid conditions in PWE were depression, developmental disorders, and stroke.
Table 1.
Characteristics of the study populationa
Prevalence of epilepsy.
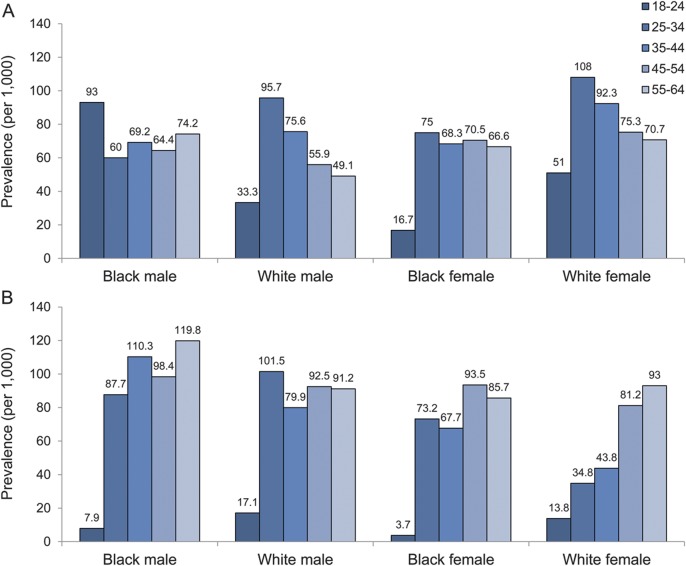
The prevalence of epilepsy among Medicaid beneficiaries was 13.2/1,000 (95% confidence interval [CI], 13.0–13.5/1,000) (table 2). The prevalence of epilepsy in men was significantly higher than in women. We noted that age-specific prevalence increased significantly with age. Blacks had significantly higher prevalence than whites. In addition, prevalence of epilepsy among subjects with preexisting disability was significantly greater than that of subjects without preexisting disability (PR, 18.2; 95% CI, 17.3–19.1). Likewise, the prevalence of epilepsy among subjects with preexisting comorbid conditions was also significantly higher than that in subjects without preexisting comorbid conditions (PR, 7.1; 95% CI, 6.8–7.4). The prevalence of epilepsy stratified by sex, age, and race in subjects with preexisting disability and in subjects with preexisting comorbid conditions is shown in figure 1.
Table 2.
Incidence and prevalence of epilepsy in Medicaid beneficiaries
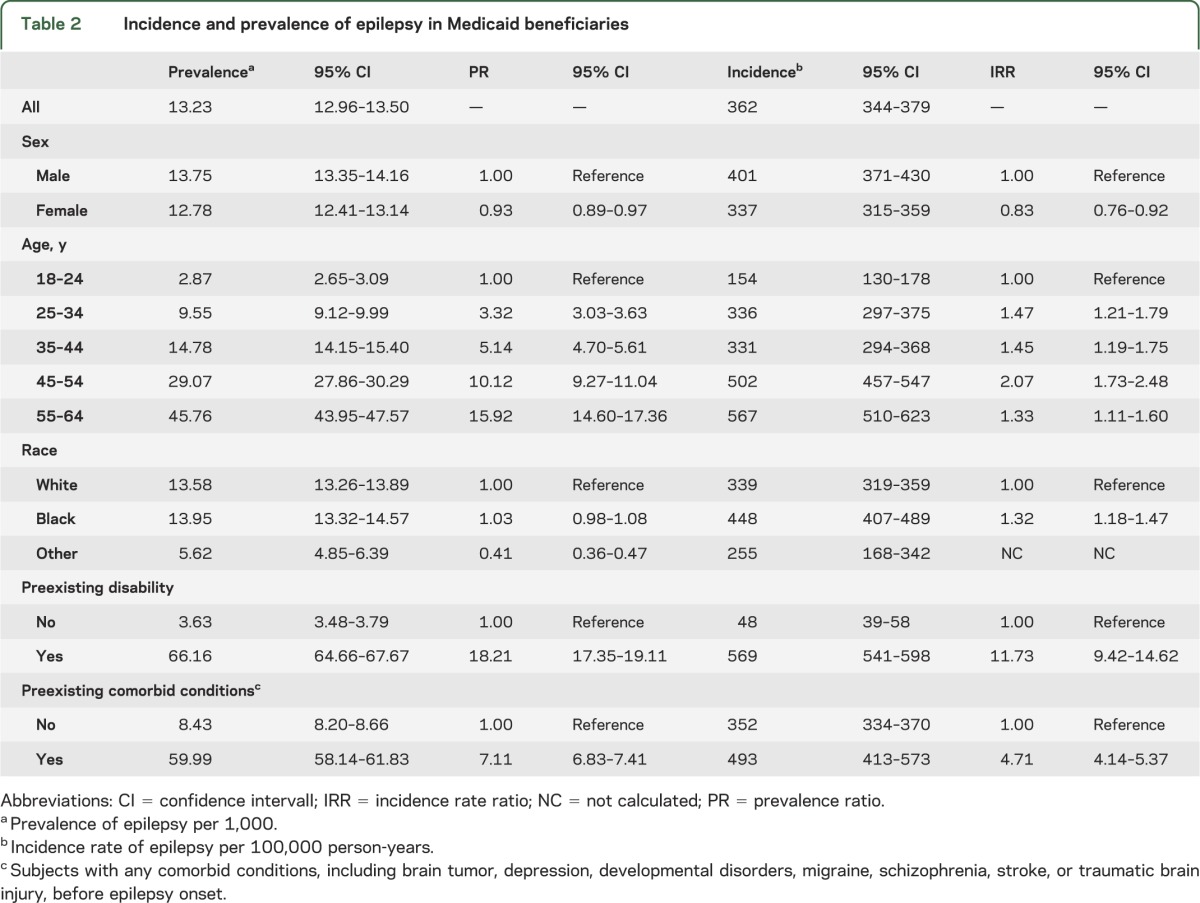
Figure 1. Prevalence of epilepsy for the low-income population in the United States between 1992 and 2006 by sex, race, and age at epilepsy diagnosis.
(A) Prevalence of epilepsy for people with preexisting disability. (B) Prevalence of epilepsy for people with preexisting brain tumor, stroke, depression, developmental disorders, migraine, schizophrenia, and/or traumatic brain injury. Note: Prevalence of epilepsy by sex, race, and age for people without preexisting disability or comorbid conditions is shown in figure e-2.
Incidence cohort.
There were 80,478 subjects who had enrolled in Medicaid for at least 5 years and met other inclusion criteria, for a total follow-up of 444,375 person-years (table 1). Similar to the prevalence sample, 57.7% were young adults (aged 18–34 years), and 73.5% were white. The mean age was 28.5 ± 13.2 years (range, 18–64 years). The proportion of subjects with preexisting disability was slightly higher than in the prevalence sample. Almost half of the subjects had preexisting disability and nearly 40% of the subjects had one or more preexisting comorbid conditions of interest. Between 1997 and 2006, 1,608 subjects were identified as incident cases of epilepsy. The mean age at epilepsy onset was 43.6 ± 12.7 years (range, 18–64 years). The majority of incident epilepsy cases had preexisting disability and/or comorbid conditions before epilepsy diagnosis. Similar to prevalent cases, depression, stroke, and developmental disorders were among the most common preexisting comorbid conditions.
Incidence of epilepsy.
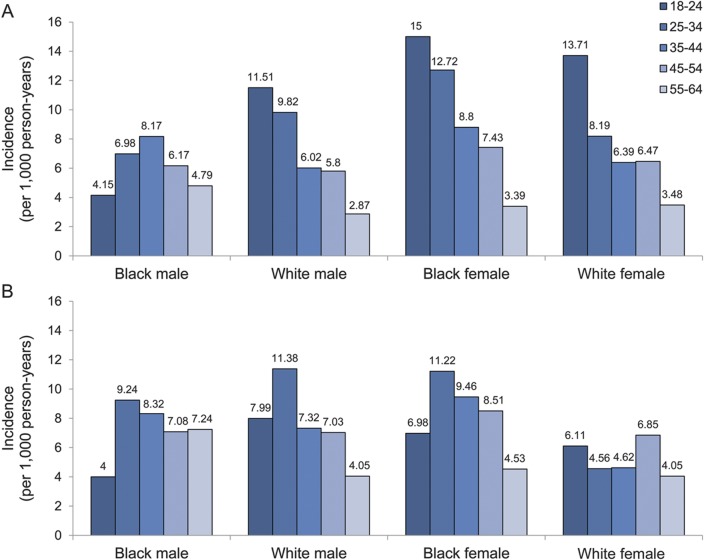
The IR of epilepsy among Medicaid beneficiaries was 360/100,000 person-years (95% CI, 340–380/100,000 person-years) (table 2). The IR significantly increased with age and was significantly higher in men. Blacks had significantly higher IRs than whites. Compared with subjects without preexisting disability, those with preexisting disability had a significantly higher risk of epilepsy (IRR, 11.7; 95% CI, 9.4–14.6). Similarly, the risk of epilepsy was significantly increased in subjects with preexisting comorbid conditions compared with those without (IRR, 4.7; 95% CI, 4.1–5.4). The incidence of epilepsy stratified by sex, age, and race in subjects with preexisting disability and in subjects with preexisting comorbid conditions is shown in figure 2.
Figure 2. Incidence rate of epilepsy for the low-income population in the United States between 1997 and 2006 by sex, race, and age at epilepsy onset.
(A) Incidence rate of epilepsy for people with preexisting disability. (B) Incidence rate of epilepsy for people with preexisting brain tumor, stroke, depression, developmental disorders, migraine, schizophrenia, and/or traumatic brain injury.
DISCUSSION
A number of studies have reported the incidence and prevalence of epilepsy but have not specifically investigated these in low-income populations in the United States. By examining the Medicaid population, this longitudinal cohort study demonstrates for the first time that epilepsy is much more common in this socioeconomic demographic than previously suspected. The incidence and prevalence of epilepsy in the general population appear to be lower than in the Medicaid population and therefore cannot be applied to this population. Our findings emphasize that data on incidence and prevalence of epilepsy specific to people on Medicaid are necessary for assessing the burden of epilepsy and planning of health services for this indigent population.
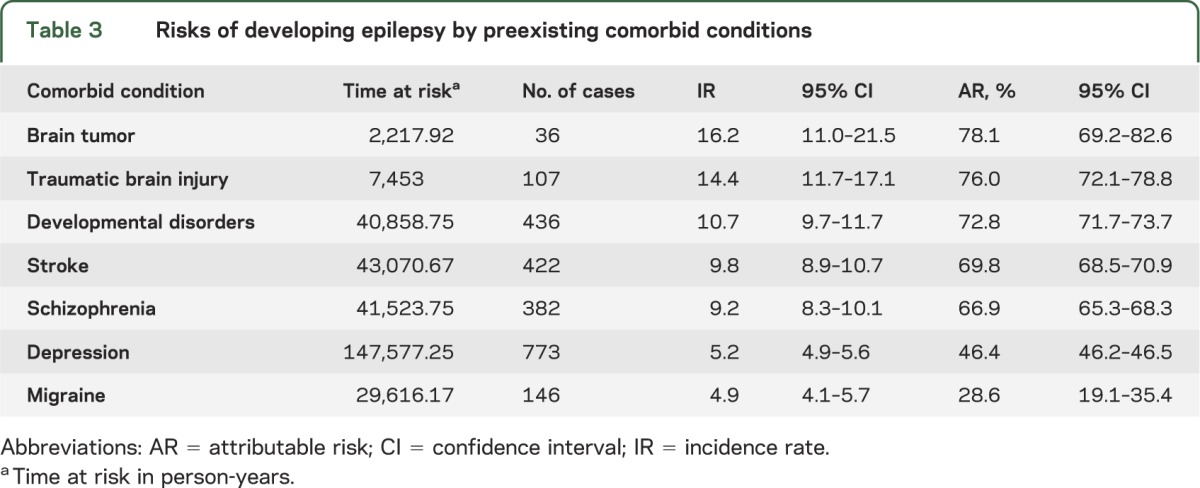
Previous studies have shown that people in lower socioeconomic class have higher incidence of several diseases,6 including epilepsy.7–9 Nonetheless, it remains unclear why socioeconomically deprived populations have a high risk of developing epilepsy. Genetic susceptibility10 and/or the presence of significant socioeconomic differentials in health-risk behaviors, exposure to occupational and environmental health hazards, or access to and use of proper medical care could potentially put people in low socioeconomic status at risk of developing epilepsy or other neurologic conditions that lead to epilepsy. A high incidence and prevalence of epilepsy in the Medicaid population is not unexpected. Medicaid is the principal safety-net health insurance program in the United States for low-income adults, disabled individuals, and the elderly.1 The majority of Medicaid enrollees have poor health and have a high prevalence of chronic medical conditions.5 Some of these chronic conditions, especially depression, migraine, and schizophrenia, might share common underlying neuropathologic or physiologic dysfunction with epilepsy.11 Other conditions, including brain tumor,12 stroke,13 and TBI,14 are well-known risk factors for symptomatic epilepsy. Our data suggest that among these preexisting conditions, brain tumor, TBI, and stroke (although less common than depression and developmental disorders) carry a higher risk of developing epilepsy (table 3).
Table 3.
Risks of developing epilepsy by preexisting comorbid conditions
The incidence of epilepsy varies substantially across studies depending on the study population, method of case ascertainment, and diagnostic accuracy.15–17 In the United States, the annual incidence of epilepsy ranges from 15 to 71/100,000 person-years.3,9,18 Nonetheless, these studies consistently show that adults aged 25–64, the healthiest subgroup of the population, have the lowest incidence of epilepsy.9,19,20 A study of people in the health maintenance organizations (HMOs) reports that the incidence of epilepsy in this age group varies between 12.4 and 20.9/100,000 person-years.20 The low incidence estimate in the HMO population is probably attributable to the fact that enrollment in HMOs is employment-based and employed individuals are likely to be healthy and therefore have low risk of developing epilepsy.20 In contrast, we found that the incidence of epilepsy is highest in young and middle-aged adults. Similar to the incidence, the prevalence of epilepsy also varies widely.15,17 The prevalence of epilepsy in the United States usually ranges from 5 to 9/1,000.3,18,21–25 Studies using door-to-door survey normally report higher prevalence than those using the medical record review or medical contact.21 A study in people who enrolled in a managed care organization shows that the prevalence of epilepsy for people aged 20 to 64 is approximately 7–8/1,000.3 We found that the prevalence of epilepsy in adult Medicaid beneficiaries is much greater, especially in those with preexisting disability. Medicaid individuals with disability are a diverse group of people with a wide variety of conditions that cause physical and/or psychological impairments and limitations.26 Many of the disabled individuals in Medicaid have several forms of disabilities. Approximately half of them have physical impairments and functional limitations, and almost 40% have severe mental illnesses.26 These conditions, such as autism and mental retardation, are closely related to epilepsy.27 Interestingly, we found that among people with preexisting disability, white women aged 25–34 have the highest prevalence of epilepsy (figure 1A). The reason for a substantially high prevalence in this subgroup is less clear. Because psychogenic, nonepileptic seizures typically present in young women,28,29 one might speculate that a high prevalence of epilepsy in this subgroup might be attributable to diagnostic error as some of the women with nonepileptic seizures might have been misdiagnosed as having epilepsy and treated with antiepilepsy drugs. It could also be that the underlying conditions resulting in disability in these young women are more severe and/or closely associated to epilepsy. However, without clinical information, these assumptions cannot be tested.
Previous epidemiologic studies show that the incidence and prevalence of epilepsy increase with increasing age.15,21 In the Medicaid population, this pattern is only seen in the subgroup of people without preexisting disability and/or comorbidities (figure e-2) and in women with preexisting comorbid conditions (figure 1B). For other subgroups, especially those with disability, we found that epilepsy incidence and prevalence actually decrease as age increases. Our data also show that the prevalence of epilepsy in the youngest subgroup of black males, black females, and white males with preexisting comorbid conditions is substantially lower than that in the older age group (figure 1B). The inverted U-shape distribution of age-specific incidence or prevalence is normally seen in reports from developing countries30 or less-developed regions of an industrialized country.31 Because the majority of Medicaid individuals have preexisting disability and/or comorbid conditions, it is possible that these conditions in persons aged 25–34 and 35–44 are more severe and therefore result in a greater risk of developing epilepsy and/or premature mortality. A prior study shows that the risk of premature death is significantly increased during the first few years after epilepsy diagnosis.32 However, whether this is also true for the Medicaid population requires further study.
The major limitation of this study is that we relied solely on ICD-9-CM diagnosis codes and pharmacy claims for epilepsy case ascertainment. The process of assigning ICD-9-CM codes is complex and is therefore subject to errors.33 To ensure the validity of a diagnostic paradigm that depends largely on ICD-9-CM codes, reviewing medical records of the identified (and the unidentified) cases is necessary. Unfortunately, under the current data user agreement, we are not allowed to link Medicaid beneficiaries to their medical records because of privacy concerns. Without precise clinical information, the validity of our case ascertainment remains unknown. Nonetheless, we used very stringent criteria to identify PWE (figure e-1). In addition, for incident epilepsy cases, we required that subjects be enrolled in Medicaid for ≥5 years before epilepsy diagnosis and that there was no epilepsy or seizure diagnosis during that time period. These criteria are conservative and therefore highly specific in the identification of PWE. Requiring a long period of enrollment before epilepsy diagnosis might introduce a selection bias favoring older populations, and genuine newly diagnosed epilepsy cases recently enrolled into the program may be missed. Because of the strict case definition, the incidence and prevalence of epilepsy in this study could have been underestimated. Nevertheless, we demonstrate a very high incidence and prevalence of epilepsy in the Medicaid population.
Whether our findings could be generalizable to the Medicaid population in other geographic areas is debatable. Each state in the United States is responsible for their own Medicaid program under regulations and guidance of the federal government.26 The states have considerable discretion for eligibility policy, benefit coverage, and program administration. In addition, many states have expanded coverage beyond what federal Medicaid law requires. Nonetheless, the sickest and poorest among the states, including children, low-income parents, and individuals with disability, account for the majority of the Medicaid population.26 Ohio Medicaid is no exception. In addition, enrollment and eligibility for adults in Ohio Medicaid are consistently ranked in the middle among other states and therefore could be considered as “typical” for Medicaid programs in the United States.34 The proportion of beneficiaries in each eligibility group in the Ohio Medicaid program is also largely comparable to national estimates.35 Consequently, the incidence and prevalence of epilepsy reported in this study can be viewed as reasonable estimates for determining health care needs and for planning service delivery for the entire Medicaid population. Nonetheless, these data should be used with caution. At a minimum, our findings indicate that epilepsy is a significant public health problem and disproportionately represented in the low-income population of an otherwise high-income country.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Mr. James Gearheart of the Ohio Department of Job and Family Services for his comments.
GLOSSARY
- CI
confidence interval
- HMO
health maintenance organization
- ICD-9-CM
International Classification of Diseases, ninth revision, Clinical Modification
- IR
incidence rate
- IRR
incidence rate ratio
- PR
prevalence ratio
- PWE
persons with epilepsy
- TBI
traumatic brain injury
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design: Dr. Kaiboriboon, Dr. Koroukian. Analysis and interpretation of data: Dr. Kaiboriboon, Dr. Bakaki, Dr. Lhatoo, Dr. Koroukian. Drafting of the manuscript: Dr. Kaiboriboon, Dr. Bakaki. Critical revision of the manuscript for important intellectual content: Dr. Kaiboriboon, Dr. Bakaki, Dr. Lhatoo, Dr. Koroukian. Statistical analysis: Dr. Bakaki, Dr. Koroukian. Administrative, technical, or material support: Dr. Kaiboriboon, Dr. Koroukian. Study supervision: Dr. Kaiboriboon, Dr. Lhatoo, Dr. Koroukian.
STUDY FUNDING
K.K. and P.M.B. are supported by the Epilepsy Foundation. This study was also supported in part by the Case Western Reserve University/Cleveland Clinic CTSA grant number UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kaiser Commission on Medicaid and the Uninsured The Medicaid program at a glance. Available at: http://www.kff.org/medicaid/upload/7235-04.pdf. Accessed March 22, 2012
- 2.Holden EW, Grossman E, Nguyen HT, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag 2005;8:1–14 [DOI] [PubMed] [Google Scholar]
- 3.Holden EW, Thanh Nguyen H, Grossman E, et al. Estimating prevalence, incidence, and disease-related mortality for patients with epilepsy in managed care organizations. Epilepsia 2005;46:311–319 [DOI] [PubMed] [Google Scholar]
- 4.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 2011;52(suppl 7):2–26 [DOI] [PubMed] [Google Scholar]
- 5.Gibson TB, Lee TA, Vogeli CS, et al. A four-system comparison of patients with chronic illness: the Military Health System, Veterans Health Administration, Medicaid, and commercial plans. Mil Med 2009;174:936–943 [DOI] [PubMed] [Google Scholar]
- 6.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: no easy solution. JAMA 1993;269:3140–3145 [PubMed] [Google Scholar]
- 7.Heaney DC, MacDonald BK, Everitt A, et al. Socioeconomic variation in incidence of epilepsy: prospective community based study in south east England. BMJ 2002;325:1013–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesdorffer DC, Tian H, Anand K, et al. Socioeconomic status is a risk factor for epilepsy in Icelandic adults but not in children. Epilepsia 2005;46:1297–1303 [DOI] [PubMed] [Google Scholar]
- 9.Benn EK, Hauser WA, Shih T, et al. Estimating the incidence of first unprovoked seizure and newly diagnosed epilepsy in the low-income urban community of Northern Manhattan, New York City. Epilepsia 2008;49:1431–1439 [DOI] [PubMed] [Google Scholar]
- 10.Ottman R, Annegers JF, Risch N, Hauser WA, Susser M. Relations of genetic and environmental factors in the etiology of epilepsy. Ann Neurol 1996;39:442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winawer MR, Hesdorffer DC. Migraine, epilepsy, and psychiatric comorbidity: partners in crime. Neurology 2010;74:1166–1168 [DOI] [PubMed] [Google Scholar]
- 12.Hildebrand J, Lecaille C, Perennes J, Delattre JY. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology 2005;65:212–215 [DOI] [PubMed] [Google Scholar]
- 13.Lossius MI, Ronning OM, Slapo GD, Mowinckel P, Gjerstad L. Poststroke epilepsy: occurrence and predictors—a long-term prospective controlled study (Akershus Stroke Study). Epilepsia 2005;46:1246–1251 [DOI] [PubMed] [Google Scholar]
- 14.Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet 2009;373:1105–1110 [DOI] [PubMed] [Google Scholar]
- 15.Banerjee PN, Filippi D, Allen Hauser W. The descriptive epidemiology of epilepsy: a review. Epilepsy Res 2009;85:31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 2010;51:883–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology 2011;77:1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroner BL, Fahimi M, Kenyon A, Thurman DJ, Gaillard WD. Racial and socioeconomic disparities in epilepsy in the District of Columbia. Epilepsy Res 2013;103:279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993;34:453–468 [DOI] [PubMed] [Google Scholar]
- 20.Annegers JF, Dubinsky S, Coan SP, Newmark ME, Roht L. The incidence of epilepsy and unprovoked seizures in multiethnic, urban health maintenance organizations. Epilepsia 1999;40:502–506 [DOI] [PubMed] [Google Scholar]
- 21.Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940–1980. Epilepsia 1991;32:429–445 [DOI] [PubMed] [Google Scholar]
- 22.Kobau R, Zahran H, Grant D, Thurman DJ, Price PH, Zack MM. Prevalence of active epilepsy and health-related quality of life among adults with self-reported epilepsy in California: California Health Interview Survey, 2003. Epilepsia 2007;48:1904–1913 [DOI] [PubMed] [Google Scholar]
- 23.Kelvin EA, Hesdorffer DC, Bagiella E, et al. Prevalence of self-reported epilepsy in a multiracial and multiethnic community in New York City. Epilepsy Res 2007;77:141–150 [DOI] [PubMed] [Google Scholar]
- 24.Kobau R, Zahran H, Thurman DJ, et al. Epilepsy surveillance among adults—19 States, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ 2008;57:1–20 [PubMed] [Google Scholar]
- 25.Kobau R, Luo Y, Zack MM, Helmers S, Thurman DJ. Epilepsy in adults and access to care—United States, 2010. MMWR Morb Mortal Wkly Rep 2012;61:909–913 [PubMed] [Google Scholar]
- 26.Schneider A, Elias R. The Medicaid Resource Book. Kaiser Commission on Medicaid and the Uninsured. Available at: http://www.kff.org/medicaid/2236-index.cfm. Accessed December 22, 2012 [Google Scholar]
- 27.Fisher B, Dezort C, Nordli DR, Berg AT. Routine developmental and autism screening in an epilepsy care setting. Epilepsy Behav 2012;24:488–492 [DOI] [PubMed] [Google Scholar]
- 28.Meierkord H, Will B, Fish D, Shorvon S. The clinical features and prognosis of pseudoseizures diagnosed using video-EEG telemetry. Neurology 1991;41:1643–1646 [DOI] [PubMed] [Google Scholar]
- 29.Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav 2003;4:205–216 [DOI] [PubMed] [Google Scholar]
- 30.Edwards T, Scott AG, Munyoki G, et al. Active convulsive epilepsy in a rural district of Kenya: a study of prevalence and possible risk factors. Lancet Neurol 2008;7:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CC, Chen LS, Yen MF, Chen HH, Liou HH. Geographic variation in the age- and gender-specific prevalence and incidence of epilepsy: analysis of Taiwanese National Health Insurance-based data. Epilepsia 2012;53:283–290 [DOI] [PubMed] [Google Scholar]
- 32.Lhatoo SD, Johnson AL, Goodridge DM, MacDonald BK, Sander JW, Shorvon SD. Mortality in epilepsy in the first 11 to 14 years after diagnosis: multivariate analysis of a long-term, prospective, population-based cohort. Ann Neurol 2001;49:336–344 [PubMed] [Google Scholar]
- 33.O'Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res 2005;40:1620–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seiber EE, Sahr TR. Public-private substitution among Medicaid adults: evidence from Ohio. Medicare Medicaid Res Rev 2011;1:E1–E17 Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/MMRR/Downloads/MMRR2011_001_01_A01.pdf. Accessed December 22, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis ER, Roberts D, Rousseau DM, Schwartz T. Medicaid enrollment in 50 States. The Kaiser Commission on Medicaid and the Uninsured. Available at: http://www.kff.org/medicaid/upload/7606-04.pdf. Accessed October 1, 2012
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.