Abstract
Objectives:
We and others showed that migraineurs are at increased risk of subclinical and clinical ischemic brain lesions. Migraineurs also have a higher prevalence of frequent syncope and orthostatic intolerance, symptoms that are associated with transient reductions in cerebral blood flow. In this study, we assessed whether these autonomic symptoms may contribute to the increased risk of brain lesions in migraine.
Methods:
Migraineurs (n = 291) and controls (n = 140) from the population-based, cross-sectional CAMERA (Cerebral Abnormalities in Migraine, an Epidemiologic Risk Analysis) cohort (aged 30–60 years, and free of other neurologic symptoms) underwent 1) brain MRI scan, and 2) structured telephone interview including questions on frequent syncope (≥5/lifetime) and orthostatic intolerance.
Results:
Frequent syncope (odds ratio [OR] = 2.7; 95% confidence interval: 1.3–5.5) and orthostatic intolerance (OR = 2.0 [1.1–3.6]) were independent risk factors for high load of deep white matter lesions. Effects were strongest in women and similar in migraineurs and controls. Migraine diagnosis did not mediate or moderate these associations. Individuals with orthostatic intolerance had higher prevalence of high periventricular white matter lesion load (OR = 1.9 [1.1–3.5]). Syncope and orthostatic intolerance were not related to subclinical infarcts or infratentorial lesions.
Conclusions:
Frequent syncope, orthostatic intolerance, and migraine independently increase the risk of white matter lesions, particularly in females.
Migraine is a common neurovascular brain disorder characterized by attacks of disabling headache, autonomic dysfunction, and sometimes aura symptoms.1 Although suffering a migraine attack is ranked among the most painful and disabling disorders,2 the disease migraine is commonly not believed to have long-term brain consequences.
However, we found in the population-based CAMERA (Cerebral Abnormalities in Migraine, an Epidemiologic Risk Analysis) study that female migraineurs are at increased risk of deep white matter hyperintense lesions (WMLs), and that both male and female migraineurs have a higher prevalence of infratentorial brain lesions, including hyperintense brainstem lesions and cerebellar infarcts.3,4 This association was not modified by cardiovascular risk factors or use of vasoconstricting antimigraine medication. These MRI lesions appear to have developed subclinically, but they might still be relevant in view of the findings of a meta-analysis showing that migraine is a risk factor for ischemic stroke.5 Furthermore, WMLs have in general a predictive value for future stroke and cognitive and functional decline.6 The mechanisms leading to increased risk of brain lesions in migraineurs remain unknown.
In the same cohort, we examined clinical presentations of autonomic nervous system (ANS) dysfunction. We found an increased lifetime prevalence of syncope, frequent syncope, and orthostatic intolerance (OI) in migraineurs without interictal signs of ANS failure.7 Abnormal reactivity of blood pressure or heart rate, i.e., orthostatic hypotension (OH)8 or postural tachy-cardia syndrome,9,10 was not found more often in migraineurs. A recent study suggested the existence of syncopal migraine (i.e., migrainous headache immediately preceding or following syncopal episodes).11
ANS dysfunction is associated with transient cerebral hypoperfusion12–14 and may predispose to brain lesions.15–20 The Atherosclerosis Risk in Communities (ARIC) study identified asymptomatic OH and orthostatic decrease of systolic and diastolic blood pressure as predictors of ischemic stroke, even after adjustment for other risk factors.19,20 An increased rate of WMLs has also been reported in patients with severe, symptomatic OH due to multiple system atrophy and Lewy body dementia.21–23 The severity of OH correlated with the degree of WMLs. A multifactorial etiology for WMLs is likely because OH often occurs together with known WML risk factors, including supine hypertension and cognitive decline.24–26 Surprisingly, there are no studies on whether the most common cause of transient cerebral hypoperfusion, i.e., reflex syncope, is harmful to the brain. Of interest, a recent study demonstrated cerebral blood flow abnormalities in patients with syncope in their asymptomatic period (i.e., between syncopal attacks).27
The purpose of this study was to evaluate whether clinical symptoms of ANS dysfunction increase the risk of WMLs, and whether this effect is influenced by the presence of migraine.
METHODS
Study population.
The population-based CAMERA study has been described previously.3,28 In brief, from 863 migraineurs and 5,628 controls who were identified according to International Headache Society criteria,1 we randomly selected 134 migraineurs without aura, 161 with aura, and 140 controls without headaches aged 30 to 60 years, frequency matched by sex and age. Cases and controls did not differ in age composition, sex proportions, and cardiovascular risk factors; this also held for responders (69%) vs nonresponders.
Participants gave written informed consent and participated without financial reimbursement. The protocol was approved by the ethics committee and included a computerized structured telephone interview, a neurologic examination, and a brain MRI study. The hospital visit took place within 10 days of the interview, in migraine cases in a headache-free period (≥3 days after a migraine attack).
Syncope-related variables.
The assessment of ANS symptoms and signs has been detailed previously.7 Briefly, syncope (“fainting”) was explained to all participants with the same standard text, as a brief loss of consciousness that may be provoked by the sight of blood or by standing for a long time in the heat, but that could occur without a clear provocation. Unconsciousness due to head injury and epileptic seizure were not counted as syncope. “Near syncope” was described to patients as the symptoms that usually precede a faint but can also occur separately and consist of dizziness, light-headedness, loss of concentration, ringing in the ears, or darkening of sight. Answers were categorized for the analyses as “syncope ever” (at least 1 attack) and “frequent syncope” (5 or more syncopal attacks over the lifetime). Additional questions explored syncope, near syncope, or avoidance during specific circumstances, including prolonged standing, long hot showers, after heavy meals, after exercise, or at the sight of blood or during a venipuncture. OI was defined as the provocation of syncope or presyncope upon standing or the avoidance of prolonged standing (see full questionnaire text [appendix e-1 on the Neurology® Web site at www.neurology.org]).7 Two autonomic reactivity tests were performed (change in blood pressure and heart rate after standing up and after venipunction), but based on the previous results were not part of the current analyses. All syncope-related variables were predefined in the previous article, blinded for the MRI results.
Magnetic resonance imaging.
Whole-brain MRI scans from a 1.0- and a 1.5-tesla unit with comparable protocols included contiguous 3-mm axial slices with combined proton density and T2-weighted fast spin-echo and fluid-attenuated inversion recovery (FLAIR) sequences (figure). Blinded to clinical status, one neuroradiologist read all images and systematically recorded topographic details of periventricular and deep WMLs (PVWMLs and DWMLs, respectively), infratentorial hyperintense lesions (IHLs), and infarcts. A complete description of the semiquantitative WML-rating methods has been provided previously.3,25 PVWML scores >3 (of 15) and the highest 20% volumes of total DWML load were classified as high load. IHLs were hyperintense on proton-density and T2 images and were not hypointense on FLAIR images.29,30 Infarcts were defined as nonmass parenchymal defects, with a vascular distribution, isointense to CSF signal on all sequences, and, when supratentorial, surrounded by a hyperintense rim on FLAIR images.31,32 There was no difference in sensitivity of both scanners to pick up lesions.3
Figure. Examples of a case with (A, woman 45 years) and a case without (B, woman, 47 years) deep white matter hyperintensities.
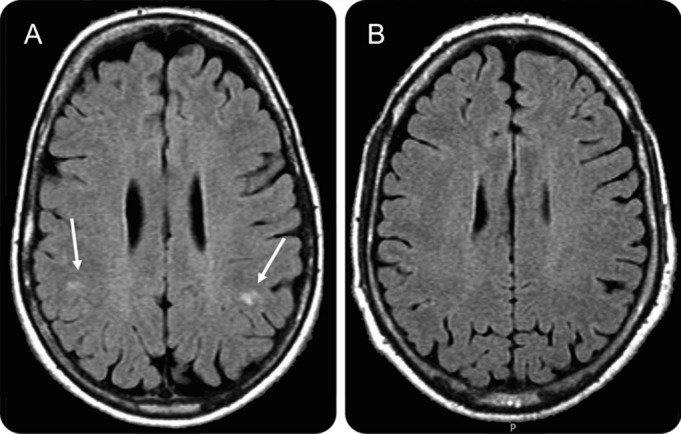
(A) With deep white matter lesions (DWMLs) (arrows); (B) without DWMLs.
Assessment of confounders, covariates, and migraine characteristics.
Sociodemographic, medical, and migraine characteristics were assessed by interview and physical examination, and are described in detail elsewhere.3 Migraine participants estimated headache and aura attack frequency, and frequency and amount of specific antimigraine medication (ergotamines, triptans) they used in the years they had migraine attacks.3
Statistics.
Syncope data were available from 140 controls and 291 migraineurs of 435 participants with complete MRI data. In a maximum of 12 participants, data on the syncope frequency were incomplete; these participants did not differ from the remainder regarding demographic variables, cardiovascular risk factors, or MRI variables. Chi-square tests, unpaired t tests, and 1-way analyses of variance were used to test for any differences in the distributions and means of measured characteristics among the study groups. The assessment of associations of migraine and its subtypes (with and without aura) to the risk of infarcts, high PVWML load, high DWML load, and IHLs was described in previous reports.3,4 It was based on logistic regression analyses controlling for age, sex, cardiovascular risk factors, and antimigraine medication. Previous and also current analyses were stratified by sex because of differences in prevalence and risk for migraine and stroke.3,5 The prevalence comparison of syncope variables between migraineurs and controls was also described previously.7 Because that group (n = 476) also included subjects without MRI data, we now recalculated the statistics for the current subgroup of subjects with MRI data (n = 431).
Current analyses focus on the association between syncope-related variables and brain lesions on MRI. This was assessed by comparing presence (yes/no) of infarcts, high DWML load, high PVWML load, and IHLs vs preselected syncope-related variables (syncope ever, frequent syncope [≥5 times], and OI). Using logistic regression analysis, we examined the risk (odds ratios [ORs], 95% confidence intervals) for the MRI outcomes by syncope variables, controlling for age, sex, and municipality (model 1), and additionally for education, body mass index, hypertension, cholesterol, alcohol use categories, and smoking (model 2), and stratified these analyses in addition by sex and migraine diagnosis. Because of the small numbers, this was possible only for the DWML outcome.
To evaluate mediation of the associations by migraine, we added migraine diagnosis (yes/no) to model 2. To assess moderation, we calculated the ORs for each diagnosis combination of syncope and migraine (adding dummy variables to model 2; e.g., risk for migraineurs without OI, risk for controls with OI, and risk for migraineurs with OI compared with controls without OI as the reference group). These analyses were a priori stratified by sex, because the established association between high DWML load and migraineurs was present only in females.3 Analyses and appropriate regression diagnostics were conducted with the Statistical Package of Social Sciences (version 16; SPSS Inc., Chicago, IL).
RESULTS
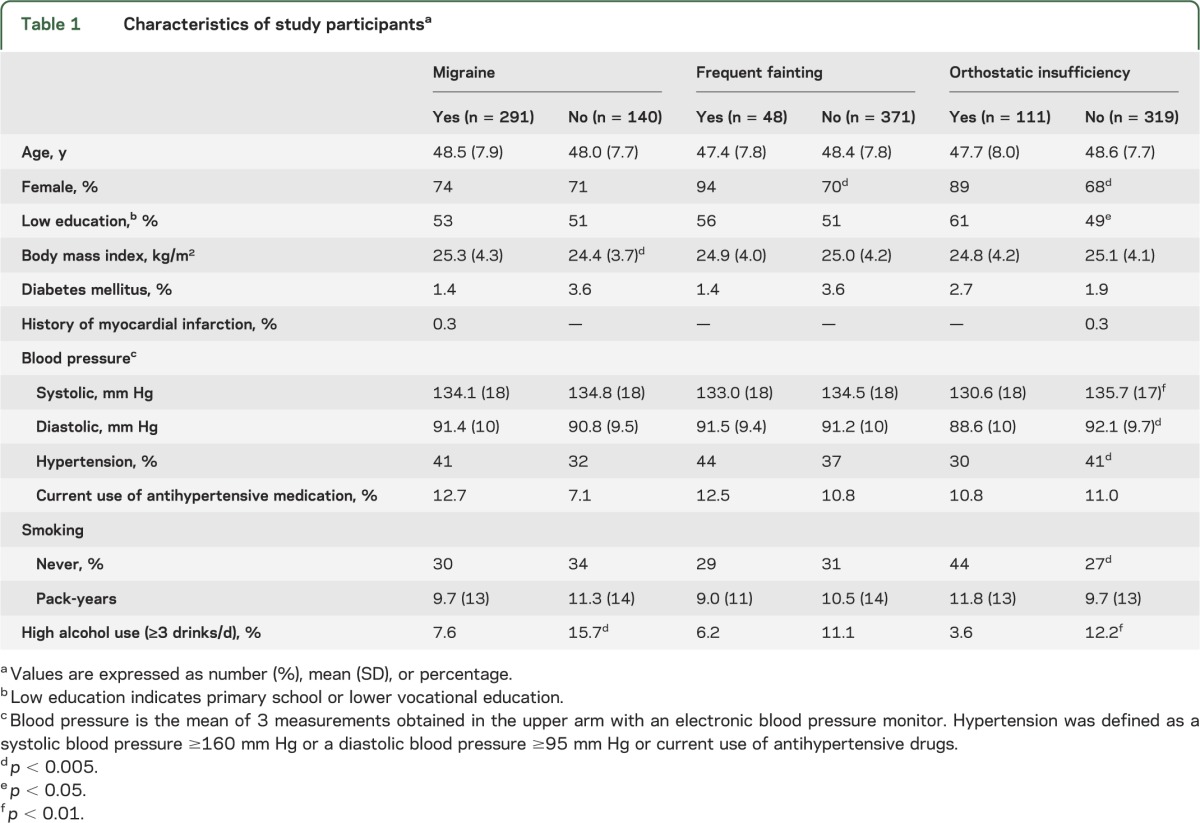
The demographic characteristics and risk factors of the participants according to their migraine diagnosis are shown in table 1. Measured blood pressures and hypertension prevalence were lower in those with OI, but not in those with a history of frequent fainting or migraine. As reported earlier,7 also in this subgroup of subjects with MRI data (n = 431), migraineurs (vs controls) had a significantly higher prevalence of “syncope ever” (49% vs 31%; p < 0.0001), “frequent syncope” (14% vs 6%; p = 0.01), and OI (32% vs 12%; p < 0.0001; see table e-1 for further prevalence data and table e-2 for results of autonomic reactivity tests). After stratification by sex, the differences remained significant only in female participants, but it should be noted that the number of men was much lower than that of women. No differences were found between migraineurs with and without aura, nor between those with fewer (<1 attack/month) and more attacks. Diabetes mellitus, history of myocardial infarction, body mass index, high alcohol intake, and use of antihypertensives did not emerge as potential risk factors for syncope. Irrespective of migraine diagnosis, there was overlap between “frequent syncope” and OI: of those with frequent syncope, 57% also had OI, vs 22% of those without frequent syncope; of those with OI, 25% were frequent fainters, vs 7% of those without OI (p < 0.001).
Table 1.
Characteristics of study participantsa
Those with and without history of “syncope ever” did not differ in prevalence of any lesions. Table 2 shows the prevalence of high DWML load, high PVWML load, and IHLs in those with and without frequent syncope and OI (crude data). Participants with frequent syncope had a higher prevalence of high DWML load (p = 0.005), which was strongest in female subjects and which seems to involve migraineurs and controls equally. In logistic regression analyses with multivariate adjustments (table 3), frequent syncope was an independent risk factor for high DWML load (model 2, OR = 2.7; 95% confidence interval: 1.3–5.5), and the association was not significantly mediated by migraine diagnosis (see table 3, model 2 + migraine). This association remained significant and similar also in stratified analyses of controls only, and in analyses of those without current use of antihypertensives (data not shown). Frequent syncope also showed a trend (table 2) toward higher prevalence of high PVWML load in all participants (p = 0.08), which was again clearest in females (p = 0.06). The number of cases with a high PVWML load was too small for multivariate analyses. Frequent syncope was not related to IHLs.
Table 2.
Prevalence of syncope, orthostatic intolerance, and orthostatic hypotension versus presence of MRI hyperintense lesions: The population-based CAMERA MRI study (n = 431)a
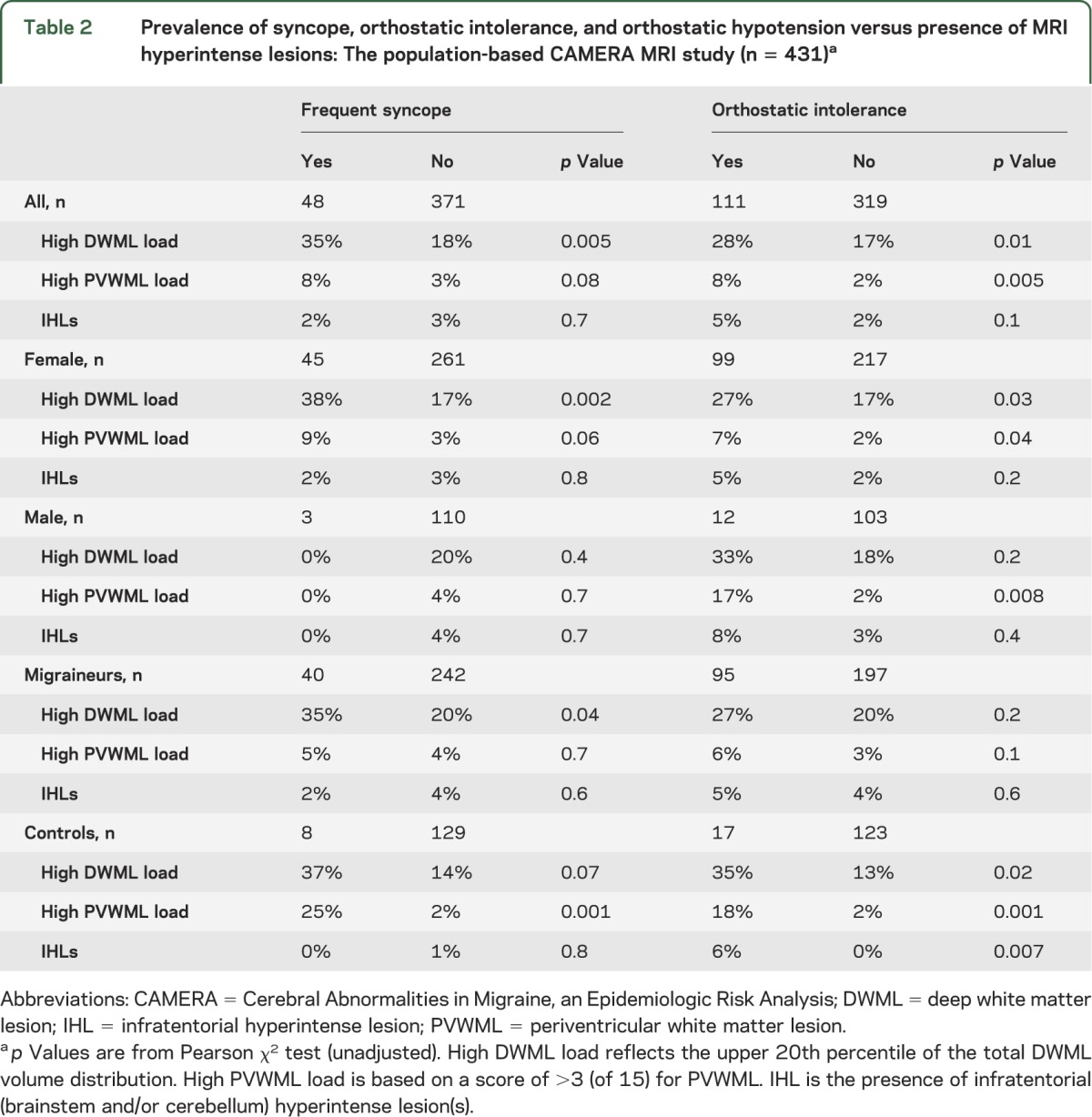
Table 3.
Risk of high DWML load by syncope-related risk factors, with and without controlling for migraine diagnosis: The population-based CAMERA MRI study (n = 431)a
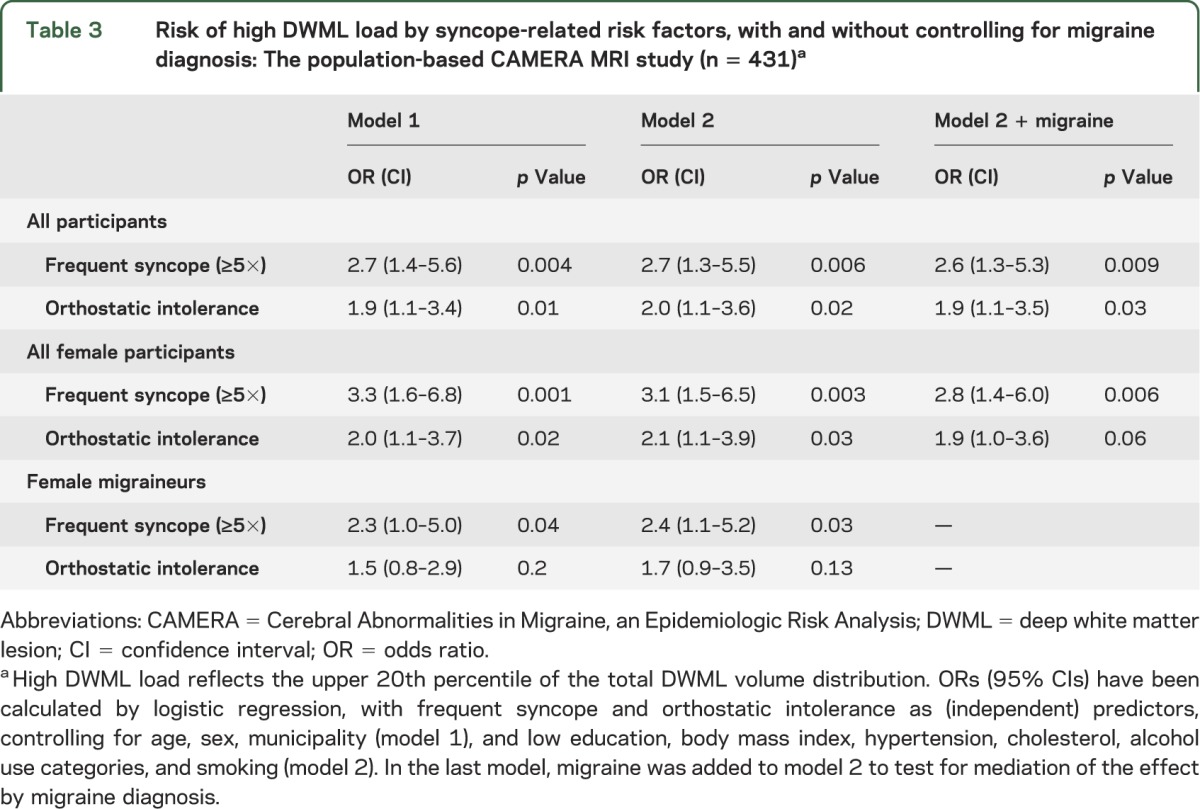
Participants with OI more often had high DWML load (table 2; p = 0.01) and high PVWML load (p = 0.005), also most explicit in females. Multivariate analyses (table 3) also identified OI as an independent risk factor for high DWML load, and this association was also not significantly mediated by migraine, and similar (and significant) in analyses in controls only. In subanalyses of controls only, OI was related to a higher prevalence of IHLs (table 2, p = 0.007).
In assessing the risk of high DWML load in subgroups of female participants based on diagnosis combinations of syncope and migraine (dummy analyses), we found similarly increased risks for migraineurs with frequent syncope (OR = 6.3 [1.0–40], model 2) and controls with frequent syncope (OR = 6.0 [2.1–17]), which excludes a significant moderating role of migraine in the association between frequent syncope vs high DWML load: female migraineurs without frequent syncope were still at increased risk (OR = 2.3 [1.0–5.3]; similar to earlier reported values3); female controls without frequent syncope served as the reference group (OR = 1.0). In the same way, female migraineurs with OI (OR = 3.9 [0.9–17]) and female controls with OI (OR = 3.5 [1.4–8.6]) were at similarly increased risk of high DWML load; female migraineurs without OI also remained at increased risk for high DWML load (OR = 2.0 [0.8–4.7]).
We found no difference in prevalence of brain or cerebellar infarcts in those without vs those with frequent syncope (8% vs 2%; p = 0.1) or OI (8% vs 5%; p = 0.4); this did not change after stratification by sex, migraine status, or migraine subtype.
DISCUSSION
We present evidence that frequent syncope and OI are independent risk factors for high DWML load and possibly also for high PVWML load and IHLs in healthy, young to middle-aged subjects from the general population. The effects were strongest in women and similar in migraineurs and nonmigraineurs. Migraine did not alter the associations. Female migraineurs without frequent syncope or OI were still at 2-fold increased risk of high DWML load.3 Therefore, a history of frequent syncope or OI does not by itself explain why female migraineurs have higher risk of brain lesions; both syncope-related ANS symptoms as well as migraine seem to be independent risk factors.
Syncope is defined as transient, brief, and self-limited loss of consciousness due to cerebral hypoperfusion, with spontaneous recovery.12,13 The 3 groups of causes of syncope are reflex syncope (synonym: neurally mediated syncope), cardiac syncope, and OH. Given the relatively young age at onset (migraineurs 21 ± 13 years; controls 23 ± 13 years) and the specific circumstances triggering (pre)syncope, it is highly likely that most cases were of reflex syncope.7 Whereas long-lasting cerebral hypoperfusion is known to cause brain damage, so far evidence has been lacking that short-lasting hypoperfusion as in reflex syncope is associated with subclinical brain changes.
WMLs are frequently observed in older subjects, and their prevalence increases with age. Their pathogenesis is still incompletely understood. Multiple mechanisms are likely involved, including episodic or chronic hypoperfusion, atherosclerosis, insufficient blood-brain barrier function, altered vascular permeability, increased endothelial activation and microglial activation due to hypoxic and inflammatory insults, and CSF accumulation.33 The regions of the white matter that are supplied by the distal ends of the penetrating arterioles (the periventricular zones and the semioval center) already have, in normal conditions, the lowest perfusion pressure.34 These zones are most vulnerable to periods of relative hypoperfusion, and perfusion may theoretically become insufficient (ischemia) either in conditions of chronically impaired local cerebral blood flow, as may happen in large- and/or small-vessel disease, or in conditions with a paroxysmal reduction in cardiac output or blood pressure, as in syncope or OH. It is therefore conceivable that repeated cerebral hypoperfusion in frequent syncope may contribute to development of WMLs. However, it should be stressed that this study is not able to prove any causative mechanism.
This is the first MRI study on WMLs in frequent syncope. Several MRI studies found associations between OH and the risk of WMLs and/or silent infarcts. Most such studies concerned older subjects with concurrent risk factors such as hypertension and diseases such as dementia.16,17,21,24,26 In such subjects, it is likely that both local factors and systemic hypotension colluded to result in WMLs. Strong evidence that OH is an independent predictor of ischemic stroke in middle-aged persons comes from the ARIC study.19,20 Our results are in line with these studies, with one exception, and that is that no such additional risk factors are likely to have influenced the results in the present relatively young group. In both reflex syncope and OH, low systemic blood pressure will result in transient cerebral hypoperfusion. However, the degree of cerebral hypoperfusion will depend on the integrity of the cerebral autoregulation. The role of autoregulation in reflex syncope and OH is still a matter of debate.14 Our study was too small to assess an eventual specific distribution of syncope-related brain lesions; this requires further study. Two previous studies indicated that the cerebral perfusion deficits during reflex syncope are most prominent in the frontal regions.35,36 Accordingly, cognitive executive functions in patients with syncope due to OH were greatly affected by an orthostatic challenge.37 However, in a separate publication on the current cohort, we found no evident effects of the identified deep WMLs on cognitive functioning.38
Strengths of the CAMERA study have been detailed and acknowledged previously.3,39 The detailed description of the cohort allowed us to control for possibly confounding effects due to cardiovascular risk factors, including diabetes and hypertension, in multivariate analyses. Eventual residual confounding by current antihypertensive treatment was thought to be unlikely after additional stratified analyses in those without current use of such treatment (either antihypertensive or as preventive migraine medication). Although there were no participants with overt heart disease, we cannot exclude the possibility that some syncope-related variables might act as a proxy for cardiovascular risk factors.19 The relatively young age of the study group suggests that this explanation is not likely. Although our syncope-related measures were historical, and thus potentially subject to recall bias, we are confident that by using relatively robust cutoff values for, e.g., “frequent syncope,” and by using different questions to assess orthostatic complaints, these data produced a valid classification of individuals with vs without clear ANS symptoms. The questions assessing OI might have included subjects with nonspecific complaints, but this does not seem to have a substantial role, given the similar effects observed for frequent syncope and OI. Because of logistical limitations inherent to a large-scale epidemiologic study, we were unable to perform a more extensive ANS test battery, e.g., with tilt-table testing; such assessments will likely allow for a more precise evaluation of subtypes of ANS dysfunction. Because data were acquired blinded to migraine diagnosis, the stratification by migraine diagnosis is also not a likely source of bias. Even if migraineurs for unknown reasons would have been more likely to answer syncope questions affirmatively, this would not explain any relationship between syncope and DWMLs in controls.
In summary, we identified frequent syncope and OI as independent risk factors for high DWML load in both controls and migraineurs, without interaction of the association by migraine. In female migraineurs, this adds to their known increased risk of high DWML load, but does not explain it. It should be stressed, however, that we studied subclinical brain lesions; the clinical relevance of these lesions in controls and migraineurs is still unknown. Additional clinical and epidemiologic studies are needed to confirm and explore these findings.
Supplementary Material
GLOSSARY
- ANS
autonomic nervous system
- ARIC
Atherosclerosis Risk in Communities
- CAMERA
Cerebral Abnormalities in Migraine, an Epidemiologic Risk Analysis
- DWML
deep white matter lesion
- FLAIR
fluid-attenuated inversion recovery
- IHL
infratentorial hyperintense lesion
- OH
orthostatic hypotension
- OI
orthostatic intolerance
- OR
odds ratio
- PVWML
periventricular white matter lesion
- WML
white matter hyperintense lesion
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
M. Kruit contributed to this study by study concept and design, acquisition of data, analysis and interpretation of data, and drafting/revising the manuscript. R. Thijs contributed by analysis and interpretation of data and drafting/revising the manuscript. M. Ferrari contributed by obtaining funding, study design, interpretation of data, and revising the manuscript. L. Launer contributed by obtaining funding, study concept and design, analysis of data, and revising the manuscript. M. van Buchem contributed by obtaining funding, study concept and design, interpretation of data, and revising the manuscript. J.G. van Dijk contributed by study design, interpretation of data, and revising the manuscript.
STUDY FUNDING
This study was supported by grant 97.108 from the Netherlands Heart Foundation.
DISCLOSURE
M. Kruit is funded by NIH grant 1R01NS061382 and at the time of the current study by grant 97.108 from the Netherlands Heart Foundation. R. Thijs has been supported by the Dutch Epilepsy Foundation (Nationaal Epilepsie Fonds the Netherlands), and received speaker fees from Medtronic. M. Ferrari is funded by grants from NIH (1R01NS061382), the Netherlands Organisation for Scientific Research (903-52-291, VICI 918-56-602, Spinoza 2009), the European Community (EUROHEAD, LSHM-CT-2004-504837), and the Centre for Medical Systems Biology in the framework of the Netherlands Genomics Initiative. He reports consultancy/industry support from Almirall, Coherex, CoLucid, Eisai, GlaxoSmithKline, Linde, MAP, Medtronic, Menarini, Merck, Minster, Pfizer, and St. Jude. L. Launer is a Senior Investigator in the Intramural Research Program, National Institute on Aging, US. M. van Buchem has been supported by the Center for Translational Molecular Medicine, 7th EU Framework Programme, ZON-MW, Reumafonds, NIH grant 1RO1NS070834, and Center for Medical Systems Biology, and received speaker fees from Philips Healthcare. J.G. van Dijk reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(suppl 1):9–160 [DOI] [PubMed] [Google Scholar]
- 2.Leonardi M, Steiner TJ, Scher AT, Lipton RB. The global burden of migraine: measuring disability in headache disorders with WHO's Classification of Functioning, Disability and Health (ICF). J Headache Pain 2005;6:429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruit MC, van Buchem MA, Hofman PA, et al. Migraine as a risk factor for subclinical brain lesions. JAMA 2004;291:427–434 [DOI] [PubMed] [Google Scholar]
- 4.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Brain stem and cerebellar hyperintense lesions in migraine. Stroke 2006;37:1109–1112 [DOI] [PubMed] [Google Scholar]
- 5.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 2009;339:b3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt R, Grazer A, Enzinger C, et al. MRI-detected white matter lesions: do they really matter? J Neural Transm 2011;118:673–681 [DOI] [PubMed] [Google Scholar]
- 7.Thijs RD, Kruit MC, van Buchem MA, Ferrari MD, Launer LJ, van Dijk JG. Syncope in migraine: the population-based CAMERA study. Neurology 2006;66:1034–1037 [DOI] [PubMed] [Google Scholar]
- 8.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology 1996;46:1470. [DOI] [PubMed] [Google Scholar]
- 9.Low PA, Opfer-Gehrking TL, Textor SC, et al. Postural tachycardia syndrome (POTS). Neurology 1995;45:S19–S25 [PubMed] [Google Scholar]
- 10.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69–72 [DOI] [PubMed] [Google Scholar]
- 11.Curfman D, Chilungu M, Daroff RB, Alshekhlee A, Chelimsky G, Chelimsky TC. Syncopal migraine. Clin Auton Res 2012;22:17–23 [DOI] [PubMed] [Google Scholar]
- 12.Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Eur Heart J 2009;30:2631–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dijk JG, Thijs RD, Benditt DG, Wieling W. A guide to disorders causing transient loss of consciousness: focus on syncope. Nat Rev Neurol 2009;5:438–448 [DOI] [PubMed] [Google Scholar]
- 14.van Dijk JG, Thijs RD. Cerebral perfusion in syncope. In: Benditt DG, Brignole M, Raviele A, Wieling W, editors. Syncope and Transient Loss of Consciousness. Oxford, UK: Blackwell Publishing; 2007:20–23 [Google Scholar]
- 15.Dobkin BH. Orthostatic hypotension as a risk factor for symptomatic occlusive cerebrovascular disease. Neurology 1989;39:30–34 [DOI] [PubMed] [Google Scholar]
- 16.Bakker SL, de Leeuw FE, de Groot JC, et al. Cerebral vasomotor reactivity and cerebral white matter lesions in the elderly. Neurology 1999;52:578–583 [DOI] [PubMed] [Google Scholar]
- 17.Eguchi K, Kario K, Hoshide S, et al. Greater change of orthostatic blood pressure is related to silent cerebral infarct and cardiac overload in hypertensive subjects. Hypertens Res 2004;27:235–241 [DOI] [PubMed] [Google Scholar]
- 18.van Osch MJ, Jansen PA, Vingerhoets RW, van der GJ. Association between supine cerebral perfusion and symptomatic orthostatic hypotension. Neuroimage 2005;27:789–794 [DOI] [PubMed] [Google Scholar]
- 19.Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D. Orthostatic hypotension as a risk factor for stroke: the Atherosclerosis Risk in Communities (ARIC) study, 1987–1996. Stroke 2000;31:2307–2313 [DOI] [PubMed] [Google Scholar]
- 20.Yatsuya H, Folsom AR, Alonso A, Gottesman RF, Rose KM. Postural changes in blood pressure and incidence of ischemic stroke subtypes: the ARIC study. Hypertension 2011;57:167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenny RA, Shaw FE, O'Brien JT, Scheltens PH, Kalaria R, Ballard C. Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry 2004;75:966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim TS, Lee PH, Kim HS, Yong SW. White matter hyperintensities in patients with multiple system atrophy. J Neurol 2009;256:1663–1670 [DOI] [PubMed] [Google Scholar]
- 23.Tha KK, Terae S, Yabe I, et al. Microstructural white matter abnormalities of multiple system atrophy: in vivo topographic illustration by using diffusion-tensor MR imaging. Radiology 2010;255:563–569 [DOI] [PubMed] [Google Scholar]
- 24.Kario K, Hoshide S, Shimada K. Extreme-dipper, abnormal diurnal blood pressure variation in the elderly hypertensive, and orthostatic hypertension. J Hum Hypertens 1998;12:141–142 [DOI] [PubMed] [Google Scholar]
- 25.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Leeuw FE, de Groot JC, Oudkerk M, et al. A follow-up study of blood pressure and cerebral white matter lesions. Ann Neurol 1999;46:827–833 [DOI] [PubMed] [Google Scholar]
- 27.Joo EY, Hong SB, Lee M, et al. Cerebral blood flow abnormalities in patients with neurally mediated syncope. J Neurol 2011;258:366–372 [DOI] [PubMed] [Google Scholar]
- 28.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology 1999;53:537–542 [DOI] [PubMed] [Google Scholar]
- 29.Pullicino P, Ostrow P, Miller L, Snyder W, Munschauer F. Pontine ischemic rarefaction. Ann Neurol 1995;37:460–466 [DOI] [PubMed] [Google Scholar]
- 30.Kwa VI, Stam J, Blok LM, Verbeeten B., Jr T2-weighted hyperintense MRI lesions in the pons in patients with atherosclerosis. Amsterdam Vascular Medicine Group. Stroke 1997;28:1357–1360 [DOI] [PubMed] [Google Scholar]
- 31.Price TR, Psaty B, O'Leary D, Burke G, Gardin J. Assessment of cerebrovascular disease in the Cardiovascular Health Study. Ann Epidemiol 1993;3:504–507 [DOI] [PubMed] [Google Scholar]
- 32.Bokura H, Kobayashi S, Yamaguchi S. Distinguishing silent lacunar infarction from enlarged Virchow-Robin spaces: a magnetic resonance imaging and pathological study. J Neurol 1998;245:116–122 [DOI] [PubMed] [Google Scholar]
- 33.Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006;37:1391–1398 [DOI] [PubMed] [Google Scholar]
- 34.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652–659 [DOI] [PubMed] [Google Scholar]
- 35.Toyry JP, Kuikka JT, Lansimies EA. Regional cerebral perfusion in cardiovascular reflex syncope. Eur J Nucl Med 1997;24:215–218 [DOI] [PubMed] [Google Scholar]
- 36.Ilgin N, Olgunturk R, Kula S, et al. Brain perfusion assessed by 99mTc-ECD SPECT imaging in pediatric patients with neurally mediated reflex syncope. Pacing Clin Electrophysiol 2005;28:534–539 [DOI] [PubMed] [Google Scholar]
- 37.Poda R, Guaraldi P, Solieri L, et al. Standing worsens cognitive functions in patients with neurogenic orthostatic hypotension. Neurol Sci 2012;33:469–473 [DOI] [PubMed] [Google Scholar]
- 38.Palm-Meinders IH, Koppen H, Terwindt GM, et al. Structural brain changes in migraine. JAMA 2012;308:1889–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tietjen GE. Stroke and migraine linked by silent lesions. Lancet Neurol 2004;3:267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


