Abstract
Objective:
To explore the association of nonmelanoma skin cancer (NMSC) and Alzheimer disease (AD) in the Einstein Aging Study, an epidemiologic study of aging in New York City.
Methods:
Community-residing volunteers aged 70 years or older were assessed annually, followed by multidisciplinary diagnostic consensus. Cancer status and type was obtained by self-report. Cox proportional hazards models were used to test associations between NMSC and subsequent risk of developing a neurocognitive disorder. To deduce a biologically specific association between AD and NMSC, we considered 3 nested outcomes groups: only AD (probable or possible AD as the sole diagnosis), any AD (probable AD or possible AD, as well as mixed AD/vascular dementia), and all-cause dementia.
Results:
We followed 1,102 adults with a mean age of 79 years at enrollment. Prevalent NMSC was associated with reduced risk of only AD (hazard ratio = 0.21; 95% confidence interval = 0.051–0.87; p = 0.031) among subjects after adjustment for demographics, hypertension, diabetes, and coronary heart disease. APOE ε4 genotypes were available in 769 individuals. The association was similar in magnitude, but nonsignificant, when the number of APOE ε4 alleles was included in the model. No significant association was found between NMSC and subsequent development of any AD or all-cause dementia.
Conclusions:
This population-based longitudinal study shows that individuals older than 70 years with NMSC have a significantly reduced risk of developing AD compared with individuals without NMSC. We deduce Alzheimer-specific neuroprotection, because the effect is attenuated or eliminated when considering less-specific diagnoses such as AD with another diagnosis (any AD) or all-cause dementia.
Previous studies observed an inverse relationship between Alzheimer disease (AD) and cancer. The Washington University AD Research Center showed reduced incidence of cancer in participants with prevalent AD, and a nonsignificant trend toward lower incidence of AD in persons with history of cancer at baseline.1 Skin cancer specifically accentuated the protective effect against dementia and AD.2 Data from the Cardiovascular Health Study found that AD, but not vascular dementia (VaD), was associated with a reduced risk of future cancer hospitalization.3 In whites, a previous cancer diagnosis conferred a reduced risk of AD, but in African Americans with cancer, an increased AD risk was reported. There was no significant association between a previous diagnosis of cancer and future VaD.3
Other research has suggested that skin cancer in particular may have specific effects. In a clinical trial, patients with AD treated with a γ-secretase inhibitor had increased rates of nonmelanoma skin cancer (NMSC).4 NMSC is the most common malignancy in the United States, with an estimated 2007 prevalence of approximately 13 million whites.5 Herein, we explore the association of NMSC and AD in the Einstein Aging Study (EAS), a longitudinal epidemiologic study of aging in New York City. We hypothesized that a history of NMSC would be associated with reduced Alzheimer risk, and that the effect would not apply to other causes of dementia.
METHODS
Design.
The EAS is a cohort study that follows community-residing, systematically sampled, dementia-free older adults in the Bronx, NY, with annual neurocognitive assessments. In the present study, we followed persons with and without a history of NMSC, assessing time to clinical dementia diagnosis. To deduce a biologically specific association between AD and NMSC, we considered nested subsets according to diagnostic subtype: only AD (probable or possible AD as the sole diagnosis), any AD (probable AD or possible AD, as well as mixed AD/VaD), and all-cause dementia.
Study population.
EAS design, enrollment procedures, and follow-up methods have been previously described.6 Inclusion criteria required that participants be aged 70 years or older, noninstitutionalized, speak English, and reside in the Bronx. Exclusion criteria included sensory impairment that would hinder neuropsychological testing, nonambulatory status, psychiatric symptomatology or substance use that would impede assessment, and prevalent dementia. Between 1993 and 2004, the population sample was derived from Health Care Financing Administration/Centers for Medicare & Medicaid Services lists and from New York City Board of Elections voter registration lists after 2004. Potential participants were systematically recruited through the use of letters explaining the study. Follow-up telephone calls were conducted to further explain the study, receive verbal consent, complete a brief medical history questionnaire, and perform a brief cognitive screening test to prevent the enrollment of prevalent dementia cases in the study.6 Eligible persons based on the telephone interview were assessed in person at the EAS clinic where final study eligibility was determined.
A total of 1,791 participants were enrolled in the EAS between October 1993 and December 2009. Participants with no follow-up (n = 679) were excluded from this study. Therefore, a total of 1,102 participants were included in this study. At baseline and at annual follow-up visits, participants were assessed via demographic and health questionnaires, psychosocial histories, neuropsychological testing, neurologic and medical evaluation, and physical measures.
Standard protocol approvals, registrations, and patient consents.
Verbal informed consent was obtained during all telephone calls and written informed consents were obtained during all clinic visits according to study protocols approved by the Committee on Clinical Investigation, the institutional review board of the Albert Einstein College of Medicine. Additionally, the Committee on Clinical Investigation approved the use of human subjects for this study.
Criteria for dementia diagnosis.
Dementia status for EAS participants was ascertained at case conferences with neuropsychology and neurology input. Dementia diagnosis was dichotomously classified and fulfilled standardized clinical criteria from the DSM-IV.7 Criteria included memory impairment plus impairment in at least one additional cognitive domain, with evidence of functional decline. Dementia was subtyped according to criteria for probable or possible AD as determined by the National Institute of Neurological and Communication Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association.8 The diagnosis of probable, possible, or mixed VaD was based on criteria set forth by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers.9 For participants with incident dementia, date of incident dementia was assigned to the office visit before consensus case conference. These were strictly clinical diagnoses, informed by comprehensive review of cognitive testing, neurologic signs and symptoms, and functional status. EAS clinical-pathologic studies demonstrated that the diagnosis of AD has a positive predictive value of 78.5% and a negative predictive value of 82.3% for pathologically verified AD (unpublished data).
Criteria for cancer diagnosis.
Participants were asked at every assessment whether he or she ever had any form of cancer. A response of “yes” would prompt additional questions ascertaining cancer type, date, and treatment. Individuals who indicated skin cancer and did not specify skin cancer of the melanoma type were classified as having NMSC. For the purposes of our study, we chose to examine only the NMSC type to clarify the specific protective effect suggested in past studies.1,2
Statistical analysis.
Cox regression models using age as the time scale, with a time-dependent predictor variable NMSC status, were then used to test the association of NMSC diagnosis (yes/no) on risk of dementia (only AD, any AD, or all-cause dementia). Study participants were classified as either prevalent NMSC or no NMSC at baseline. For participants with prevalent NMSC, persons were considered at-risk from the date of study entry to the date of either the first dementia diagnosis or to the end of the participant's follow-up, whichever was earlier, and NMSC status was positive for the entire follow-up period. Participants who did not have NMSC at baseline and who did not develop NMSC during the course of the study were considered free of NMSC for the entire follow-up period. For participants who did not have NMSC at baseline, but who developed incident NMSC during the study follow-up period, the time-dependent predictor NMSC status was derived as follows: person-time participants were considered free of NMSC before diagnosis and were considered exposed subsequent to diagnosis until the date of dementia diagnosis or to the end of the participant's follow-up period, whichever was earlier. For example, a participant followed for 3 years before and 2 years after a diagnosis of NMSC would have had an NMSC-negative status for 3 years and an NMSC-positive status for 2 years.
Four different models were used for this analysis to correct for potential confounders. The first model included sex as a covariate. The second model included demographic factors (sex and education) as covariates. The third model included the demographic factors with the addition of occupation and also risk factors associated with VaD (history of hypertension, diabetes, and coronary heart disease). The fourth model included the demographic factors and also risk factors associated with AD and VaD (number of APOE ε4 alleles, history of hypertension, diabetes, and coronary heart disease); however, APOE data were available for only 769 individuals. Education is a continuous variable that indicates years of schooling. Comorbid medical conditions were self-reported based on yes or no questions. Because NMSC is very rare among African Americans,10 this analysis was repeated using only white participants to eliminate a potential confounder. These analyses were then repeated with any diagnosis of cancer (all-cause) rather than NMSC as the primary predictor of interest.
RESULTS
We followed 1,102 persons initially free of dementia for an average of 3.7 years and median of 3.0 years (maximum 15.5 years). At baseline, 109 of the 1,102 persons had a history of NMSC. Additionally, during the course of the study, 32 participants developed incident NMSC (table 1). Accordingly, these 141 individuals contributed a total of 509 NMSC-positive person-years to the NMSC history cohort, and 993 individuals contributed 3,543 NMSC-negative person-years to the cohort.
Table 1.
Demographic and neurocognitive profile of study participants by nonmelanoma skin cancer status at baseline and follow-up
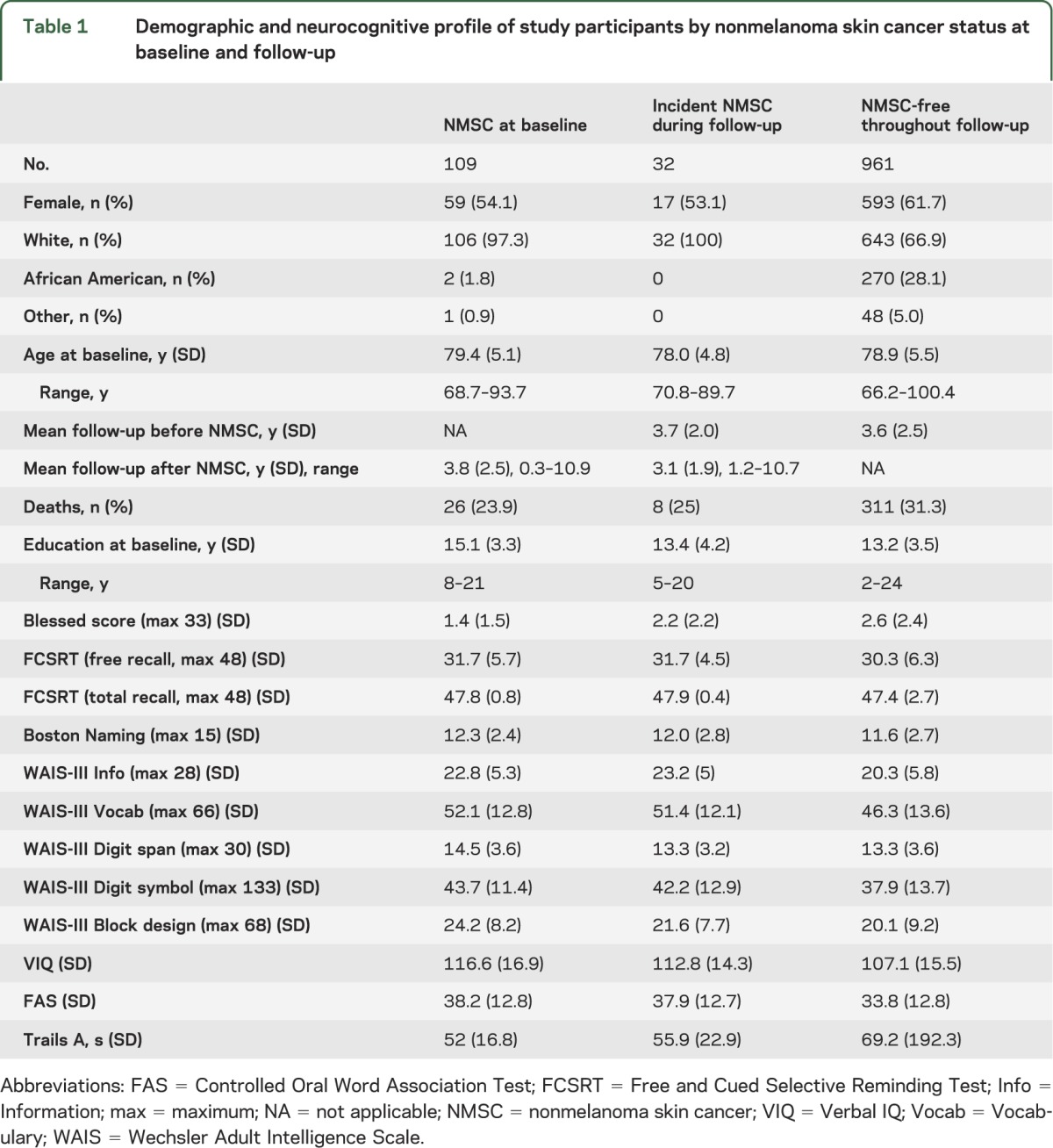
Comparison of baseline characteristics by NMSC status.
Table 1 shows a comparison of the samples with NMSC at baseline, those with incident NMSC, and those who remained NMSC-free throughout follow-up. These 3 groups did not differ in sociodemographic characteristics other than race, as African Americans had far less skin cancer. There were, however, differences over a range of baseline cognitive assessments. These are only descriptive comparisons, and no formal statistical tests were performed, for nonsignificant differences between groups can still result in confounding. These differences were in global mental status (Blessed Information–Memory-Concentration Test,11 which assesses memory, attention, concentration, and the ability to complete activities of daily living) as well as a range of neurocognitive instruments assessing memory, language, visuospatial, and executive functions: Free and Cued Selective Reminding Test12; Boston Naming Test13; FAS letter fluency14; and WAIS-III (Information, Vocabulary, Digit Span, Block Design, Digit Symbol, and Verbal IQ).15
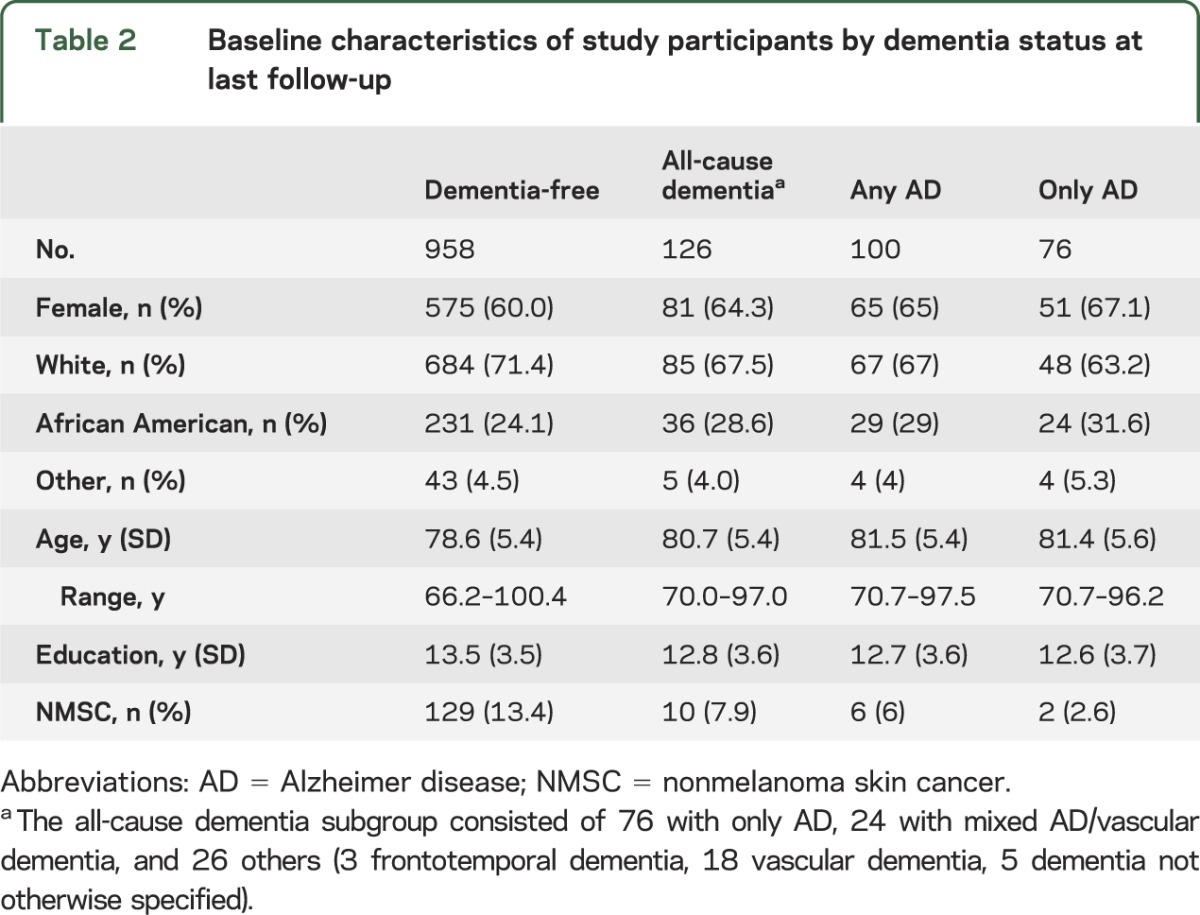
Comparison of baseline characteristics by dementia status.
During the course of the study, 958 people remained dementia-free throughout follow-up and 126 developed dementia, the all-cause dementia group. Of the incident dementia cases, 76 had only AD; 24 had mixed AD/VaD resulting in a total of 100 people with any AD. In addition, 26 subjects met criteria for dementia but did not have an AD diagnosis. The most common diagnoses in the other dementia group included VaD and frontotemporal dementia (table 2).
Table 2.
Baseline characteristics of study participants by dementia status at last follow-up
Development of a neurocognitive disorder as a function of NMSC status.
All Cox regression models used in the analyses apply age as the time scale. The results of model 1 (adjusting for sex), model 2 (adjusting for sex and education), model 3 (adjusting for demographic and vascular risk factors), and model 4 (adjusting for demographic and vascular risk factors and the number of APOE ε4 alleles) are shown in table 3. Model 3 demonstrates that NMSC showed reduced Alzheimer risk (hazard ratio [HR] for developing only AD was 0.21, p = 0.031) (figure). Only 2 individuals with NMSC developed only AD. There was a nonsignificant reduced risk of developing any AD, with an attenuated HR of 0.50 (p = 0.11). When considering the outcome of all-cause dementia, the apparent protective effect was further diminished (HR = 0.68, p = 0.24). When these analyses were repeated using only white participants, NMSC continued to be associated with a reduced risk of developing only AD, albeit with diminished statistical confidence (table e-1 on the Neurology® Web site at www.neurology.org). Model 4 shows a substantial but nonsignificant reduced risk of developing only AD (HR = 0.18, p = 0.094), any AD (HR = 0.60, p = 0.35), and all-cause dementia (HR = 0.75, p = 0.50).
Table 3.
Results of Cox proportional hazards modelsa testing time to diagnosis of dementia or AD as a function of history of nonmelanoma skin cancer in all participants (N = 1,134)
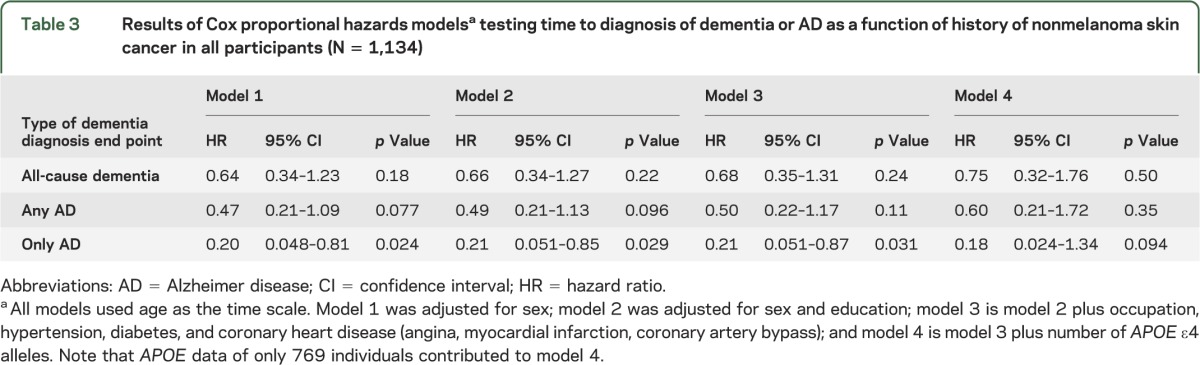
Figure. Kaplan-Meier failure estimates showing time to the onset of AD as a function of NMSC status.
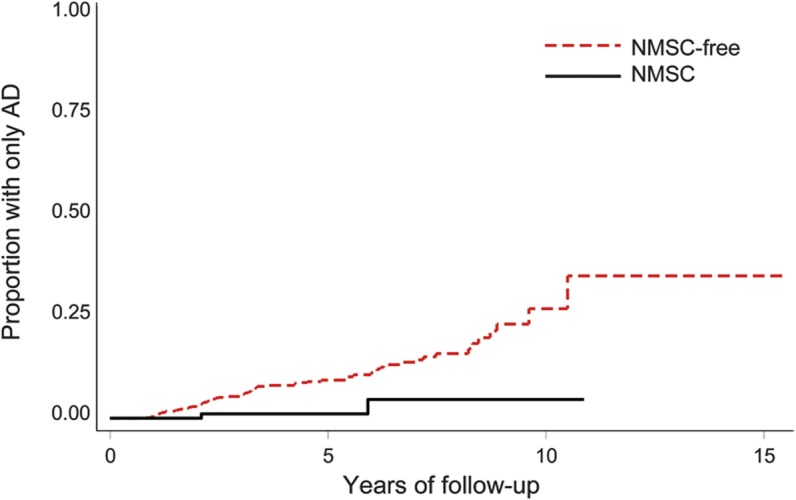
AD = Alzheimer disease; NMSC = nonmelanoma skin cancer.
Development of a neurocognitive disorder as a function of cancer status.
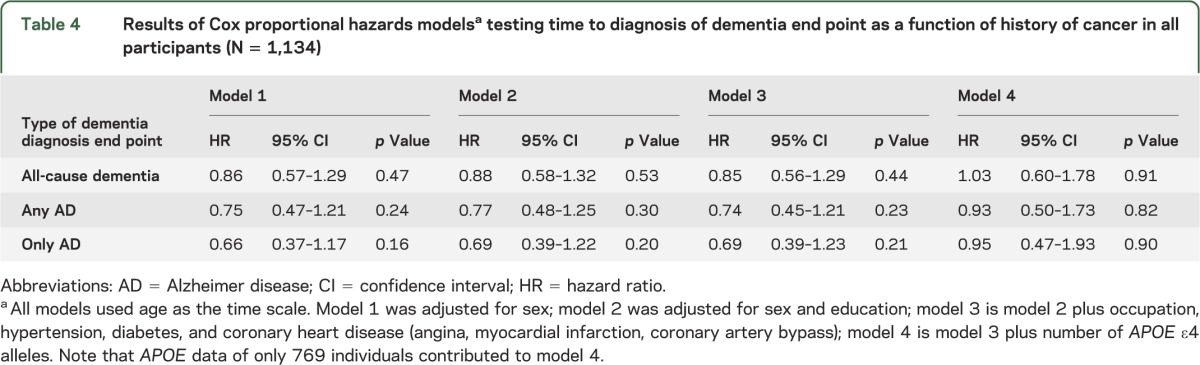
Model 3 shows that there was no significant effect for reduced risk of developing all-cause dementia (HR = 0.85, p = 0.44), any AD (HR = 0.74, p = 0.23), or only AD (HR = 0.69, p = 0.21) for history of any cancer (table 4). Model 4 shows a nonsignificant increase in the risk of developing all-cause dementia (HR = 1.30, p = 0.91), and a nonsignificant decrease in any AD (HR = 0.93, p = 0.90) and only AD (HR = −0.95, p = 0.90) for history of any cancer. When these analyses were repeated using only white participants, a similar trend was observed.
Table 4.
Results of Cox proportional hazards modelsa testing time to diagnosis of dementia end point as a function of history of cancer in all participants (N = 1,134)
DISCUSSION
This population-based longitudinal study shows that individuals older than 70 years with NMSC have a significantly reduced risk of developing AD compared with individuals free of NMSC. We deduce Alzheimer-specific neuroprotection, because the effect is attenuated or eliminated when considering inclusive outcomes such as all-cause dementia. Adjusting for hypertension, diabetes, and coronary heart disease did not significantly change the results. When adjusting for the number of APOE ε4 alleles, the magnitude of the HR is unchanged but statistical significance is lost, possibly because of reduced power.
Repeating the analysis for whites only also did not significantly change the results. Skin cancer is the most common cancer among whites; however, it represents a very small percentage of cancers for African Americans. Additionally, the incidence of skin cancer has been increasing for whites, but remains low among African Americans.10
This study focused solely on NMSC. Other studies have found an inverse association between cancer of any type and AD.1,3 Our results showed nonsignificant trends and therefore can neither substantiate nor refute these findings. It is possible that the putative protective effect of cancer of any type suggested in some studies is driven primarily by the strong influence of NMSC, with its high prevalence in light-skinned populations. For example, in the Washington University study, no participants with a history of skin cancer developed dementia during the follow-up period.2
Our study has also shown that participants with a history of NMSC perform better than NMSC-free participants on baseline cognitive assessments. This finding is consistent with previous research that has shown that one form of NMSC, basal cell carcinoma, is associated with higher socioeconomic status, income, and education. That study also showed that another form of NMSC, squamous cell carcinoma, is weakly associated with higher income, but not education.16 Lower education is associated with reduced reporting of medical illness.17 These baseline cognitive differences suggest that NMSC might be a marker for a factor or factors that protect against the development of AD. In the subset of the sample with no NMSC history at baseline and throughout follow-up, there may be an increased rate of persons with predementia AD. This would account for both the reduced mean performance and higher levels of variability in the NMSC-free subjects.
The apparent protective effect of NMSC has several possible explanations. Perhaps NMSC and reduced risk of AD are linked through a confounder such as education.18 Better education encourages healthier lifestyle choices,19 including more health checkups,17 whereby the presence of cancer could be detected. After adjusting for education and occupation, the results remained robust. Other potential confounders include personality, psychosocial variables, and physical activity.20,21 Physical activity is protective against cognitive decline and dementia.18 Outdoor physical activity may lead to increased exposure to ultraviolet radiation, which increases skin cancer risk.22
It is also possible that the protective effect of NMSC is conferred by biological factors. None of the psychosocial factors discussed above are known to confer a risk reduction near the magnitude we report for NMSC. The protective effect is largest and most robust for the outcome of only AD, a diagnostic group likely to be enriched with Alzheimer biology and pathology. There are several plausible mechanisms. The relationship between cancer and AD may reflect differences in DNA methylation,23 activity of the tumor suppressor gene p53, the enzyme Pin1, or the Wnt signaling pathway.20 Additionally, NMSC-specific associations with AD can be attributed to mechanisms involving γ-secretase signaling21,24,25 through a Notch 1 signaling pathway.26,27
This study has a number of limitations. First, NMSC diagnoses were identified based on self-report. While collecting cancer data, we did not specifically ask about NMSC; therefore, it is possible that a percentage of our NMSC cohort contains cases of melanoma that were misclassified. Because melanoma is not protective against AD, if this error occurred, it would cause us to underestimate our results. Self-reported cancer histories in the United States are generally accurate,28,29 although their use in epidemiologic studies30 has been challenged. In older adults, forgetting prior diagnoses is a possibility and differential forgetting in those who go on to develop AD is plausible. However, we excluded prevalent dementia from the inception cohort. If this effect occurs, it would again attenuate our findings. Diagnostic confirmation of NMSC is problematic because most state and national cancer registries do not track NMSC diagnoses. Future studies in this area should apply more rigorous ascertainment of NMSC status.
Second, we used date of study enrollment and not date of NMSC onset to start calculating person-time for risk of AD in persons who self-reported a history of NMSC at baseline. Although we did ask participants to report a date of diagnosis, such a date would be subject to recall bias, and the follow-up for the purpose of dementia outcomes began with study enrollment, so we elected not to use it. Because most of the NMSC-positive participants were prevalent for the disease, this decision probably served to attenuate our results. Third, we had limited genotype (APOE ε4 allele) data on our patients. This served to reduce power in model 4 and attenuated our results.
Fourth, this study utilized clinical neurocognitive diagnosis. Although clinical-neuropathologic correlation in EAS is excellent, some level of misclassification in dementia status is inevitable. This misclassification would be likely to attenuate the reported associations if anything. Lastly, the EAS exclusively studies individuals older than 70 years, and our results may not apply to other samples.
Our study also has a number of strengths. The EAS is a large, population-based, prospective study that uses well-established procedures to ascertain dementia outcomes and achieve excellent retention. Our findings meet several of the criteria for causation,31 including strength, temporality, specificity, and plausibility. The present study has shown that persons who report a diagnosis of NMSC have a reduced risk of developing Alzheimer dementia. Further investigation is necessary to elucidate the biological and psychosocial basis for the reduced Alzheimer risk associated with NMSC.
ACKNOWLEDGMENT
The authors thank the participants, investigators, and staff of the Einstein Aging Study.
GLOSSARY
- AD
Alzheimer disease
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- EAS
Einstein Aging Study
- HR
hazard ratio
- NMSC
nonmelanoma skin cancer
- VaD
vascular dementia
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
R.S. White: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis. R.B. Lipton: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, obtaining funding. C.B. Hall: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis. J.R. Steinerman: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision.
STUDY FUNDING
This research was supported by grant 2P01AG003949 from the National Institute of Aging, the Einstein Aging Study; and grant 5P50CA013330 from the National Cancer Institute, the Albert Einstein Cancer Center.
DISCLOSURE
R.S. White reports no disclosures. C.B. Hall receives or has received research support from the National Institute of Aging (P01 AG03949, P01 AG027734, R01 AG022092, R01 AG034087, R01 AG034119, R21 AG036935), the National Center for Research Resources (1-UL1-RR025750-01), the National Cancer Institute (P30 CA13330-35), the National Institute of Occupational Safety and Health (5-U011O-OH008242, U01 OH010411, U01 OH010412, and contracts 200-2011-39378 and 200-2011-39489), and Endo Pharmaceuticals, has consulted to research projects at the University of Connecticut Health Center, is and has been a member of Data and Safety Monitoring Committees at Columbia University, received honoraria from Washington University St. Louis and from Oregon Health and Science University, has received travel funding from Washington University St. Louis, Yale University, Oregon Health and Science University, and the University of Victoria, and is a member of the American Statistical Association Media Experts Panel. R.B. Lipton receives research support from the NIH (PO1 AG03949 [Program Director, Project and Core Leader], PO1AG027734 [Project Leader], RO1AG025119 [Investigator], RO1AG022374-06A2 [Investigator], RO1AG034119 [Investigator], RO1AG12101 [Investigator], K23AG030857 [Mentor], K23NS05140901A1 [Mentor], and K23NS47256 [Mentor]), the National Headache Foundation, and the Migraine Research Fund; has reviewed for the NIA and National Institute of Neurological Disorders and Stroke; holds stock options in Neuralieve Inc. (a company without commercial products); serves as consultant, advisory board member, or has received honoraria from Allergan, American Headache Society, Autonomic Technologies, Boston Scientific, Bristol-Myers Squibb, CogniMed, Diamond Headache Clinic, Eli Lilly, Endo, GlaxoSmithKline, Merck, Nautilus Neuroscience, Neuralieve, and Novartis. J.R. Steinerman is the founding scientist of ProGevity Neuroscience and an employee of CRI Lifetree. He is a member of a Data and Safety Monitoring Committee at Columbia University. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC. Alzheimer disease and cancer. Neurology 2005;64:895–898 [DOI] [PubMed] [Google Scholar]
- 2.Behrens MI, Roe C, Morris JC. Inverse association between cancer and dementia of the Alzheimer’s type. In: von Bernhardi R, Inestrosa NC, editors. Neurodegenerative Diseases: From Molecular Concepts to Therapeutic Targets. New York: Nova Science Publishers; 2008:111–120 [Google Scholar]
- 3.Roe CM, Fitzpatrick AL, Xiong C, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology 2010;74:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings J. What can be inferred from the interruption of the semagacestat trial for treatment of Alzheimer's disease? Biol Psychiatry 2010;68:876–878 [DOI] [PubMed] [Google Scholar]
- 5.Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol 2010;146:279–282 [DOI] [PubMed] [Google Scholar]
- 6.Katz MJ, Lipton RB, Hall CB, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord 2012;26:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 9.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology 1992;42:473–480 [DOI] [PubMed] [Google Scholar]
- 10.Bradford PT. Skin cancer in skin of color. Dermatol Nurs 2009;21:170–177 [PMC free article] [PubMed] [Google Scholar]
- 11.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 1968;114:797–811 [DOI] [PubMed] [Google Scholar]
- 12.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology 1988;38:900–903 [DOI] [PubMed] [Google Scholar]
- 13.Tombaugh TN, Hubley AM. The 60-item Boston Naming Test: norms for cognitively intact adults aged 25 to 88 years. J Clin Exp Neuropsychol 1997;19:922–932 [DOI] [PubMed] [Google Scholar]
- 14.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999;14:167–177 [PubMed] [Google Scholar]
- 15.Taylor MJ, Heaton RK. Sensitivity and specificity of WAISIII demographically corrected factor scores in neuropsychological assessment. J Int Neuropsychol Soc 2001;7:867–874 [PubMed] [Google Scholar]
- 16.Steding-Jessen M, Birch-Johansen F, Jensen A, Schüz J, Kjær SK, Dalton SO. Socioeconomic status and non-melanoma skin cancer: a nationwide cohort study of incidence and survival in Denmark. Cancer Epidemiol 2010;34:689–695 [DOI] [PubMed] [Google Scholar]
- 17.Ross CE, Wu CL. The links between education and health. Am Sociol Rev 1995;60:719–745 [Google Scholar]
- 18.Wang L, Larson EB, Bowen JD, van Belle G. Performance-based physical function and future dementia in older people. Arch Intern Med 2006;166:1115–1120 [DOI] [PubMed] [Google Scholar]
- 19.Mirowsky J, Ross CE. Education, personal control, lifestyle and health. Res Aging 1998;20:415–449 [Google Scholar]
- 20.Behrens MI, Lendon C, Roe CM. A common biological mechanism in cancer and Alzheimer's disease? Curr Alzheimer Res 2009;6:196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T, Wen H, Brayton C, et al. Moderate reduction of gamma-secretase attenuates amyloid burden and limits mechanism-based liabilities. J Neurosci 2007;27:10849–10859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer—the role of sunlight. In: Sunlight, Vitamin D and Skin Cancer. Reichrath J, editor. New York: Springer; 2008:89–103 [DOI] [PubMed] [Google Scholar]
- 23.Tremolizzo L, Rodriguez-Menendez V, Brighina L, Ferrarese C. Is the inverse association between Alzheimer's disease and cancer the result of a different propensity to methylate DNA? Med Hypotheses 2006;66:1251–1252 [DOI] [PubMed] [Google Scholar]
- 24.Kelleher RJ, Shen J. Gamma-secretase and human disease. Science 2010;330:1055–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serneels L, Van Biervliet J, Craessaerts K, et al. Gamma-secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer’s disease. Science 2009;324:639–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watt FM, Estrach S, Ambler CA. Epidermal notch signalling: differentiation, cancer and adhesion. Curr Opin Cell Biol 2008;20:171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet 2003;33:416–421 [DOI] [PubMed] [Google Scholar]
- 28.Bergmann MM, Calle EE, Mervis CA, Miracle-McMahill HL, Thun MJ, Heath CW. Validity of self-reported cancers in a prospective cohort study in comparison with data from state cancer registries. Am J Epidemiol 1998;147:556–562 [DOI] [PubMed] [Google Scholar]
- 29.Dominguez FJ, Lawrence C, Halpern EF, et al. Accuracy of self-reported personal history of cancer in an outpatient breast center. J Genet Couns 2007;16:341–345 [DOI] [PubMed] [Google Scholar]
- 30.Parikh-Patel A, Allen M, Wright WE; California Teachers Study Steering Committee Validation of self-reported cancers in the California Teachers Study. Am J Epidemiol 2003;157:539–545 [DOI] [PubMed] [Google Scholar]
- 31.Hill A. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300 [PMC free article] [PubMed] [Google Scholar]


