Abstract
Background
Elements of volume resuscitation from hemorrhagic shock, such as amount of blood product and crystalloid administration, have been shown to be associated with Multiple Organ Dysfunction (MOD). However, it is unknown whether these are causative factors or merely markers of an underlying requirement for large-volume resuscitation. We sought to further delineate the relevance of the major individual components of early volume resuscitation to onset of MOD after severe blunt traumatic injury.
Methods
We performed a secondary analysis of a large, multi-center prospective observational cohort of severely injured blunt trauma patients, the NIGMS Trauma Glue Grant, to assess the relevance of individual components of resuscitation administered in the first 12 hours of resuscitation including packed red blood cells (PRBC), fresh frozen plasma (FFP) and isotonic crystalloid, to the onset of MOD within the first 28 days after injury. Deaths within 48 hours of injury were excluded. We utilized a two-tiered, exhaustive logistic regression model search technique to adjust for potential confounders from clinically relevant MOD covariates, including indicators of shock severity, injury severity, comorbidities, age and gender.
Results
The study cohort consisted of 1,366 severely injured blunt trauma patients (median NISS=34). Incidence of 28-day Marshall MOD was 19.6%. Transfusion of ≥10 Units of PRBC in the first 12 hrs (OR 2.06, 95% CI 1.44 - 2.94), but not FFP (≥8 U) or large volume crystalloid administration (≥12L), was independently associated with onset of 28-day Marshall MOD. PRBC:FFP ratio in the first 12 hours was not significantly associated with MOD.
Conclusions
When controlling for all major components of acute volume resuscitation, massive-transfusion volumes of PRBC’s within the first 12 hours of resuscitation are modestly associated with MOD, while FFP and large volume crystalloid administration are not independently associated with MOD. Previous reported associations of blood products and large-volume crystalloid with MOD may be reflecting overall resuscitation requirements and burden of injury rather than independent causation.
Keywords: Multiple Organ Dysfunction, Blood, Trauma, Injury
Introduction
The development of multiple organ dysfunction (MOD) is associated with significantly increased morbidity and mortality after severe blunt traumatic injury.1, 2 Advances in trauma systems, volume resuscitation, damage control surgical procedures and advanced critical care supportive measures over the past five decades continue to contribute to the survival of patients with progressively increasing severity of injuries. However, even as these interventions improve upon survival in the immediate post-injury period, MOD continues to impact the long term morbidity and mortality of severely injured patients.3-5
Multiple organ dysfunction is associated with an early, systemic hyperinflammatory response to severe injury known as the systemic inflammatory response syndrome (SIRS).6-9 Despite continuing active investigation, the triggers of this immunologic response and why some patients, but not others, mount a dysfunctional robust response remain unknown. Specifically, the role of the individual components of volume resuscitation in systemic inflammation and the development of MOD remains unclear. The development of aggressive volume resuscitation techniques utilizing crystalloid solutions and blood products during the wartime conflicts of the 20th century have saved countless numbers of patients in hemorrhagic shock. However, over-aggressive crystalloid resuscitation has been associated with complications such as worsening of acute coagulopathy and the development of pulmonary edema.10-12 In addition, both crystalloid solutions and blood products, such as packed red blood cells (PRBC) and fresh frozen plasma (FFP) have been shown to possess immunomodulatory properties.10, 13-15 This has led some to propose that blood may be a causative factor of SIRS and MOD.16-18 However, retrospective analyses of recent experience from the combat theatres of Iraq and Afghanistan showing improved survival with early and aggressive blood-based resuscitation of hemorrhagic shock in patients requiring massive transfusion suggests that early blood-based resuscitation may be the optimal resuscitative strategy.12, 19-23
The role of the individual components of acute volume resuscitation in the development of MOD has yet to be fully elucidated. We sought to test the current assumption that the early usage of blood products during acute volume resuscitation is directly associated with MOD after severe blunt traumatic injury. To address this question we studied the individual components of resuscitation, including blood products, crystalloid and colloid solutions utilized in a severely injured blunt traumatic cohort, to determine their association with the development of post-traumatic MOD. We hypothesized that the early usage of blood products would not have a strong association with increased risk for MOD after severe blunt traumatic injury when controlling for other components of resuscitation including crystalloid, as well as other known risk factors.
Methods
Overview/Study Population
To determine the role of individual components of acute volume resuscitation on MOD, we performed a retrospective secondary analysis of data obtained from a multicenter, prospective cohort of severely injured blunt trauma patients in hemorrhagic shock (National Institute of General Medical Sciences Inflammation and the Host Response to Injury Collaborative Program). The study cohort consists of male and female patients ≥13 years of age evaluated at Level One trauma centers. Inclusion criteria required a blunt traumatic mechanism with an abbreviated injury score (AIS) ≥2 outside the head region, base deficit ≥6 mmol/L, systolic blood pressure <90 mmHg pre-hospital or within 60 minutes of emergency department arrival, and blood product transfusion within 12 hours of injury. Exclusion criteria consisted of those with significant mortality risk from severe head injury (AIS head >4), those evaluated at the trauma center >6 hours from time of injury, cervical spinal cord injury, and thermal burns >20% total body surface area. An extensive dataset was prospectively collected on each patient in the cohort including demographics, injury severity and pattern, fluid and blood product resuscitation parameters, serial laboratory values and multiple outcomes, including MOD. After being compiled and validated, de-identified data is included in the Glue Grant investigator-accessible Trauma Related Database (TRDB) for secondary analysis.
Patients who expired <48 hours from time of injury were not included in the analysis. This was done to exclude patients who likely died from irreversible hemorrhagic shock or non-survivable traumatic brain injury. The primary outcome of interest was defined as incidence of at least one episode of MOD within the first 28 days after injury. The Marshall scoring system, excluding the neurological/GCS component, was utilized to classify MOD in this study.24, 25 An episode of MOD was defined as ≥2 consecutive days with a Marshall score ≥6 occurring at least 48 hours from time of admission.
The primary predictors of interest (Table 1) include total units of PRBC, total units of FFP, and total amount of crystalloid, administered in the first 12 hours of resuscitation. To simplify interpretation of results, blood product resuscitation component volumes were translated from the raw data of milliliters (ml) to average volumes of standard component therapy in Units (U): 1U PRBC = 350mL, 1U FFP = 300 mL, 1U platelets = 350 mL, 1U cryoprecipitate = 150 mL. The prospective cohort data collection and secondary analysis were both approved by the IRB at UT Southwestern.
Table 1.
Model Covariates
| Primary Covariates | |
|---|---|
| Total PRBC administered 0-12 hrs. (U) | continuous |
| Total FFP administered 0-12 hrs. (U) | continuous |
| Total Crystalloid administered 0-12 hrs. (L) | continuous |
| Secondary Covariates | |
| Age | continuous |
| Gender | male/female |
| New Injury Severity Score (NISS) | continuous |
| AIS Head region ≥3 | yes/no |
| AIS Abdomen region ≥3 | yes/no |
| AIS Thorax region ≥3 | yes/no |
| AIS Extremity region ≥3 | yes/no |
| AIS Spine region ≥3 | yes/no |
| PRBC:FFP ratio 0-12 hrs. | continuous |
| APACHE II admission score | continuous |
| Max. serum Lactate 0-6 hrs. | continuous |
| Max. Base deficit 0-6 hrs. | continuous |
| Vasopressor use 0-24 hrs. | yes/no |
| Body mass index (BMI) | continuous |
| Comorbidity: COPD | yes/no |
| Comorbidity: Diabetes | yes/no |
| Comorbidity: Chronic renal disease | yes/no |
| Comorbidity: Hypertension | yes/no |
| Comorbidity: Liver disease | yes/no |
| Glucose >200 0-24 hrs | yes/no |
PRBC - packed red blood cells, FFP - fresh frozen plasma, AIS - Abbreviated Injury Score (U) - units, (L) - Liters, Total platelets administered 0-12 hrs and Total cryoprecipitate were analyzed in prior searches but not reported here due to poor performance.
Statistical Modeling
Associations between primary predictors and MOD were adjusted for clinically relevant covariates such as injury severity, age, gender, and shock severity indications (Table 1), employing a two-tiered model search of optimally recoded variables using information theoretic criteria with subsequent 5-fold cross validation that accounts for possible model over fit, model misspecification, experiment-wise error rates, spurious correlations, and multicollinearity. Missing values were estimated using a stochastic single imputation method that replaced missing values by randomly sampling from actual joint response and variable distribution. Subsequent transformations were performed on the data containing imputed values.
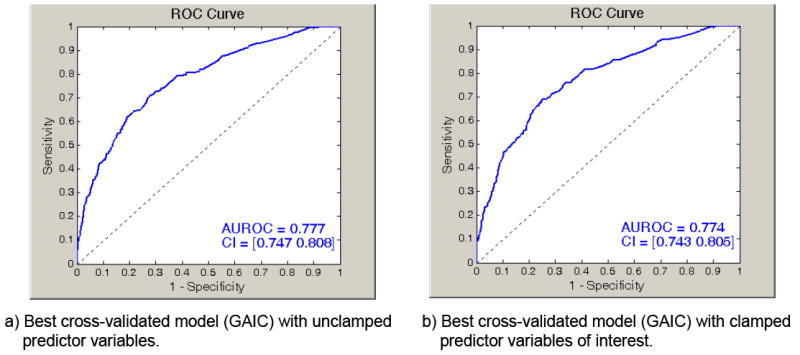
The search for a best final model predicting MOD proceeded as follows. First, 23 independent variables (Table 1) were taken from a list of covariate predictors that were both available from the dataset and considered clinically relevant from existing literature. Next, 10 of the 23 variables that were continuous were dichotomized using a single bootstrapped cut-point that was designed to optimize the fit between the resulting dichotomized variable and MOD.26 Optimally recoding continuous variables manages non-linear effects and simplifies the logistic regression results to simple odds ratios. Next, we applied an “all possible models” stochastic search that randomly visited all possible models comprising variables from the recoded covariate pool (i.e., 223=8,388,608).27-29 This strategy is superior to stepwise regression that often omits potentially important models from consideration. The performance of these variables in actual models was assessed by how well the estimated models fit the data based on the Akaike Information Criteria.30 Variables that had a high presence in models with poor fit were removed until a computationally tractable number of 15 covariates remained in the variable pool. All possible models were exhaustively created, estimated (215 = 65,536) using study data, and evaluated and ranked based on the Generalized Akaike Information Criterion (GAIC).31 The GAIC offers an unbiased estimate of the log-likelihood that is robust for small sample sizes in the presence of model misspecification. To correct for multicollinearity, models with condition numbers for the Hessian, Outer Product Gradient, or Robust Covariance matrices that were greater than 100,000 were removed from further consideration. Next, we selected the top 2,048 models based on GAIC and, for each model, recomputed all parameter estimates using a 5-fold cross-validation.32 The model among the 2,048 models observed to have the best fit, based on cross-validated GAIC, was selected as the final model. The final cross-validated model (Table 3) was statistically significant (LRT p < .0001) when fitted to the original sample (-2LL = 1157.7, GAIC = 1181.6) with an AUROC = 0.777 (95% CI 0.747-0.808). For comparison purposes, we applied other cross-validation criteria to select a best model, including the best log-likelihood, Area under the Receiver Operating Characteristic Curve (AUROC), Classification Rate, Youden Index (J), and Kappa (κ).33-35 Findings that required the predictors of interest be in all the top cross-validated models using these five alternative criteria did not vary from those reported here.
Table 3.
Best model Robust Odds Ratio Estimates for 28-day MOD
| OR | 95% CI | |
|---|---|---|
| Male gender | 2.20 | 1.55 - 3.10* |
| Serum Lactate >6.2 (mm/L) 0-6 hrs. | 2.05 | 1.49 - 2.81* |
| APACHE II >33 | 2.07 | 1.49 - 2.88* |
| Vasopressor utilization 0-24 hrs. | 2.08 | 1.49 - 2.89* |
| PRBC administration >9.5 (U) 0-12 hrs. | 2.06 | 1.44 - 2.94* |
| NISS >34 | 1.83 | 1.36 - 2.49* |
| BMI >28 | 1.60 | 1.20 - 2.14* |
| Comorbidity: Liver disease | 2.52 | 1.32 - 4.81* |
| Comorbidity: Diabetes | 1.14 | 0.74 - 1.77 |
| Comorbidity: COPD | 1.69 | 0.89 - 3.22 |
| FFP administration >7.8 (U) 0-12 hrs. | 1.14 | 0.74 - 1.77 |
OR - Robust Odds Ratio estimates, CI - confidence interval, (U) - Units, PRBC - packed red blood cells, FFP - fresh frozen plasma, NISS - New injury severity score, BMI - Body mass index, COPD - Chronic obstructive pulmonary disease,
p<0.05
The above “unclamped” analyses allowed all variables, including primary predictor variables, to freely enter and leave models during the search process (unclamped). For hypothesis testing, we repeated the above search while clamping the three primary predictor variables of interest (Table 1) so that these variables would automatically appear in the final model (clamped). The final cross-validated model (Table 4) was statistically significant (LRT p < .0001) when fitted to the original sample (-2LL = 1168.6, GAIC = 1190.7) with an AUROC = 0.774 (95% CI 0.743 – 0.805).
Table 4.
Best model Robust Odds Ratio with clamped hypothesis testing
| OR | 95% CI | |
|---|---|---|
| Male gender | 2.23 | 1.58 - 3.16* |
| Serum Lactate >6.2 (mmol/L) 0-6 hrs. | 2.01 | 1.46 - 2.76* |
| Vasopressor utilization 0-24 hrs. | 1.97 | 1.41 - 2.74* |
| APACHE II >33 | 1.97 | 1.40 - 2.76* |
| NISS >34 | 1.77 | 1.31 - 2.39* |
| BMI >34 | 1.62 | 1.21 - 2.17* |
| Age >49 (yrs.) | 1.50 | 1.10 - 2.04* |
| Clamped variables | ||
| PRBC administration >9.5 (U) 0-12 hrs. | 1.91 | 1.32 - 2.76* |
| FFP administration >7.8 (U) 0-12 hrs. | 1.00 | 0.78 - 1.86 |
| Crystalloid administration > 12.4 (L) 0-12 hrs. | 1.23 | 0.89 - 1.70 |
OR - Robust Odds Ratio estimates, CI - confidence interval, NISS - New injury severity score, BMI - Body mass index, PRBC - packed red blood cells, FFP - fresh frozen plasma, (U) - Units, (L) - Liters,
p<0.05
Finally, we experimented with alternative predictor variables, including total platelets administered 0-12 hrs (U) and Total Cryoprecipitate administered 0-12 hrs (U), applying the same clamped and unclamped approaches. Results were excluded from these presentations due to poor performance, but are available from the authors.
Results
Study population/characteristics
The sampled population consisted of 1,467 patients with severe blunt traumatic injury who were enrolled in the prospective cohort, completed data validation, and were entered into the TRDB database over a seven year period between January 2002 and February 2009. A total of 101 injured patients (6.9%) expired within 48 hours of presentation and were excluded from the final study population as per the previously defined exclusion criteria. Thus, the final population available for analysis consisted of 1,366 severely injured patients.
Population demographics (Table 2) confirm that this was a severely injured, predominantly male cohort (65.3%), with physiologic signs of hemorrhagic shock. The median age for this cohort was 41 years. The leading mechanism of injury was motor vehicle crash (54.7%), followed by motorcycle crash (15.3%), pedestrian struck by automobile (12.7%), fall (8.6%), and other unspecified blunt mechanism (8.7%). Overall, this cohort required a significant amount of acute volume resuscitation with a median 12-hour volume requirement of 9.8 liters of crystalloid solution, and median PRBC transfusion volume of 4.6 Units (Table 2). The 28-day mortality rate for the cohort was 10% with median time to death from injury of 6 days (interquartile range, 4-11).
Table 2.
Study Population
| Demographics | Median | IQR |
|---|---|---|
| Age | 41 | 26 - 54 |
| NISS | 34 | 27 - 48 |
| Max. base deficit 0-6 hrs. | -9.35 | (-12.65) - (-6.7) |
| BMI | 26.8 | 23.8 - 31.2 |
| Acute care LOS (d) | 19 | 11 - 31 |
| Resuscitation parameters | ||
| PRBC administered 0-12 hrs. (U) | 4.64 | 2.14 - 8.28 |
| FFP administered 0-12 hrs. (U) | 1.66 | 0 - 5.00 |
| Plts administered 0-12 hrs. (U) | 0 | 0 - 0.77 |
| Cryo administered 0-12 hrs. (U) | 0 | 0 - 0 |
| PRBC:FFP ratio 0-12 hrs. | 2.00 | 1.20 - 3.56 |
| PRBC:FFP ratio 0-24 hrs. | 2.00 | 1.25 - 3.75 |
| Crystalloid administered 0-12 hrs. (L) | 9.77 | 6.91 - 13.88 |
| Colloid administered 0-12 hrs. (L) | 0 | 0 - 0 |
NISS - New injury severity score, BMI - Body mass index,(d) - days, (U) - Units, (L) –Liters PRBC - packed red blood cells, FFP - fresh frozen plasma, Plts - platelets, Cryo-cryoprecipitate
Association of resuscitation components with MOD
The overall incidence of at least one episode of 28-day MOD in the study population was 19.6%. The best unclamped model is shown in Table 3, with optimal cut values for continuous variables indicated in the variable name. Significant covariates with associations to 28-day MOD included male gender, peak serum lactate >6.2 mmol/L in the first 6 hours, APACHE II score >33, vasopressor utilization within the first 24 hours, new Injury Severity Score (NISS) >34, body mass index (BMI) >28, and coexistent comorbidities of diabetes mellitus, chronic obstructive pulmonary disease (COPD) and underlying chronic liver dysfunction. Of the primary resuscitation variables of interest, only the administration of greater than 9.5 Units of PRBC within the first 12 hours of resuscitation was associated with onset of MOD (OR 2.06, 95% CI 1.44 - 2.94). Although it was included as a covariate in the best model, administration of greater than 7.8 Units of FFP within the first 12 hours of resuscitation did not have a statistically significant independent association with MOD (OR 1.14, 95% CI 0.74 - 1.77).
Similar results were obtained when the variables of interest (blood product and crystalloid administration) were “clamped” into the final model (Table 4). Of the clamped variables of interest, administration of PRBC remains associated with MOD (OR 1.91, 95% CI 1.32 - 2.76), while 12-hour FFP and crystalloid volume administration fail to show a statistically significant association to 28-day MOD. Receiver Operating Characteristic (ROC) curves for both models are shown in Figure 1.
Figure 1. Receiver Operating Characteristic Curves for Logistic Regression Models.
Discussion
The most effective methodology for acute volume resuscitation from hemorrhagic shock remains an area of intense investigation and ongoing controversy. With the evolution of the concept of “damage control resuscitation”, it has become clear that while immediate and aggressive crystalloid resuscitation in the trauma bay may bring rapid improvement to the vital signs of both patient and treating physician, the overall effect on outcome may be much less comforting. The resuscitating physician must also be cognizant of early post-trauma coagulopathy, volume overload and the delayed physiologic and immunological effects associated with large volume resuscitation and massive transfusion of blood products. A thorough understanding of these physiologic processes, as well as the potential short and long term risks and benefits of different resuscitation components utilized early after injury is therefore necessary for the development of optimal volume resuscitation strategies and protocols.
In this study, our goal was to further discriminate between the individual associations of resuscitation components utilized in early volume resuscitation of the severely injured blunt trauma patient with post-resuscitation MOD. Specifically, our analysis focused on the utilization of PRBC’s, FFP, and isotonic crystalloid within the first 12 hours of resuscitation. We found a modest association of massive transfusion volumes of PRBC administered within this time period with 28-day MOD. This association is consistent with previously published work suggesting an association between blood and MOD.2, 3, 16, 18, 36 However, in our analysis this association was significantly weaker than previously described by authors such as Moore and Sauaia.16, 18 In addition, the other two resuscitation components of interest in this analysis, FFP and crystalloid volume, were not independently associated with MOD. This also differs from other previous studies suggesting an association between administration of these volume components and MOD.37, 38 Despite previous descriptions of associative relationships between blood product and crystalloid administration with MOD, controversy persists over whether these relationships are truly representative of a causal relationship. It is possible, perhaps likely, that these relationships are merely surrogates for severity of injury burden. In other words, the administration of large amounts of intravascular volume repletion and blood product components are associated with, but not causative for, post-injury MOD because they serve as markers for a high injury burden and severity of physiologic derangement.
There are several aspects unique to our analysis which we believe may account for the differences between our results and previous reports. First, the multi-institution Trauma Glue Grant cohort represents the most comprehensive and extensive prospective data collection available to date on patients treated for severe blunt traumatic injury at risk for MOD. This allowed us to analyze a highly accurate and thoroughly vetted dataset to assess the individual components of the first 12 hours of volume resuscitation. Other studies have looked at individual components, such as PRBC or FFP, but did not control for all relevant components of resuscitation during the same time period.16-18, 38 Thus, as discussed previously, the relationship of those selected components may reflect an overall volume requirement of those patients likely to proceed to MOD, rather than be a causative agent in and of itself. A recent analysis of a smaller subset of the Trauma Glue Grant cohort found associations between FFP administration volumes in the first 24-hours with MOD and ARDS.38 However, there are several differences that exist between these two studies including the analytic methodology, resuscitation covariates, MOD criteria, as well as differing resuscitation time frames that were analyzed which may explain these differences.
Interestingly, while increasing volumes of plasma have been described to be associated with adverse outcomes such as MOD, the PRBC:FFP ratio within the first 12 hours of resuscitation was not independently associated with MOD in this study. As shown in Table 2, the PRBC:FFP ratios after 12 and 24 hours of resuscitation are similar to those in other recent studies.39, 40 It must be noted, however, that our study population differs from most other studies analyzing PRBC:FFP ratio in that the majority of these patients, although severely injured, did not receive massive transfusion amounts of blood products and suffered from blunt mechanisms of injury only. In addition, this analysis focuses on the initial 12 hours of resuscitation, and product ratios during this time frame may be significantly different than those over the entire transfusion course. Therefore, it may not be possible to extrapolate this finding specifically to those patients undergoing massive transfusion volumes of blood products. The upcoming Prospective Randomized Optimum Platelet and Plasma Ratios (PROPPR) study is a multi-center randomized controlled trial specifically testing the impact of PRBC:FFP ratios on outcomes in massively transfused patients.
Our model building process also is a novel approach to analyzing this population cohort and brings several advantages to other previous analytical approaches. The study utilizes a very computationally intensive strategy to adjust for the confounding effects of clinically relevant covariates on the measured association between independent components of early volume resuscitation on MOD. Unlike previous studies, our maximum likelihood approach used a two-tiered stochastic/exhaustive search that accounts for non-linear trends, model overfitting and misspecification, missing data, experiment-wise error rates, spurious correlations, and multicollinearity. We “test” for association of blood products and crystalloid administration on multiple organ dysfunction by either freely searching models to see if the covariate of interest appears in the final best model (unclamped best model), or clamping the covariates of interest into every model throughout the search process and testing for any significant associations in the final best model (clamped best model). Our findings indicated that while PRBC administration was modestly significant (Table 3, Table 4), in general blood products and crystalloid administration were not strongly associated with MOD in either clamped or unclamped searches for two (unclamped, clamped) best approximating models. The fact that there is no single resuscitation component with a strong association to MOD after this type of multi-level analysis brings into question previous theories of causality between these products and MOD.
The findings of our study may have several implications for the evolving field of post-injury resuscitation. There is an evolving paradigm shift towards the early and aggressive utilization of blood products brought about by recent literature supporting the concepts of “damage control resuscitation”. Recent retrospective studies have shown there to be a survival benefit in the administration of blood products in patients in hemorrhagic shock in ratios approaching that of whole blood.22, 39, 41, 42 It is thought that this benefit is largely due to the prevention and treatment of the now recognized early coagulopathy associated with severe injury and hemorrhagic shock. Given the historical literature on the association of blood product transfusion with systemic immune modulation and adverse outcomes, there is understandable concern on how the incorporation of damage control resuscitation principles will impact post-injury outcomes such as MOD. However, as mentioned previously, limitations on existing studies prevent the certainty of a causal relationship between blood product administration and MOD. Our data further suggests that the associations between blood products and crystalloid administration and MOD may be confounding markers of patients at high-risk of MOD rather than causal agents themselves.
There are several limitations of this study which must be acknowledged. Although the Trauma Glue Grant dataset is arguably the most thorough and precise prospective cohort study of severely injured patients at risk for MOD to date, this is a secondary analysis and was not designed nor powered to be a definitive study to define the role of early volume resuscitation components to the ultimate development of post-injury MOD. Furthermore, information on the age and leukoreduction status of transfused PRBC units was not available for inclusion in the model. Despite the ongoing advancement and refinement of modeling techniques such as those utilized here, observational analyses of populations with the multi-factorial complexity of disease processes such as severe blunt traumatic injury and MOD may never be able to fully link causality to outcome. The answer of whether or not resuscitation components such as blood products and crystalloid have any causal link to outcomes such as MOD and mortality in this severely injured patient population will only be answered through carefully designed prospective interventional trials of post-injury volume resuscitation protocols.
Conclusion
The most effective strategy for early volume resuscitation from traumatic hemorrhagic shock remains controversial. In this study, we sought to further elucidate the potential relationships of the individual major volume resuscitation components, including blood products and crystalloid solution, with post-injury MOD. When controlling for all major components of acute volume resuscitation, massive-transfusion volumes of PRBC’s within the first 12 hours of resuscitation are modestly associated with MOD, while FFP and large volume crystalloid administration are not independently associated with MOD. Previous reported associations of blood products and large-volume crystalloid with MOD may be reflecting overall resuscitation requirements and injury severity rather than independent causation. Ultimately, prospective interventional trials of clearly defined volume resuscitation protocols will be necessary to elucidate the optimal early resuscitation strategy to achieve adequate tissue perfusion, prevent and correct coagulopathy, while also minimizing risk of post-resuscitative complications such as MOD.
Acknowledgments
Sources of Support:
This project was supported by NIH NIGMS Grant Number U54 GM062119-10, as well as NCRR-NIH Grant Number UL1RR024982, titled, “North and Central Texas Clinical and Translational Science Initiative” (Milton Packer, M.D., PI). This research was also made possible by grants from the National Cancer Institute (NCI) (R44CA139607, Steven S. Henley, M.S., PI) and the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (R43AA013670, Steven S. Henley, M.S., PI) under the Small Business Innovation Research (SBIR) program. The authors wish to gratefully acknowledge this support. However, this paper does not necessarily reflect the views or opinions of the National Institutes of Health, including NCRR-NIH, NIH NIGMS, NCI, NIAAA, or the Department of Veterans Affairs.
References
- 1.Barie PS, Hydo LJ. Epidemiology of multiple organ dysfunction syndrome in critical surgical illness. Surg Infect (Larchmt) 2000 Fall;1(3):173–185. doi: 10.1089/109629600750018105. discussion 185-176. [DOI] [PubMed] [Google Scholar]
- 2.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996 Apr;40(4):501–510. doi: 10.1097/00005373-199604000-00001. discussion 510-502. [DOI] [PubMed] [Google Scholar]
- 3.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005 May;140(5):432–438. doi: 10.1001/archsurg.140.5.432. discussion 438-440. [DOI] [PubMed] [Google Scholar]
- 4.Durham RM, Moran JJ, Mazuski JE, Shapiro MJ, Baue AE, Flint LM. Multiple organ failure in trauma patients. J Trauma. 2003 Oct;55(4):608–616. doi: 10.1097/01.TA.0000092378.10660.D1. [DOI] [PubMed] [Google Scholar]
- 5.Mayr VD, Dunser MW, Greil V, et al. Causes of death and determinants of outcome in critically ill patients. Crit Care. 2006;10(6):R154. doi: 10.1186/cc5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobb JP, O’Keefe GE. Injury research in the genomic era. Lancet. 2004 Jun 19;363(9426):2076–2083. doi: 10.1016/S0140-6736(04)16460-X. [DOI] [PubMed] [Google Scholar]
- 7.Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997 Nov;25(11):1813–1819. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Miller-Graziano CL, Szabo G, Griffey K, Mehta B, Kodys K, Catalano D. Role of elevated monocyte transforming growth factor beta (TGF beta) production in posttrauma immunosuppression. J Clin Immunol. 1991 Mar;11(2):95–102. doi: 10.1007/BF00917745. [DOI] [PubMed] [Google Scholar]
- 9.Roumen RM, Hendriks T, van der Ven-Jongekrijg J, et al. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993 Dec;218(6):769–776. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deb S, Sun L, Martin B, et al. Lactated ringer’s solution and hetastarch but not plasma resuscitation after rat hemorrhagic shock is associated with immediate lung apoptosis by the up-regulation of the Bax protein. J Trauma. 2000 Jul;49(1):47–53. doi: 10.1097/00005373-200007000-00007. discussion 53-45. [DOI] [PubMed] [Google Scholar]
- 11.Alam HB, Rhee P. New developments in fluid resuscitation. Surg Clin North Am. 2007 Feb;87(1):55–72. vi. doi: 10.1016/j.suc.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007 Feb;62(2):307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 13.Locker GJ, Staudinger T, Knapp S, et al. Prostaglandin E1 inhibits platelet decrease after massive blood transfusions during major surgery: influence on coagulation cascade? J Trauma. 1997 Mar;42(3):525–531. doi: 10.1097/00005373-199703000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JL, Moore EE, Gonzalez RJ, Fedel N, Partrick DA, Silliman CC. Alteration of the postinjury hyperinflammatory response by means of resuscitation with a red cell substitute. J Trauma. 2003 Jan;54(1):133–139. doi: 10.1097/00005373-200301000-00016. discussion 139-140. [DOI] [PubMed] [Google Scholar]
- 15.Escobar GA, Cheng AM, Moore EE, et al. Stored packed red blood cell transfusion up-regulates inflammatory gene expression in circulating leukocytes. Ann Surg. 2007 Jul;246(1):129–134. doi: 10.1097/01.sla.0000264507.79859.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997 Jun;132(6):620–624. discussion 624-625. [PubMed] [Google Scholar]
- 17.Napolitano L. Cumulative risks of early red blood cell transfusion. J Trauma. 2006 Jun;60(6 Suppl):S26–34. doi: 10.1097/01.ta.0000199979.95789.17. [DOI] [PubMed] [Google Scholar]
- 18.Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC. Early predictors of postinjury multiple organ failure. Arch Surg. 1994 Jan;129(1):39–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 19.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007 Oct;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 20.Fox CJ, Gillespie DL, Cox ED, et al. Damage control resuscitation for vascular surgery in a combat support hospital. J Trauma. 2008 Jul;65(1):1–9. doi: 10.1097/TA.0b013e318176c533. [DOI] [PubMed] [Google Scholar]
- 21.Fox CJ, Gillespie DL, Cox ED, et al. The effectiveness of a damage control resuscitation strategy for vascular injury in a combat support hospital: results of a case control study. J Trauma. 2008 Feb;64(2 Suppl):S99–106. doi: 10.1097/TA.0b013e3181608c4a. discussion S106-107. [DOI] [PubMed] [Google Scholar]
- 22.Spinella PC, Perkins JG, Grathwohl KW, et al. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma. 2008 Feb;64(2 Suppl):S69–77. doi: 10.1097/TA.0b013e318160ba2f. discussion S77-68. [DOI] [PubMed] [Google Scholar]
- 23.Spinella PC, Perkins JG, Grathwohl KW, et al. Fresh whole blood transfusions in coalition military, foreign national, and enemy combatant patients during Operation Iraqi Freedom at a U.S. combat support hospital. World J Surg. 2008 Jan;32(1):2–6. doi: 10.1007/s00268-007-9201-5. [DOI] [PubMed] [Google Scholar]
- 24.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995 Oct;23(10):1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Sauaia A, Moore EE, Johnson JL, Ciesla DJ, Biffl WL, Banerjee A. Validation of postinjury multiple organ failure scores. Shock. 2009 May;31(5):438–447. doi: 10.1097/SHK.0b013e31818ba4c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashner T, Henley S, Golden R, Rush A, Jarrett R. Assessing the preventive effects of cognitive therapy following relief of depression: A methodological innovation. Journal of Affective Disorders. 2007;104:251–261. doi: 10.1016/j.jad.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Clyde M, George E. Model uncertainty. Statistical Science. 2004;19(1):81–94. [Google Scholar]
- 28.Dellportas P, Forster J, Ntzoufras I. One Bayesian model and variable selection using MCMC. Statistics and Computing. 2002;12:27–36. [Google Scholar]
- 29.Viallefont V, Raftery A, Richardson S. Variable selection and Bayesian model averaging in case-control studies. Statistics in Medicine. 2001;20:3215–3230. doi: 10.1002/sim.976. [DOI] [PubMed] [Google Scholar]
- 30.Akaike H. Information theory and an extension of the maximum likelihood priniciple. In: Petrov BN, C F, editors. Second International Symposium on Information Theory. Budapest: Academiai Kiado; 1973. [Google Scholar]
- 31.Bozdogan H. Akaike’s Information Criterion and recent developments in information complexity. Journal of Mathematical Psychology. 2000;44:62–91. doi: 10.1006/jmps.1999.1277. [DOI] [PubMed] [Google Scholar]
- 32.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning. 2. New York: Springer-Verlag; 2009. [Google Scholar]
- 33.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20(1):37–46. [Google Scholar]
- 34.Pepe M. The Statistical Evaluation of Medical Tests for Classification and Prediction. New York: Oxford University Press; 2003. [Google Scholar]
- 35.Youden W. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 36.Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma. 2005 Sep;59(3):717–723. [PubMed] [Google Scholar]
- 37.Heckbert SR, Vedder NB, Hoffman W, et al. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998 Sep;45(3):545–549. doi: 10.1097/00005373-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 38.Watson GA, Sperry JL, Rosengart MR, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009 Aug;67(2):221–227. doi: 10.1097/TA.0b013e3181ad5957. discussion 228-230. [DOI] [PubMed] [Google Scholar]
- 39.Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009 Jan;66(1):41–48. doi: 10.1097/TA.0b013e31819313bb. discussion 48-49. [DOI] [PubMed] [Google Scholar]
- 40.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008 Sep;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 41.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009 Apr;66(4 Suppl):S69–76. doi: 10.1097/TA.0b013e31819d85fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009 May;197(5):565–570. doi: 10.1016/j.amjsurg.2008.12.014. discussion 570. [DOI] [PubMed] [Google Scholar]


