Abstract
Background
The traditional paradigm is that deep venous thrombosis (DVT) and pulmonary embolus (PE) are different temporal phases of a single disease process, most often labeled as the composite endpoint venous thromboembolism (VTE). However, we theorize that after severe blunt injury, DVT and PE may represent independent thrombotic entities rather than different stages of a single pathophysiologic process and therefore exhibit different clinical risk factor profiles.
Methods
We examined a large, multi-center prospective cohort of severely injured blunt trauma patients to compare clinical risk factors for DVT and PE, including indicators of injury severity, shock, resuscitation parameters, comorbidities and VTE prophylaxis. Independent risk factors for each outcome were determined by cross-validated logistic regression modeling using advanced exhaustive model search procedures.
Results
The study cohort consisted of 1,822 severely injured blunt trauma patients (median ISS = 33, median base deficit = −9.5). Incidence of DVT and PE were 5.1% and 3.9% respectively. Only 9 of 73 (5.7%) patients with a PE were also diagnosed with DVT. Independent risk factors associated with DVT include prophylaxis initiation within 48 hours (OR 0.57, 95% CI 0.36–0.90) and thoracic AIS ≥ 3 (OR 1.82, 95% CI 1.12–2.95), while independent risk factors for PE were serum lactate >5 (OR 2.33, 95% CI 1.43–3.79) and male gender (OR 2.12, 95% CI 1.17–3.84). Both DVT and PE exhibited differing risk factor profiles from the classic composite endpoint of VTE.
Conclusion
Deep venous thrombosis and pulmonary embolus exhibit differing risk factor profiles following severe injury. Clinical risk factors for diagnosis of DVT after severe blunt trauma include the inability to initiate prompt pharmacologic prophylaxis and severe thoracic injury, which may represent overall injury burden. In contrast, risk factors for PE are male gender and physiologic evidence of severe shock. We hypothesize that post-injury DVT and PE may represent a broad spectrum of pathologic thrombotic processes as opposed to the current conventional wisdom of peripheral thrombosis and subsequent embolus.
Level of Evidence
Prognostic study, Level III
Keywords: Venous thromboembolism, Deep venous thrombosis, Pulmonary embolus, Trauma, Injury
Introduction
Venous thromboembolic complications, such as deep venous thrombosis (DVT) and pulmonary embolism (PE) remain significant contributors to morbidity and mortality following traumatic injury. Despite the widespread adoption of venous thromboembolism prophylaxis protocols, incidence of DVT and PE are reported as high as 44% and 24% respectively during post-injury hospitalization in high risk patients 1–5 With regards to prophylaxis and treatment, DVT and PE are most often grouped together as a single entity, venous thromboembolism (VTE). Risk factors for VTE after injury have been well characterized, and include advancing age, long bone and pelvic fractures, spinal cord and traumatic brain injury, prolonged immobilization and delay of prophylaxis initiation, among others.1,4 However, recent evidence suggests that risk factors may differ between DVT and PE after injury.6 In addition, many PE are being diagnosed within the first few days and a significant number are being diagnosed as early as the first 24 hours after injury.7–10 These findings bring into question whether the conventional wisdom of peripheral thrombosis and subsequent embolus is an oversimplification of thromboembolic pathophysiology after injury.
We theorized that rather than being different temporal points within a single disease process, DVT and PE may represent distinct pathophysiologic mechanisms with different clinical risk factors. Mechanistically, we postulate that while predisposition to DVT and PE may share in common a post-traumatic hypercoagulopathic state, their discordance may be secondary to differences in local factors such as tissue injury, stasis and endothelial damage. To investigate this question we examined a large prospective cohort of severely injured patients to determine if DVT and PE exhibit differing independent risk factor profiles when analyzed as independent outcomes.
Methods
Overview/Study population
To assess for potential differences in risk factor profiles for DVT and PE diagnosed after severe injury, we performed a secondary analysis of data obtained from a multicenter, prospective cohort of severely injured blunt trauma patients in hemorrhagic shock (The Inflammation and the Host Response to Injury Collaborative Program; “Trauma Glue Grant”). The study cohort consisted of male and female patients greater than or equal to 13 years of age evaluated at five urban, academic Level One trauma centers. Inclusion criteria required a blunt traumatic mechanism with an abbreviated injury score (AIS) ≥ 2 outside the head region, base deficit ≥ 6 mmol/L, systolic blood pressure <90 mmHg pre-hospital or within 60 minutes of emergency department arrival, and blood product transfusion within 12 hours of injury. Exclusion criteria consisted of those with significant mortality risk from severe head injury (AIS head >4), those evaluated at the trauma center >6 hours from time of injury, cervical spinal cord injury, and thermal burns >20% total body surface area. Consistency of patient care between centers was optimized with the development and implementation of standard operating procedures (SOP) for initial resuscitation and supportive care, including VTE prophylaxis.11–19 Data were prospectively collected and rigorously audited and include patient demographics, injury pattern and severity, volume resuscitation parameters, serial laboratory values and multiple outcomes, including DVT and PE. Data were compiled, validated, de-identified and collated into the Trauma Glue Grant investigator-accessible Trauma Related Database (TRDB) for secondary analysis. For these analyses, we did not include patients who expired <48 hours from time of injury to exclude patients who likely died from irreversible hemorrhagic shock or non-survivable traumatic brain injury.
The primary outcomes for this study were deep venous thrombosis (DVT) and pulmonary embolus (PE) within 28 days of injury. Diagnosis of DVT was defined as venous thrombosis confirmed by autopsy, venogram, duplex ultrasound or other non-invasive vascular evaluation. An occurrence of PE was defined as a diagnostic confirmation by at least one of the following: confirmation of pulmonary embolus via diagnostic angiography, computed tomography, or moderate to high probability ventilation/perfusion radionucleotide scan. The utilization of DVT screening practices, as well as clinical criteria used to initiate workup of suspected DVT and PE were institution and provider specific and not uniformly protocolized across centers. The modality utilized for screening and/or suspected diagnosis of DVT and PE were also institution and provider dependent. For comparison we also included the traditional composite endpoint of venous thromboembolism (VTE), defined as all patients with DVT, PE, or both. We chose 28-day outcome measures to focus on thromboembolic complications within the acute phase after injury, rather than the chronic rehabilitation phase. Established and suspected clinical risk factors for DVT and PE, as well as potential confounding covariates such as indicators of injury and shock severity, volume resuscitation parameters, and implementation of pharmacologic thromboembolic prophylaxis and inferior vena cava filter placement were defined prior to the risk factor model building analysis (Table 1).
Table 1.
Population Demographics (n=1,882)
| (median) | (IQR) | |
|---|---|---|
| Age (y) | 41.0 | 26 – 55 |
| Total PRBC 0–24 h (U) | 5.0 | 2.8 – 9.0 |
| Total FFP 0–24 h (U) | 2.0 | 0 – 5.3 |
| Crystalloid 0–24 h (L) | 12.5 | 9.0 – 17.4 |
| Total resuscitation 0–24 h (L) | 18.1 | 13.2 – 25.5 |
| Totalresuscitation 0–24 h (L) | 24.7 | 17.7 – 34.1 |
| Max. serum lactate 0–6 h | 4.6 | 3.2 – 6.5 |
| Max. serum base deficit 0–6 h | −9.5 | (−7) – (−12.7) |
| ISS | 33 | 22 – 41 |
| BMI | 26.8 | 23.7 – 31.2 |
| LOS (d) | 20 | 11 – 31 |
| (n) | (%) | |
| Male gender | 1249 | 66.4 |
| Pelvic fracture | 911 | 48.4 |
| ≥1 Long bone extremity fracture | 669 | 35.6 |
| 28-day Mortality | 195 | 10.4 |
y, years; h, hours; PRBC, packed red blood cells; FFP, fresh frozen plasma; U, units; L, liters; ISS, injury severity score; LOS, hospital length of stay; BMI, body mass index.
Statistical Modeling
Independent risk factor models for DVT, PE and VTE were developed by employing a multi-staged model development methodology using binary logistic regression. An “all possible models” exhaustive search methodology utilizing optimally recoded or transformed variables with subsequent 5-fold cross validation was utilized in order to develop a “best possible model” of independent risk factors for each outcome.9 Specifically, 19 independent variables were taken from a list of available predictors that were considered clinically relevant from existing literature. All variables containing missing values were completed using a marginal stochastic imputation method that replaces missing data with values found by randomly sampling from the set of all observed sample values for that variable. Each of these variables were then fit into a univariate logistic regression model and tested for model specification (i.e., goodness-of-fit) using the Log Eigenspectrum and Log Generalized Akaike Information Criterion (GAIC) Generalized Information Tests (GIMTs)20,21 If model misspecifcation was detected, continuous or ordinal variables were recoded by dichotomizing using a single bootstrapped cut-point that was designed to optimize the fit between the resulting dichotomized variable and each specific outcome (DVT, PE and VTE).20 Alternatively, if model misspecification was not detected, continuous variables were linearly transformed to the unit interval.21 These recoded or transformed variables were then entered into a covariate pool for subsequent modeling.21
Final risk factor models were determined using an exhaustive “all possible models” search methodology over the covariate pool. Exhaustive searches were performed to minimize the risk of omitting important models from consideration.9,22–24 This algorithm estimated all possible logistic regression models that can be constructed, consisting of 219 (542,288) models, based on the variables within the dataset using a second order variant of the Akaike Information Criterion (AICc), which controls for model complexity and provides a small sample bias adjustment. All models were then ranked using the Generalized Akaike Information Criterion (GAIC) which is robust in the presence of possible model misspecification and controls for model complexity. Finally, we utilized a 5-fold cross-validation with performance measures for model fit, classification and discrimination for the highest probability models to identify and rank the top 5% of independent risk factor models for DVT, PE and VTE.21,25,26 Detailed methodological descriptions are available from the authors upon request.
Results
Study population/characteristics
The study population consisted of 1,882 patients treated after blunt traumatic injury that were prospectively enrolled over a 9 year period from 2002 to 2011. Demographics of the study population confirm that this was a severely injured cohort with physiologic signs of hemorrhagic shock (Table 1). The leading mechanism of injury was motor vehicle crash occupant (53.5%), followed by motorcycle crash (15.5%), pedestrian struck by motor vehicle (13.5%), fall (8.6%), and other blunt mechanism (8.9%). Acute volume resuscitation requirements were high, with median transfusion amounts of 5 units of packed red blood cells and 12.5 liters of crystalloid solution resuscitation in the first 24 hours. The overall 28-day mortality of the cohort was 10.4% (Table 1).
Post-injury venous thromboembolic complications
Venous thromboembolic (VTE) complications were diagnosed in 8.5% of these severely injured patients within the first 28 days (Table 2). More specifically, DVT and PE were diagnosed in 5.1% (95/1882) and 3.9% (73/1882) of all patients, respectively. Of the 73 patients with PE, only 9 (12.3%) were also diagnosed with DVT during the 28-day study period. Time to event data for DVT and PE is shown in Figure 1. Median times to diagnosis of DVT and PE were similar at approximately 10 days. Pharmacologic VTE prophylaxis was initiated in less than 48 hours from time of presentation in 42% of patients (Table 2). Prophylactic inferior vena cava filters were placed in 16% of all patients. Risk factors identified by unadjusted univariate analysis of all pre-selected variables for the composite outcome of VTE were consistent with previously described risk factors including indicators of injury severity, shock severity, obesity, large volume resuscitation requirements and the inability to initiate pharmacologic prophylaxis within 48 hours (Table 3).
Table 2.
Thromboembolic complications and prophylaxis
| (n) | (%) | |
|---|---|---|
| VTE | 159 | 8.45 |
| DVT | 95 | 5.05 |
| PE | 73 | 3.88 |
| Prophylaxis initiation <48 h1 | 751 | 41.98 |
| Prophylaxis initiation <72 h1 | 883 | 54.27 |
| Prophylactic IVC filter | 299 | 15.89 |
-subcutaneous heparin/low-molecular weight heparin; VTE, venous thromboembolism; DVT, deep venous thrombosis; PE, pulmonary embolus; h, hours; IVC, inferior vena cava filter.
Figure 1. Time to event for DVT and PE.
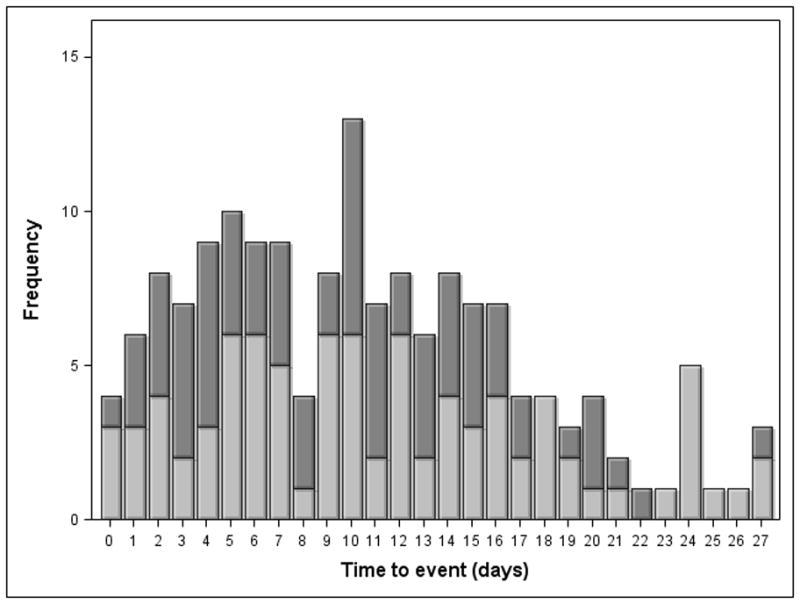
1a) Time to event data for DVT. 1b) Time to event data for PE.
Table 3.
Univariateanalysis of venous thromboembolism risk factors
| No VTE | VTE | ||||
|---|---|---|---|---|---|
| (%) | (%) | (p) | |||
| Male Gender | 65.7 | 73.6 | 0.044* | ||
| AIS Head ≥ 3 | 34.3 | 40.5 | 0.12 | ||
| AIS Thoracic ≥ 3 | 64.0 | 75.3 | 0.004* | ||
| AIS Abdomen ≥ 3 | 44.2 | 53.2 | 0.031* | ||
| AIS Spine ≥ 3 | 12.9 | 12.0 | 0.75 | ||
| AIS Extremity ≥ 3 | 68.7 | 69.7 | 0.81 | ||
| Pelvic Fracture | 48.1 | 52.2 | 0.32 | ||
| Long Bone Extremity Fracture | 35.6 | 34.6 | 0.86 | ||
| Prophylaxis initiation < 48 h1 | 43.0 | 31.0 | 0.004* | ||
| Prophylaxis initiation < 72 h1 | 55.4 | 43.2 | 0.004* | ||
| Prophylactic IVC filter | 15.6 | 18.9 | 0.31 | ||
| 28-day Mortality | 10.6 | 8.1 | 0.41 | ||
| median | IQR | median | IQR | (p) | |
| Age | 41 | (26–55) | 42 | (25–56) | 0.75 |
| BMI | 26.7 | (23.7–31.0) | 27.8 | (24.7–31.8) | 0.010* |
| ISS | 33 | (22–41) | 34 | (27–43) | 0.002* |
| Total PRBC 24 hrs 0–24 h (U) | 5.0 | (2.8–9.0) | 7.0 | (3.4–12.0) | 0.004* |
| Total FFP 0–24 h (U) | 1.9 | (0–5.3) | 3.1 | (0–7.5) | 0.003* |
| Total Crystalloid 0–24 h (L) | 12.3 | (8.9–17.2) | 14.1 | (9.3–19.1) | 0.063 |
| Total Resuscitation 0–24 h (L) | 18.0 | (13.1–25.1) | 19.4 | (14.1–29.9) | 0.014* |
| Total Resuscitation 0–48 h (L) | 24.6 | (17.6–33.6) | 27.2 | (19.5–39.2) | 0.018* |
| Max. Lactate 0–6 h | 4.5 | (3.2–6.4) | 5.6 | (3.9–7.4) | 0.001* |
| Max. Base deficit 0–6 h | 9.4 | (7.0–12.7) | 10.0 | (6.9–13.7) | 0.19 |
| APACHE II | 28 | (24–33) | 30 | (26–34) | 0.015* |
| Overall LOS | 19 | (11–31) | 27 | (19–38) | <0.001* |
-subcutaneous heparin/low-molecular weight heparin; AIS, abbreviated injury score; h, hours; IVC, inferior vena cava; BMI, body mass index; ISS, injury severity score; NISS, new injury severity score; PRBC, packed red blood cells; FFP, fresh frozen plasma; U, units; L, liters; LOS, hospital length of stay. IQR interquartile range
Post-injury DVT/PE multivariate risk factor modeling
Table 4 presents the final top performing models identified after the exhaustive model search of independent risk factors for DVT, PE and the composite endpoint VTE. The table provides estimates for the odds ratio and associated robust confidence intervals for each factor.27,28 The number of missing values in the datasets was negligible for binary variables at approximately 2.0%. The missing values for most continuous variables was negligible at 0.1% to 4.4%. The variable for serum lactate had 23.6% missing values. C-statistic values as measures of goodness of fit for the final VTE, DVT and PE models were 0.92 [95% CI 0.89, 0.93], 0.95 [95% CI 0.93, 0.97] and 0.96 [95%CI 0.95, 0.98], respectively. Independent risk factors for VTE were similar to the unadjusted analysis results and included serum lactate within the first 6 hours after presentation, the inability to initiate pharmacologic prophylaxis within the first 48 hours after injury, body mass index greater than 26, and thoracic abbreviated injury score greater than or equal to three. When looked at individually, DVT and PE exhibited differences in their independent risk factor profiles. Risk factors for DVT were identified as failure to initiate pharmacologic prophylaxis in the first 48 hours following injury and thoracic AIS greater than or equal to three. In contrast, risk factors for PE were serum lactate greater than 5 mmol/L and male gender (Table 4).
Table 4.
Multivariate best approximating risk factor models.
| VTE | |||
| OR | 95% CI3 | p | |
| Serum Lactate 0–6 hrs.1 | 1.09 | [1.04,1.15] | <0.001 |
| Prophylaxis initiation <48 h2 | 0.60 | [0.42, 0.85] | <0.001 |
| AIS Thorax ≥ 3 | 1.67 | [1.41,2.44] | 0.008 |
| BMI >26 | 1.53 | [1.09, 2.14] | 0.014 |
| AIS Spine ≥ 3 | 0.79 | [0.47, 1.31] | 0.36 |
| DVT | |||
| OR | 95% CI | p | |
| Prophylaxis initiation < 48 h2 | 0.57 | [0.36,0.90] | 0.015 |
| AIS Thorax ≥ 3 | 1.82 | [1.12, 2.95] | 0.016 |
| AIS Abdomen ≥ 3 | 1.42 | [0.94, 2.14] | 0.10 |
| PE | |||
| OR | 95% CI | p | |
| Serum Lactate > 5 (mmol/L) 0–6 h | 2.33 | [1.43,3.79] | <0.001 |
| Male Gender | 2.12 | [1.17, 3.84] | 0.013 |
| AIS Thorax ≥ 3 | 1.63 | [0.95,2.81] | 0.079 |
-Odds ratio not shown for lactate in the VTE model as analysis determined variable dichotomization was not appropriate due to linearity of association;
-subcutaneous heparin/low-molecular weight heparin; VTE, venous thromboembolism;
-CI, confidence intervals are robust based standard errors from the Robust Variance-Covariance Estimator; DVT, deep venous thrombosis; PE, pulmonary embolus; OR, odds ratio; h, hours.
It is possible that an important variable which appears frequently in other top cross-validated models may not appear as a risk factor in the best final model. Table 5 presents for each outcome the percent of the top cross-validated models in which each covariate appears as a significant predictor. With only one exception, the most frequently occurring variables across all evaluated models were found as an independent covariate of the best final model. The exception was body mass index (BMI), which appeared as an independent predictor in 91 percent of all evaluated DVT models, but was not a predictor in the best final model for DVT.
Table 5.
Covariate occurrence across all top cross-validated outcome models
| Covariate occurrence1 (%)
|
|
|---|---|
| VTE | |
| Max. lactate 0–6 h | 99.7 |
| Prophylaxis initiation < 48 h3 | 99.4 |
| BMI >26 | 92.6 |
| AIS Thorax ≥ 3 | 87.4 |
| Male Gender2 | 57.8 |
| AIS Abdomen ≥ 3 | 56.2 |
| DVT | |
| Chemical prophylaxis init. < 48 h | 90.8 |
| BMI >28 | 90.7 |
| AIS Thorax ≥ 3 | 75.6 |
| ISS >24 | 58.4 |
| Total PRBC 0–24 h >7 (U) | 51.7 |
| PE | |
| Lactate > 5 (mmol/L) 0–6 h | 100 |
| Male Gender | 97.6 |
| AIS Thorax ≥ 3 | 68.1 |
| Crystalloid 0–24 h > 15 (L) | 64.6 |
| BMI > 26 | 62.6 |
-Percentage of models that covariate was a significant predictor of outcome.
-variables in italics were not significant covariates in each of the best final models.,
-subcutaneous heparin/low-molecular weight heparin; h, hours; BMI, body mass index, AIS, abbreviated injury score; ISS, injury severity score; PRBC, packed red blood cells; L, liters;
Discussion
In this study, we found that in a population of severely injured patients there are significant differences in risk factors predicting diagnosis of DVT and PE after blunt traumatic injury. Independent predictors for diagnosis of DVT were identified as delay of pharmacologic thromboembolic prophylaxis for more than 48 hours and thoracic AIS score greater than or equal to three. In contrast, independent predictors for diagnosis of PE were serum lactate levels greater than 5 mmol/L and male gender. The risk factor profiles for DVT and PE differed not only from each other, but from the classic composite outcome of VTE. This differentiation in clinical risk factor profiles stimulates us to hypothesize that there may be differences in the post-injury pathophysiology of DVT and PE after injury. The potential decoupling of DVT from PE would represent a significant change in thought process from the traditional linear conventional wisdom for venous thromboembolism of distal thrombosis and subsequent embolization. Based on the findings presented here, and that of others discussed below, we hypothesize that DVT and PE clinically diagnosed after injury are representative of a broad spectrum of venous embolic and localized thrombotic processes which currently are classified as the broad composite outcome of post-injury venous thromboembolism (VTE).
Our risk factor modeling approach is based on an exhaustive logistic regression search methodology that is designed to evaluate all possible predictive models for a given outcome. After performing 5-fold cross-validation, a “best final model” was selected describing predictive risk factor covariates for DVT, PE, and VTE. Most often, significant predictors in the best final model are the same as covariates that appear consistently amongst all top performing models, supporting the validity of the best final model. This was indeed the case with our final models, with the exception that body mass index (BMI), while predictive of DVT in 90% of top models, was not a predictor of DVT in the best final model (Table 5). Our finding that BMI may be an important predictor is consistent with other studies showing obesity as a predictor of DVT.3,29,30
Notably absent as significant predictors of DVT, PE or VTE in this study are pelvic or long bone extremity fractures, which are commonly cited risk factors for VTE after trauma.14,31 We believe this is due to the fact that this analysis focused on a cohort of severely injured patients with evidence of hemorrhagic shock. This population often has a significant multi-system injury burden, including multiple orthopedic injuries. This is in contrast to most existing literature on risk factors for VTE which retrospectively utilized local trauma registries or national databases inclusive of patients covering the full spectrum of injury severity.4,6,32 In contrast, our study cohort consisted of only the most severely injured blunt trauma patients. Given that pelvic and long bone fractures are highly prevalent amongst these patients, they are much less likely to be discriminating risk factors for DVT and PE.
The risk factors shown to be predictive of the composite outcome VTE in our best final multivariate model are consistent with findings in other studies.2–4,6,8,32 What is unique to this study was that our findings appear to show that when analyzed independently there are significant differences in the risk factor profiles of DVT and PE that are diagnosed in severely injured patients. This suggests a potential decoupling of DVT and PE pathophysiology, and questions the conventional wisdom that the two are merely different temporal points in the same disease process. In our analysis, only 12% of patients with PE were also diagnosed with DVT. Granted, in this cohort there was no protocol across institutions to assure screening for DVT was performed after PE were diagnosed. However, these findings are similar to those described by Velmahos et al where injured patients evaluated for suspected PE were evaluated concurrently with computed tomography (CT) angiography of the pulmonary arteries and delayed phase CT venography. In their series, only 15% of injured patients with PE had concurrent DVT.8
Another finding supporting differences in risk factors between DVT and PE include a recent analysis by Knudsen et al of data from the National Trauma Data Bank (NTDB).6 They identified subtle differences in the strength of independent risk factors for DVT and PE, including a stronger association of severe chest injury (AIS thorax ≥ 3) with PE.6 Other reports have also found chest injury to be a risk factor for subsequent PE.5,33 In our study, a thoracic AIS ≥ 3 was the only significant injury severity metric predictive for DVT and the composite endpoint VTE. Although it was a covariate in the best final model for PE, it was marginally significant (p=0.079) (Table 4). However, it must be noted that AIS thorax ≥ 3 was the third most common covariate included across all top cross-validated models of PE, occurring as a significant covariate in 68% of models (Table 5). As described for BMI and DVT, it is possible that AIS Thorax ≥ 3 is an important predictor of PE even though it was not less than the conventional level of statistical significance (α=0.05) in the best final model.
Our results and those of Knudsen et al suggest that severe thoracic injury is a predictor of thromboembolic events. Both Knudsen and Velmahos postulated that thoracic injury may potentiate in situ pulmonary arterial thrombosis through local inflammation and subsequent activation of pulmonary venous endothelium.6,8 However, we conjecture that a thoracic AIS ≥ 3 may be representative of the severity of overall injury burden rather than being specific to chest injury. Chest injuries and high thoracic injury scores have been shown to be significantly correlated with overall injury severity and mortality.34,35 A potential problem with the assumption that thoracic AIS is independently associated with PE is that individual injury severity metrics are highly likely to be collinear with each other, meaning they exhibit strong correlation. Accounting for covariate multicollinearity is important when developing valid risk models. Excluding collinear variables from the final predictive model may give the impression that this single remaining covariate is an independent predictor, when in actuality it is merely the strongest of all the collinear predictive variables representing the same underlying mechanism. In this case, a high thoracic AIS score may be the most strongly associated injury metric representative of severe torso or overall injury severity. While we did examine the frequency that each potential variable was found to be a predictor among the top models, further analyses are needed to test the multicollinearity of these injury severity metrics.
In contrast to the NTDB-based data we found a severe post-injury shock state was the strongest independent predictor of PE. The presence of hypocoagulopathy after trauma has been well described in the literature. However, a hypercoagulable state has also been identified in patients following severe injury.36 These observational findings have been reinforced with experimental data demonstrating a hypercoagulable state in porcine models of hemorrhagic shock.37–39 This hypercoagulopathic state is often not well represented by commonly utilized laboratory assays such as activated partial thromboplastin time (aPTT) and prothrombin time (PT), but can be detected via thromboelastography (TEG).36,37 Furthermore, there is emerging literature showing that PE are being diagnosed much earlier than previously described. Many are identified within the first several days, and a significant proportion are being diagnosed within 24 hours of injury.7–10 When taken together, these findings suggest that future studies should examine whether PE occur immediately or shortly following injury secondary to a hypercoagulopathic state caused by tissue injury and a state of severe hemorrhagic shock.
There are limitations of this study that must be acknowledged. Although data from this cohort was collected and audited prospectively, this is a secondary analysis and was not designed to be a definitive study to fully elucidate mechanistic differences between post-injury DVT and PE. While standard operating procedures for VTE prophylaxis were established for all centers, there were no defined protocols specified for screening, diagnosis, and treatment of DVT and PE.11 Screening or diagnostic evaluation was based upon individual clinical suspicion and local practice patterns. Additionally, pursuit of further workup for asymptomatic DVT after diagnosis of PE was also not standardized and was determined by individual providers across institutions. It is therefore likely that many, if not most, of DVT and PE diagnosed in this cohort were symptomatic, although it has been shown that up to 10% of PE diagnosed in trauma patients are found incidentally.9 Given these factors, it is probable that overall rates of DVT as well as concurrent DVT/PE are underestimated as many asymptomatic thromboembolic events will have gone undiagnosed. Finally, while a complication rate of 4–8% seems relatively high from a clinical standpoint, statistically these are relatively rare events (i.e. <10%). This represents a challenge in the development of a single best approximating risk factor model. By construction, several of the highest performing models are going to have different predictor variables, which may differ from the top-performing “best final model”. This is why we feel it is important to compare each final model to the covariate occurrence frequencies among the top cross-validated models to assure that potentially significant predictors are identified, even if they are not present in the best final model (Table 5).
Acknowledgments
Sources of Support:
This project was supported by National Institutes of Health (NIH) NIGMS Grant Number U54 GM062119-10, as well the National Cancer Institute (NCI) (R44CA139607, Steven S. Henley, M.S., PI) under the Small Business Innovation Research (SBIR) program. The authors wish to gratefully acknowledge this support. However, this manuscript does not necessarily reflect the views or opinions of the National Institutes of Health or the National Cancer Institute.
Footnotes
Author contributions:
Scott Brakenridge: Study design, data collection, data analysis, data interpretation, manuscript writing.
Steven Henley: Data analysis, data interpretation, manuscript writing.
Michael Kashner: Data analysis, data interpretation, manuscript writing.
Richard Golden: Data analysis, data interpretation.
Dae-Hyun Paik: Data analysis, data interpretation.
Herb Phelan: Critical revision.
Mitchell Cohen: Critical revision.
Jason Sperry: Critical revision.
Ernest E. Moore: Critical revision.
Joseph P. Minei: Critical revision.
Ronald Maier: Study design, data interpretation, critical revision.
Joseph Cuschieri: Study design, data interpretation, critical revision.
The authors have no conflicts of interest to report.
Contributor Information
Steven S. Henley, Email: stevenh@martingale-research.com.
T. Michael Kashner, Email: Michael.Kashner@va.gov.
Richard M. Golden, Email: golden@utdallas.edu.
Herb A. Phelan, Email: Herb.phelan@utsouthwestern.edu.
Mitchell J. Cohen, Email: MCohen@sfghsurg.ucsf.edu.
Jason L. Sperry, Email: sperryjl@upmc.edu.
Joseph P. Minei, Email: Joseph.Minei@UTSouthwestern.edu.
Ronald V. Maier, Email: ronmaier@u.washington.edu.
Joseph Cuschieri, Email: jcuschie@u.washington.edu.
References
- 1.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 2.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331:1601–6. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 3.Meissner MH, Chandler WL, Elliott JS. Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability? The Journal of trauma. 2003;54:224–31. doi: 10.1097/01.TA.0000046253.33495.70. [DOI] [PubMed] [Google Scholar]
- 4.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240:490–6. doi: 10.1097/01.sla.0000137138.40116.6c. discussion 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz DJ, Brasel KJ, Washington L, et al. Incidence of asymptomatic pulmonary embolism in moderately to severely injured trauma patients. The Journal of trauma. 2004;56:727–31. doi: 10.1097/01.ta.0000119687.23542.ec. discussion 31–3. [DOI] [PubMed] [Google Scholar]
- 6.Knudson MM, Gomez D, Haas B, Cohen MJ, Nathens AB. Three thousand seven hundredthirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann Surg. 2011;254:625–32. doi: 10.1097/SLA.0b013e3182300209. [DOI] [PubMed] [Google Scholar]
- 7.Menaker J, Stein DM, Scalea TM. Incidence of early pulmonary embolism after injury. The Journal of trauma. 2007;63:620–4. doi: 10.1097/TA.0b013e31812f60aa. [DOI] [PubMed] [Google Scholar]
- 8.Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Archives of surgery. 2009;144:928–32. doi: 10.1001/archsurg.2009.97. [DOI] [PubMed] [Google Scholar]
- 9.Brakenridge SC, Toomay SM, Sheng JL, Gentilello LM, Shafi S. Predictors of early versus late timing of pulmonary embolus after traumatic injury. American journal of surgery. 2011;201:209–15. doi: 10.1016/j.amjsurg.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer Netto F, Tien H, Ng J, et al. Pulmonary emboli after blunt trauma: Timing, clinical characteristics and natural history. Injury. 2012;43:1502–6. doi: 10.1016/j.injury.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Cuschieri J, Freeman B, O’Keefe G, et al. Inflammation and the host response to injury a large-scale collaborative project: patient-oriented research core standard operating procedure for clinical care X. Guidelines for venous thromboembolism prophylaxis in the trauma patient. The Journal of trauma. 2008;65:944–50. doi: 10.1097/TA.0b013e3181826df7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathens AB, Johnson JL, Minei JP, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: Patient-Oriented Research Core--standard operating procedures for clinical care. I. Guidelines formechanical ventilation of the trauma patient. The Journal of trauma. 2005;59:764–9. [PubMed] [Google Scholar]
- 13.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core--standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. The Journal of trauma. 2006;60:1106–13. doi: 10.1097/01.ta.0000220424.34835.f1. discussion 13. [DOI] [PubMed] [Google Scholar]
- 14.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care. III. Guidelines for shock resuscitation. The Journal of trauma. 2006;61:82–9. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 15.West MA, Shapiro MB, Nathens AB, et al. Inflammation and the host response to injury, a large-scale collaborative project: Patient-oriented research core-standard operating procedures for clinical care. IV. Guidelines for transfusion in the trauma patient. The Journal of trauma. 2006;61:436–9. doi: 10.1097/01.ta.0000232517.83039.c4. [DOI] [PubMed] [Google Scholar]
- 16.Harbrecht BG, Minei JP, Shapiro MB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care: VI. Blood glucose controlin the critically ill trauma patient. The Journal of trauma. 2007;63:703–8. doi: 10.1097/TA.0b013e31811eadea. [DOI] [PubMed] [Google Scholar]
- 17.O’Keefe GE, Shelton M, Cuschieri J, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core--standard operatingprocedures for clinical care VIII--Nutritional support of the trauma patient. The Journal of trauma. 2008;65:1520–8. doi: 10.1097/TA.0b013e3181904b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West MA, Moore EE, Shapiro MB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care VII--Guidelines for antibiotic administration in severely injured patients. The Journal of trauma. 2008;65:1511–9. doi: 10.1097/TA.0b013e318184ee35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans HL, Cuschieri J, Moore EE, et al. Inflammation and the host response to injury, a Large-Scale Collaborative Project: patient-oriented research core standard operating procedures for clinical care IX. Definitions for complications of clinical care of critically injured patients. The Journal of trauma. 2009;67:384–8. doi: 10.1097/TA.0b013e3181ad66a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Efron B. 1977 Rietz Lecture -Bootstrap Methods -Another Look at the Jackknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 21.Golden R, Henley S, White H, Kashner T. New Directions in Information Matrix Testing: Eigenspectrum Tests. In: Swanson, editor. Festschrift Volume in Shonor of Halbert L White, Jr -Causality, Prediction and Specification Analysis: Recent Advances and Future Directions. New York: Springer; 2012. [Google Scholar]
- 22.Edwards D, Havranek T. A fast model selection procedure for large families of models. Journal of the American Statistical Association. 1987;82:205–13. [Google Scholar]
- 23.Viallefont V, Raftery A, Richardson S. Variable selection and Bayesian model averaging in case-control studies. Statistics in Medicine. 2001;20:3215–30. doi: 10.1002/sim.976. [DOI] [PubMed] [Google Scholar]
- 24.Clyde M, George E. Model uncertainty. Statistical Science. 2004;19:81–94. [Google Scholar]
- 25.Bozdogan H. Akaike’s Information Criterion and recent developments in information complexity. Journal of Mathematical Psychology. 2000;44:62–91. doi: 10.1006/jmps.1999.1277. [DOI] [PubMed] [Google Scholar]
- 26.Pepe M. The Statistical Evaluation of Medical Tests for Classification and Prediction. New York: Oxford University Press; 2003. [Google Scholar]
- 27.White H. Maximum likelyhood estimation of misspecified models. Econometrica. 1982;50:1–25. [Google Scholar]
- 28.White H. Estimation, inference and specification analysis. New York: Cambrige University Press; 1994. [Google Scholar]
- 29.Newell MA, Bard MR, Goettler CE, et al. Body mass index and outcomes in critically injured blunt trauma patients: weighing the impact. Journal of the American College of Surgeons. 2007;204:1056–61. doi: 10.1016/j.jamcollsurg.2006.12.042. discussion 62–4. [DOI] [PubMed] [Google Scholar]
- 30.Sharma OP, Oswanski MF, Joseph RJ, et al. Venous thromboembolism in trauma patients. The American surgeon. 2007;73:1173–80. doi: 10.1177/000313480707301121. [DOI] [PubMed] [Google Scholar]
- 31.Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? The Journal of trauma. 2011;70:1381–8. doi: 10.1097/TA.0b013e318215b928. [DOI] [PubMed] [Google Scholar]
- 32.Velmahos GC, Kern J, Chan LS, Oder D, Murray JA, Shekelle P. Prevention of venous thromboembolism after injury: an evidence-based report--part I: analysis of risk factors and evaluation of the role of vena caval filters. The Journal of trauma. 2000;49:132–8. doi: 10.1097/00005373-200007000-00020. discussion 9. [DOI] [PubMed] [Google Scholar]
- 33.Winchell RJ, Hoyt DB, Walsh JC, Simons RK, Eastman AB. Risk factors associated with pulmonary embolism despite routine prophylaxis: implications for improved protection. The Journal of trauma. 1994;37:600–6. doi: 10.1097/00005373-199410000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Hill AB, Fleiszer DM, Brown RA. Chest trauma in a Canadian urban setting--implications for trauma research in Canada. The Journal of trauma. 1991;31:971–3. doi: 10.1097/00005373-199107000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Cudnik MT, Werman HA, White LJ, Opalek JM. Prehospital factors associated with mortality in injured air medical patients. Prehospital emergency care : official journal of the National Association of EMS Physicians and the National Association of State EMS Directors. 2012;16:121–7. doi: 10.3109/10903127.2011.615011. [DOI] [PubMed] [Google Scholar]
- 36.Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. The Journal of trauma. 2009;67:266–75. doi: 10.1097/TA.0b013e3181ae6f1c. discussion 75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todd SR, Malinoski D, Muller PJ, Schreiber MA. Hextend attenuates hypercoagulability after severe liver injury in swine. The Journal of trauma. 2005;59:589–93. discussion 93–4. [PubMed] [Google Scholar]
- 38.Mulier KE, Greenberg JG, Beilman GJ. Hypercoagulability in porcine hemorrhagic shock is present early after trauma and resuscitation. The Journal of surgical research. 2012;174:e31–5. doi: 10.1016/j.jss.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Kashuk JL, Moore EE, Sabel A, et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009;146:764–72. doi: 10.1016/j.surg.2009.06.054. discussion 72–4. [DOI] [PubMed] [Google Scholar]


