Abstract
Background
Congestive heart failure in the setting of a preserved left ventricular (LV) ejection fraction is increasing in prevalence among the senior population. The underlying pathophysiologic abnormalities in ventricular function and structure remain unclear for this disorder. We hypothesized that patients with heart failure with preserved ejection fraction (HFPEF) would have marked abnormalities in LV diastolic function with increased static diastolic stiffness and slowed myocardial relaxation compared with age-matched healthy controls.
Methods and Results
Eleven highly screened patients (4 men, 7 women) aged 73±7 years with HFPEF were recruited to participate in this study. Thirteen sedentary healthy controls (7 men, 6 women) aged 70±4 years also were recruited. All subjects underwent pulmonary artery catheterization with measurement of cardiac output, end-diastolic volumes, and pulmonary capillary wedge pressures at baseline; cardiac unloading (lower-body negative pressure or upright tilt); and cardiac loading (rapid saline infusion). The data were used to define the Frank-Starling and LV end-diastolic pressure-volume relationships. Doppler echocardiographic data (tissue Doppler velocities, isovolumic relaxation time, propagation velocity of early mitral inflow , E/A-wave ratio) were obtained at each level of cardiac preload. Compared with healthy controls, patients with HFPEF had similar LV contractile function and static LV compliance but reduced LV chamber distensibility with elevated filling pressures and slower myocardial relaxation as assessed by tissue Doppler imaging.
Conclusions
In this small, highly screened patient population with hemodynamically confirmed HFPEF, increased end-diastolic static ventricular stiffness relative to age-matched controls was not a universal finding. Nevertheless, patients with HFPEF, even when well compensated, had elevated filling pressures, reduced distensibility, and increased diastolic wall stress compared with controls. In contrast, LV relaxation as assessed by tissue Doppler variables appeared consistently impaired in patients with HFPEF.
Keywords: heart failure, ventricular end-diastolic volume, aging, echocardiography Doppler, hemodynamics
Congestive heart failure (CHF) in the setting of a preserved ejection fraction has been described as an epidemic in the senior population, accounting for up to one half of all hospital admission for CHF.1,2 Despite these statistics, limited progress has been made in elucidating the pathophysiology of heart failure with preserved ejection fraction (HFPEF), particularly when compared to the study of CHF due to left ventricular (LV) systolic dysfunction. Investigation in this field has been hampered by inconsistent diagnostic criteria and challenges with the quantification of diastolic function.3 To date, no single unifying theory has emerged to fully explain the etiology of HFPEF.
The term diastolic heart failure often has been used interchangeably with HFPEF in the literature, and data from relatively younger, predominantly male subjects have implicated increased static LV stiffness and impaired lusitropic function as the primary source of symptoms in these patients.4 However, our laboratory has demonstrated that static LV stiffness and dynamic myocardial relaxation are markedly abnormal even in otherwise healthy sedentary seniors compared with young controls.5,6 These data suggest that the presence of abnormal (ie, not youthful) diastolic function is not pathognomonic for HFPEF, but instead, these findings may represent an aging-related substrate that when coupled with additional comorbid conditions such as hypertension, ischemic heart disease, or diabetes, leads to CHF. Furthermore, numerous studies have suggested alternative or additive contributing mechanisms, including elevation of LV end-diastolic volume (LVEDV), subtle impairments in LV systolic function, and increased ventricular-arterial stiffening.7–9 The lack of a comprehensive paradigm applicable to all patients suggests that the hemodynamic derangements responsible for this disorder may be quite heterogeneous.
In the present study, we performed a comprehensive, detailed characterization of hemodynamics and LV structure and function in a group of senior, mostly female patients with HFPEF, using healthy, sedentary age-matched individuals as controls. We hypothesized that the patients with HFPEF would have increased static LV stiffness and slower myocardial relaxation than the controls, leading to severely impaired ventricular filling and elevated diastolic filling pressures.
Methods
Subjects
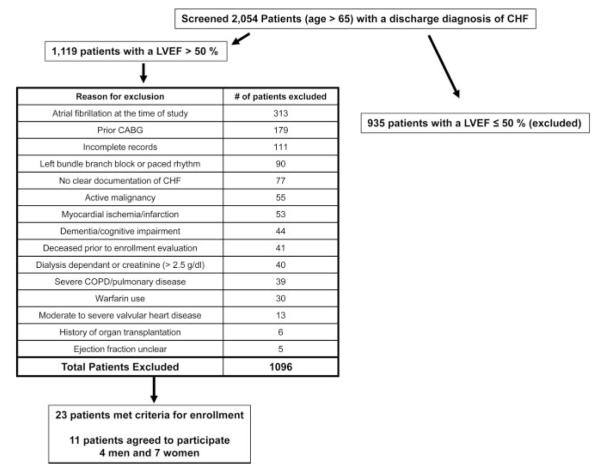
A total of 2054 patients aged >65 years with a hospital discharge diagnosis of CHF were screened for inclusion in this study. Patients with HFPEF were defined as having a clear history of CHF by Framingham criteria with an ejection fraction >50% documented by transthoracic echocardiography at the time of their index hospitalization.10 Exclusion criteria were the presence of atrial fibrillation at the time of the study, prior coronary artery bypass graft, active myocardial ischemia, unrevascularized obstructive coronary disease (>50% lesions by prior angiography), stable angina, recent (<1 year) myocardial infarction, renal failure (creatinine >2.5 g/dL) or dialysis dependence, severe chronic obstructive pulmonary disease or pulmonary disease, active malignancy, moderate or severe valvular heart disease, and warfarin use. A total of 1119 patients had an ejection fraction of >50%, and of these patients, 23 met criteria for enrollment in the study, and 11 (4 men, 7 women) aged 73±7 years agreed to participate (Figure 1). Thirteen healthy, sedentary controls (7 men, 6 women) aged 70±4 years used previously in our studies also were included. The baseline data for all subjects are presented in Table 1. All patients at the time of their index diagnosis met Framingham criteria of CHF,11 and all had either an elevated brain natriuretic peptide level (median value, 459 pg/mL) or documented pulmonary congestion by chest radiograph or right heart catheterization. The controls were the same patients (by design) who had their hemodynamic parameters, static LV compliance, and Doppler echocardiographic data reported previously.5,6 The controls were screened carefully for hypertension and cardiac disease, including structural heart and hemodynamically significant obstructive coronary disease, using a history, physical examination, and resting and postexercise transthoracic echocardiograms. Additional exclusion criteria for this group included valvular heart disease, atrial flutter/fibrillation, renal insufficiency, chronic lung disease, regular cigarette smoking within the past 10 years, and cardiovascular medication. Both groups of subjects underwent measurement of maximal oxygen uptake using previously described methods.5 All subjects signed an informed consent approved by the institutional review boards of the University of Texas Southwestern Medical Center at Dallas, Medical City Hospital (Dallas, Tex), Doctors Hospital (Dallas, Tex), or Presbyterian Hospital of Dallas.
Figure 1.
Patient enrollment flowchart. CABG indicates coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction.
Table 1.
Baseline Subject Characteristics
| HFPEF | Control | P | |
|---|---|---|---|
| No. subjects | 11 | 13 | |
| Baseline characteristics | |||
| Age, y | 73.0±6.8 | 70.2±3.5 | 0.259 |
| Female sex | 7 | 6 | 0.414 |
| BSA, m2 | 1.99±0.27 | 1.85±0.17 | 0.159 |
| VO2 max, mL/kg per min | 13.7±3.4 | 21.6±3.6 | <0.001 |
| VO2 max, L/min | 1.23±0.51 | 1.56±0.34 | 0.075 |
| Comorbid conditions | |||
| Hypertension | 11 (100) | . . . | . . . |
| Diabetes | 6 (55) | . . . | . . . |
| Coronary artery disease | 0 (0) | . . . | . . . |
| Renal insufficiency | 1 (9) | . . . | . . . |
| Hyperlipidemia | 9 (82) | . . . | . . . |
| Index hospitalization evaluation |
|||
| Dyspnea on exertion | 11 (100) | . . . | . . . |
| Pulmonary edema or rales |
9 (82) | . . . | . . . |
| Lower-extremity edema | 8 (73) | . . . | . . . |
| Serum creatinine, mg/dL | 1.2±0.23 | . . . | . . . |
| BNP, pg/mL | 448±374 | . . . | . . . |
| Medications | |||
| Diuretic | 10 (91) | . . . | . . . |
| β-blocker | 6 (55) | . . . | . . . |
| Calcium channel blocker | 5 (45) | . . . | . . . |
| ACE-I/ARB | 9 (82) | . . . | . . . |
| HMG-CoA reductase inhibitor |
9 (82) | . . . | . . . |
Data are presented as mean±SD or no. (%), unless otherwise indicated. ACE-I indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; BNP, brain natriuretic peptide; BSA, body surface area; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; VO2 max, maximal oxygen consumption.
Experimental Protocol
Subjects were studied in the resting, supine, or left lateral position. A 6-F balloon-tipped fluid-filled catheter was placed using fluoroscopic guidance through an antecubital vein into the pulmonary artery. The catheter was connected to a physiological pressure transducer with the 0 reference point set at 5.0 cm below the sternal angle. The wedge position of the catheter tip was confirmed by fluoroscopy as well as by the presence of an appropriate pulmonary capillary wedge pressure (PCWP) waveform.
After at least 30 minutes of quiet supine rest, baseline data were collected. Subsequently, cardiac filling was first decreased using lower-body negative pressure (LBNP) as previously described.12 Two levels of LBNP used were −15 and −30 mm Hg. Due to large body habitus limiting use of the LBNP apparatus in 2 of the patients with HFPEF, head-up tilt at (20° and 40° or 60°) was used instead of LBNP, with the pressure transducer 0 position carefully adjusted to the level of the right atrium documented by fluoroscopy and echocardiography.13 Measurements of mean PCWP, immediately followed by Doppler echocardiographic measurements, were made after 5 minutes at each level of cardiac unloading. After release of the negative pressure (or return to supine position) and confirmed return to hemodynamic baseline, cardiac filling was increased through a rapid infusion of warm (37°C) isotonic saline solution at 100 to 200 mL/min. Measurements were repeated after the infusion of 10 and 20 mL/kg. At each level of cardiac preload, hemodynamic measurements, including heart rate, blood pressure, and cardiac output, by the acetylene rebreathing method were made.14
Echocardiography
For all subjects at each level of cardiac loading and unloading, a transthoracic echocardiogram was obtained using an Advanced Technology Laboratories HDI 5000CV (software version 10.1) or an iE33 echocardiograph. Apical 4-chamber views were used to make each measurement. Volumes (LVEDV and LV end-systolic volume [LVESV]) were determined using a modified Simpson method, which also was used in our previous studies.6 All images were evaluated off line by a blinded experienced sonographer.
Doppler Measurements
Pulse-waved Doppler imaging, using a sample volume of 2.0 mm placed at the tips of the mitral valve leaflets, was used to determine peak velocities of mitral inflow (E- and A-wave velocities). Using a 5-chamber apical view, the interval between aortic outflow during systole and opening of the mitral valve (isovolumic relaxation time [IVRT]) was determined after the sample volume was increased to 4.0 mm. In the apical 4-chamber view, the septal wall was first highlighted in the tissue Doppler imaging (TDI) mode. Using pulse-wave Doppler imaging, a sample volume of 4.0 mm was placed at the septal side of the mitral annulus. The resulting early diastolic waveform velocity was recorded, and the process was repeated for the lateral wall. Values were averaged to obtain TDI Emean.15 A color M-mode image of LV inflow was obtained, with the sampling area positioned to extend from midatrium to the apex directly through the mitral valve orifice. The scale was reduced sufficiently to result in clear aliasing within the early portion of the mitral inflow. The resulting mitral inflow spatiotemporal velocity profile pattern was used to derive the early propagation velocity of mitral inflow. This technique has been described previously.16
Cardiac MRI Measurements
MRI was performed on a 1.5-T Philips NT MRI scanner. Short-axis, gradient-echo, cine MRI sequences with a temporal resolution of 39 milliseconds were obtained to calculate LV masses and volumes as previously described.5 LV mass was computed as the difference between epicardial and endocardial areas multiplied by the density of heart muscle, 1.05 g/mL. For LV volume determination, the endocardial border of each slice was identified manually at end diastole and end systole, and volumes were calculated by summation. LV volumes were calculated by use of the Simpson rule technique as previously described.17 LV ejection fraction was computed as (LVEDV–LVESV)/LVEDV.
Physiological Definitions
The following physiological definitions were used in the present study. Static LV chamber stiffness (or its inverse, chamber compliance) refers to the overall relationship between LV filling pressure and LVEDV as described by the stiffness constant a in the exponential equation described later. Operating or dynamic stiffness (or its inverse, operating compliance) is defined as change in diastolic LV pressure relative to diastolic LV volume or the instantaneous change in LV filling pressure relative to change in LVEDV. LV chamber distensibility refers to the LVEDV for any given LV filling pressure independent of static or operating compliance. Thus, with use of this terminology, an upward and leftward shift in an end-diastolic pressure-volume curve with the same slope, shape, and stiffness constant would have reduced distensibility but similar static chamber stiffness.
Data Analysis
The LVEDV (determined by echocardiography) and PCWP data were used to construct LV end-diastolic pressure-volume curves using the following exponential model, which has been described previously5: P=P∞ (expa(V–V0)−1) where P is PCWP; P∞, pressure asymptote of the curve; V, LVEDV index; V0, equilibrium volume or the volume at which P=0 mm Hg, and a is a constant that characterizes the chamber stiffness. LV end-diastolic transmural pressure-volume curves also were constructed using estimated transmural pressure (PCWP–right atrial pressure).18 The PCWP and stroke volume (SV) data obtained by the acetelyene rebreathing method were used to construct Frank-Starling curves. The LVEDV, SV, and mean atrial pressure data were used to construct preload recruitable stroke work(PRSW) relationships. Circumferential LV wall stress (σc) and strain were determined as previously described5 by use of the modified Laplace relation: σc=Pb/h[1–(h/2b)][1–(hb/2a2)], where P is estimated transmural pressure; h, LV midwall thickness; a, major semiaxis; and b, minor semiaxis. The LV midwall thickness and semiaxis measurements were calculated from the transthoracic echocardiographic images. Ventricular strain was calculated as follows: strain=(V–Vmin)/Vmin, where the smallest end-diastolic volume measured during cardiac unloading (Vmin) was determined. This value was subtracted from the end-diastolic volume at each loading and unloading condition (V–Vmin). The resulting data were used to construct stress-strain plots, which were modeled by an exponential equation (y=aebx). Total arterial compliance was estimated by the ratio between the acetylene rebreathing-derived SV and pulse pressure.19 Effective arterial elastance was estimated as the LV end-systolic pressure divided by SV, where LV end-systolic pressure was estimated as 0.9×systolic blood pressure.20,21
Statistical Methods
Numeric data are presented as mean±SD; in graphics, the SEM is used. Results for individual characteristics between the controls and patients with HFPEF were compared by use of Student t test. For data obtained over the course of cardiac unloading and loading, 2-way repeated-measures ANOVA (group, loading condition) was applied to evaluate the differences between the 2 groups for normally distributed data. The Mann-Whitney rank sum test was used for nonnormally distributed data. Linear regression analysis was performed to assess the relationship between stroke work and LVEDV in both groups as well as the relationship between PCWP and Doppler data. All analyses were performed with statistical software. Given the relatively small sample size, P values are reported and interpreted according to American Physiological Society guidelines.22
The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Results
Subject Characteristics
The baseline characteristics of the study subjects are presented in Table 1. Detailed data for the controls have been published previously.5,6 The subjects were similar in terms of age, although the patients with HFPEF were more obese and had markedly lower maximal oxygen uptakes. The patients were all hypertensive by history, and the majority (55%) had diabetes mellitus. During their index hospitalization they had clear evidence of CHF, and the majority had markedly elevated brain natriuretic peptide levels (55%; mean, 448±374 pg/mL). Most patients were on evidence-based medical therapies for CHF (Table 1). β-blockers were held for at least 24 to 48 hours before all studies and diuretics were delayed to the end of the study on the morning of examination.
Ventricular-Vascular Characteristics
The ventricular-vascular characteristics of the study subjects are summarized in Table 2. Both groups had similar resting index LVEDVs; however, indexed LVESVs were significantly smaller in the patients with HFPEF than in controls(14.3±5.5 mL/m2 versus 20.3±3.1 mL/m2; P=0.004), resulting in a higher ejection fraction in the HFPEF group (74.1±7.5% versus 68.2±2.7%; P=0.021). Although comparable in indexed LV mass and indexed LVEDV, the mass/volume ratio was significantly higher in the patients with HFPEF (1.23±0.32 g/mL versus 0.96±0.15 g/mL; P=0.017). Measures of vascular function, including systemic vascular resistance, total arterial compliance, and effective arterial elastance, were similar in both groups. Pulse pressure was wider in the HFPEF group (74.4±10.4 mm Hg versus 59.3±8.56 mm Hg; P<0.001) owing to a lower diastolic blood pressure (70.2±10.4 mm Hg versus 78.2±7.8 mm Hg; P=0.042) (Table 3).
Table 2.
Baseline Ventricular-Vascular Function
| HFPEF | Control | P | |
|---|---|---|---|
| LV characteristics | |||
| EDV, mL* | 111.5±25.7 | 118.2±22.7 | 0.526 |
| EDV index, mL/m2 | 56.3±12.1 | 63.7±8.4 | 0.109 |
| ESV, mL | 27.5±9.3 | 37.5±7.7 | 0.012 |
| ESV index, mL/m2 | 14.3±5.5 | 20.3±3.1 | 0.004 |
| SV, mL | 82.2±23.3 | 80.7±15.8 | 0.856 |
| SV index, mL/m2 | 41.3±10.0 | 43.5±5.9 | 0.525 |
| EF, % | 74.1±7.5 | 68.2±2.7 | 0.021 |
| LV mass, g | 140.1±58.9 | 114.0±26.4 | 0.181 |
| LV mass index, g/m2 | 69.3±21.8 | 61.0±9.4 | 0.249 |
| LV mass/EDV, g/mL | 1.23±0.32 | 0.96±0.15 | 0.017 |
| LV stiffness constant | 0.041±0.038 | 0.061±0.030 | 0.172 |
| LV equilibrium volume, mL | 9.2±10 | 21.2±8.9 | 0.006 |
| Pressure asymptote, mm Hg |
9.4±8.5 | 5.5±4.3 | 0.124 |
| Operating stiffness during cardiac unloading, mm Hg×m2/mL |
0.60±0.7 | 0.63±1.3 | 0.258 |
| Operating stiffness during cardiac loading, mm Hg×m2/mL |
0.88±1.1 | 1.3±1.5 | 0.258 |
| Vascular function | |||
| SVR, dyne×s×cm−5 | 1375±740 | 1583±280 | 0.357 |
| SVRi, dyne×s×cm−5×m2 | 706±390 | 872±222 | 0.205 |
| PP, mm Hg | 74.4±10.4 | 59.3±8.56 | <0.001 |
| TAC, mL/mm Hg | 1.17±0.27 | 1.31±0.45 | 0.379 |
| Ea, mm Hg/mL | 1.60±0.52 | 1.75±0.50 | 0.490 |
Data are presented as mean±SD. Ea indicates effective arterial elastance; EF, ejection fraction; PP, pulse pressure; SVR, systemic vascular resistance; SVRi, systemic vascular resistance index; TAC, total arterial compliance.
LV volumes and mass obtained by MRI.
Table 3.
Baseline Resting Hemodynamics
| HFPEF | Control | P | |
|---|---|---|---|
| SBP, mm Hg | 144.6±11.5 | 137.5±14.2 | 0.198 |
| DBP, mm Hg | 70.2±10.4 | 78.2±7.8 | 0.042 |
| MAP, mm Hg | 95.0±9.6 | 98.0±9.6 | 0.461 |
| Qreb, L/min | 6.72±2.90 | 5.03±0.63 | 0.052 |
| Qrebi, L · min−1 · m−2 | 3.41±1.54 | 2.73±0.27 | 0.129 |
| HR reb, bpm | 75.8±21.5 | 68.9±10.3 | 0.316 |
| SV reb, mL/min | 86.7±21.1 | 75.5±19.8 | 0.193 |
| SVi reb, mL · min−1 · m−2 | 44.4±14.1 | 40.7±8.9 | 0.440 |
| PCWP, mm Hg | 15.2±5.1 | 11.4±2.0 | 0.021 |
| RAP, mm Hg | 9.55±3.3 | 7.98±1.9 | 0.160 |
| ETMP, mm Hg | 5.66±3.0 | 3.39±1.4 | 0.023 |
Data are presented as mean±SD. DBP indicates diastolic blood pressure; ETMP, estimated transmural pressure; HR, heart rate; MAP, mean arterial pressure; Qreb, cardiac output by acetylene rebreathing technique; RAP, right atrial pressure; reb, rebreathing; SBP, systolic blood pressure; SVi, stroke volume index.
Hemodynamics
The baseline hemodynamics are detailed in Table 3. There were no significant differences in resting heart rate, cardiac index, or systolic blood pressure between the 2 groups. The baseline resting PCWPs were significantly higher in the patients with HFPEF than in controls (15.2±5.1 mm Hg versus 11.4±2.0 mm Hg; P=0.021). Norepinephrine levels increased modestly in response to LBNP in the HFPEF group (baseline, 413±196; highest level of cardiac loading at baseline, 601±330 pg/mL; P=0.001) and control group (baseline, 279±127; highest level of cardiac unloading, 418±217 pg/mL; P=0.016).
LV Contractility and Systolic Function
Overall contractile function was similar between the 2 groups because there were no differences noted in the LV Frank-Starling relationship (second-order regression analysis, r=0.99 for both groups; P=0.664) (Figure 2A) or in the PRSW relationship (linear regression analysis, r=0.97 and r=0.98 for patients with HFPEF and controls, respectively; P=0.995) (Figure 2B).
Figure 2.
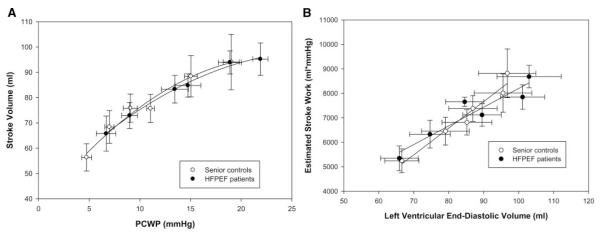
A, Frank-Starling relationship. Overall contractile function was similar between the patients with HFPEF and controls (second-order regression analysis, r=0.99 for both groups; P=0.664). B, PRSW. No differences were noted in PRSW (linear regression analysis, r=0.97 and r=0.98 for patients with HFPEF and controls, respectively; P=0.421).
LV End-Diastolic Pressure-Volume Index Relationship
The grouped mean data demonstrated an upward and leftward shift of the end-diastolic pressure-volume curve for the patients with HFPEF compared with controls (Figure 3A), indicating decreased distensibility (higher pressure for the same volume). However, there was no difference in overall static chamber compliance; evaluation of the stiffness constant a revealed no significant difference between the 2 groups (HFPEF, 0.041±0.038; controls, 0.061±0.030; P=0.172). This relationship persisted when estimated transmural pressure was used in place of PCWP (Figure 3B). Equilibrium volumes were smaller in the patients with HFPEF than in controls (9.2±10 mL versus 21.2±8.9 mL; P=0.006).
Figure 3.
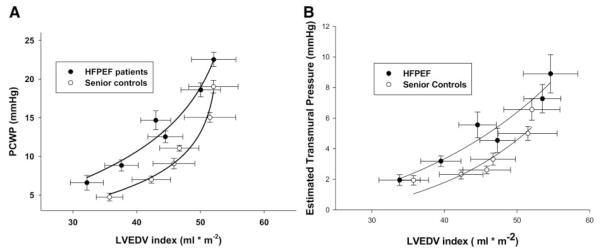
A, End-diastolic pressure-volume relationships showing decreased distensibility in the patients with HFPEF versus controls, with no significant differences in overall chamber compliance. B, End-diastolic transmural pressure-volume relationships. The relationship is maintained even when PCWP is substituted for by estimated transmural pressure (PCWP–right atrial pressure).
End-Diastolic Stress-Strain Relationship
Baseline circumferential wall stress was higher in the patients with HFPEF than in controls (26.5±14.4 kdynes/cm2 versus 7.5±5.2 kdynes/cm2; P<0.001), and this relationship was maintained across loading conditions (Figure 4). Accordingly, at 0 strain, end-diastolic wall stress was higher in the patients with HFPEF (10.9±5.1 kdynes/cm2 versus 2.9±1.5 kdynes/cm2; P=0.015). For any equivalent degree of ventricular deformation, the patients with HFPEF had a higher wall stress during cardiac unloading (P=0.024) but not during saline loading (P=0.339).
Figure 4.
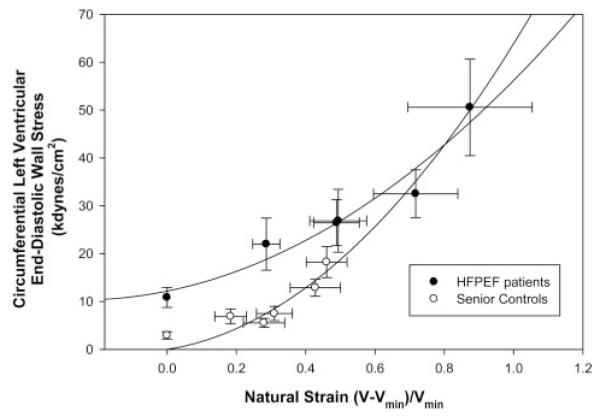
End-diastolic stress-strain relationship. Baseline circumferential wall stress was higher in the patients with HFPEF than in controls (P<0.001). For any equivalent degree of ventricular deformation, the patients with HFPEF had a higher wall stress during cardiac unloading (P=0.024), but not during saline loading (P=0.339), than controls.
Doppler Measures of Diastolic Function
TDI Velocities
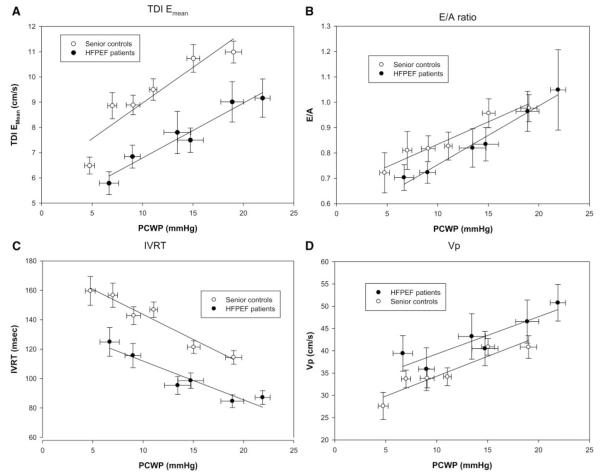
The resting baseline TDI Emean velocities were substantially slower for the patients with HFPEF than in controls (7.62±1.53 cm/s versus 9.49±1.61 cm/s; P=0.014). This significant difference was present across all loading conditions (P=0.013). The increased TDI Emean velocities in the controls were driven by faster TDI Eseptal velocities. At baseline, TDI Eseptal velocity was 6.42±1.31 cm/s in the patients with HFPEF and 8.40±1.68 cm/s in the controls (P=0.008). The overall difference between the 2 groups for TDI Eseptal was highly significant (P=0.002). In contrast, the difference in TDI Elateral velocities between the 2 groups at baseline was less (HFPEF, 8.82±2.13 cm/s; controls, 10.6±2.15 cm/s; P=0.068), with a lower degree of statistical difference across all loading conditions (P=0.078). Data for TDI Emean are shown in Figure 5A.
Figure 5.
A, Mean of lateral and septal TDI mitral annular velocities (TDI Emean), which were significantly slower in the patients with HFPEF than in controls across loading conditions (P=0.013). B, E/A ratio showing no difference across loading conditions between the patients with HFPEF and controls (P=0.431). C, IVRT was prolonged in the controls versus the patients with HFPEF across loading conditions (P=0.002). D, Vp was faster in the controls than in the patients with HFPEF across loading conditions (P=0.023).
Transmitral Flow Velocities: Peak E- and A-Wave Velocity and E/A Ratio
In the patients with HFPEF, the resting baseline peak E-wave velocities were elevated compared with that of the controls (76.5±25.8 cm/s versus 56.8±13.9 cm/s; P=0.031). Similarly, the baseline resting A-wave velocities were higher in the HFPEF group (100.7±28.9 cm/s versus 70.7±17.6 cm/s; P=0.006). The concomitant increase in both peak E- and peak A-wave velocities in the patients with HFPEF resulted in no difference in the resting E/A ratio between the 2 groups at baseline or across loading conditions (Figure 5B).
IVRT
Baseline IVRT was shorter in the patients with HFPEF than in the controls (96.4±35.4 milliseconds versus 146.7±18.8 milliseconds; P<0.001). This difference was present across all preload conditions (P=0.002) (Figure 5C).
Propagation Velocity of Early Mitral Inflow
Baseline propagation velocity of early mitral inflow (Vp) was faster in the HFPEF group than in the control group (42.9±12.8 cm/s versus 34.2±7.2 cm/s; P=0.051). The Vp values were also significantly higher in the patients with HFPEF at the lowest filling pressures (39.4±12.6 cm/s versus 27.6±8.6 cm/s; P=0.038) and at the highest filling pressures (50.8±10.8 cm/s and 40.8±8.8 cm/s; P=0.043). There was a significant difference in the overall relationship between preload and Vp between the 2 groups (P=0.023) (Figure 5D).
Sex Differences in Diastolic Function
Doppler Data
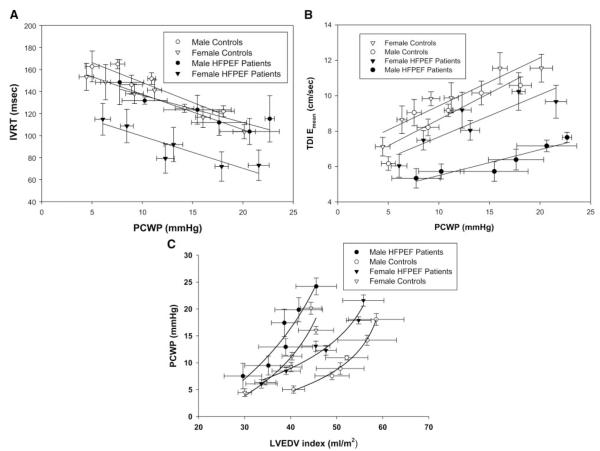
Of the 11 patients with HFPEF, 4 were men, and 7 were women. The Doppler data were analyzed by group (HFPEF or control) and by sex. There were no sex-based differences in the E/A ratio (P=0.868) or Vp (P=0.292). In contrast, IVRT was shortest in the female patients with HFPEF across loading conditions than in the female controls (P=0.008), male controls (P<0.001), and male patients with HFPEF (P=0.030) (Figure 6A). TDI Emean velocities were slower in the female patients with HFPEF versus female controls (P=0.017) and slower in the male patients with HFPEF than in all other subjects (P<0.001) (Figure 6B).
Figure 6.
A. Sex-based differences in IVRT. IVRT was shortest in the female patients with HFPEF across loading conditions than in female controls (P=0.008), male controls (P<0.001), and male patients with HFPEF (P=0.030). B, Sex-based differences in TDI Emean velocities. TDI Emean velocities were slower in the female patients with HFPEF than in the female controls (P=0.017) and slower in the male patients with HFPEF than in all other subjects (P<0.001). C, Sex differences in end-diastolic pressure-volume relationships. Male patients with HFPEF had a leftward shift of their end-diastolic pressure-volume relationship compared with female patients with HFPEF. In contrast, female controls and female patients with HFPEF had little difference in static LV compliance.
LV End-Diastolic Pressure-Volume Index Relationships
Despite the small numbers of subjects, there were large differences noted in the static LV compliance curves between male and female patients with HFPEF (Figure 6C). Male patients had a prominent leftward and upward shift of their end-diastolic pressure-volume relationship compared with male controls. In contrast, female controls and female patients with HFPEF had little difference in static LV compliance. Moreover, the curves for male patients with HFPEF and female controls appeared quite similar; both shifted similarly upward and to the left compared to male controls. The stiffness constants for the male patients appeared higher than for the female patients (0.070±0.053 and 0.025±0.010, respectively); however, statistical comparisons between the groups was limited by the small sample size of the sex subgroups.
Discussion
The primary purpose of the present study was to determine whether patients with HFPEF have clinically meaningful abnormalities of diastolic function; therefore, we were quite rigorous in excluding patients who might have alternative reasons for the development of heart failure. For example, we excluded patients with atrial fibrillation at the time of the study because it may trigger or exacerbate HFPEF, especially if heart rate was uncontrolled.23–26 Secondly, we excluded patients with ischemic heart disease because ischemia clearly elevates filling pressures and alters diastolic function.24–26 Furthermore, we excluded patients with prior coronary artery bypass graft because loss of the pericardium alters LV compliance and interventricular interactions, and these patients may be prone to incomplete revascularization.27 Finally, we excluded patients with renal insufficiency who may have elevated plasma volume and, thereby, elevated filling pressures. As a consequence of these strict criteria, we selected a cohort characterized by the clear presence of CHF but without any alternative explanation for diastolic dysfunction. Furthermore, to our knowledge, this study is the only one to use a healthy age-matched cohort not being catheterized for the presence of angina to compare invasive measurements of diastolic function.
Static LV Stiffness Is Not Increased in All Patients With HFPEF
There are few studies that have examined LV chamber compliance in patients with HFPEF using invasively derived data. Kawaguchi et al,8 studied mostly female and nonsenior patients (9 women, 1 man; mean age, 60.5 years). Using inferior vena cava occlusion and a single-beat method to extrapolate the LV diastolic pressure-volume relationship in 6 of the patients with HFPEF, they demonstrated higher LV end-diastolic pressures but similar stiffness constants among the patients. In contrast, using multiple data points within single-beat and mathematically deriving diastolic pressures, Zile et al4 demonstrated a marked increase in static LV stiffness in younger patients with HFPEF (16 women, 31 men; mean age, 59 years). Data from a group of German investigators also reported elevated stiffness constants (inferior vena cava occlusion, conductance catheter) in young (mean age, <60 years), mostly female patients with HFPEF versus mostly male controls without CHF but with chest pain.28,29 Two additional, completely noninvasive studies warrant mention. Lam et al,30 using Doppler imaging to estimate end-diastolic pressure, demonstrated that senior, mostly female patients with HFPEF (134 women, 110 men; age, 76 years) from a population-based study showed increased passive LV diastolic stiffness compared to controls with and without hypertension. He et al31 compared noninvasively derived end-diastolic pressure-volume relationships in patients with HFPEF (n=128; age, 72 years) with controls (n=93) without hypertension or CHF and noted a slight rightward shift of the end-diastolic pressure-volume curve in the HPPEF group but no difference in the static compliance relationship.
In some, but not all, of these studies, the increased passive elastance of the HFPEF group is striking, with stiffness constants 2 to 3 times higher than controls. Our data in an older population with confirmed HFPEF are notably different. In the present study, the HFPEF group demonstrated modestly decreased LV distensibility but no significant difference in static LV compliance relative to the sedentary controls. Our data extend these previous studies by providing an important signal that excessive static LV stiffness may not be a universal finding in all patients with HFPEF when compared to healthy, sedentary, age-matched individuals.
Doppler Measures of Diastolic Function
There has been considerable debate regarding Doppler measures of diastolic function in the diagnosis of HFPEF. For example, Oh et al32 suggested that in the appropriate clinical setting, the diagnosis of HFPEF can be confirmed if Doppler transmitral velocity patterns and myocardial tissue velocities suggest impaired LV relaxation and compliance. In contrast, Maurer et al33 argued that Doppler patterns do not adequately characterize the static LV end-diastolic pressure-volume relationship or the intrinsic relaxation properties of the myocardium. Previous studies have been inconsistent with regard to both the magnitude and the direction of change in specific Doppler variables.9,28,30,34,35 This disparity is likely multifactorial and influenced by patient age, level of clinical compensation, heterogeneity in the diagnostic inclusion criteria, and inconsistent control groups.
The present study provides further evidence that the differences in conventional Doppler parameters of diastolic function, such as E- and A-wave velocities, the E/A ratio, and IVRT, between healthy sedentary seniors and patients with HFPEF are not sufficient to differentiate these 2 groups. For example, in the present study, patients with HFPEF had higher transmitral E- and A-wave velocities (but similar E/A ratios) and shorter IVRT across loading conditions. These findings are likely explained by the higher left atrial pressure driving faster early and late filling velocities (higher atrioventricular gradients) and earlier opening of the mitral valve.6 The normal E/A ratio, shorter IVRT, and slightly faster Vp in these patients highlight the limitations of traditional Doppler variables to accurately quantify LV relaxation.
Nevertheless, despite higher left atrial pressures, patients with HFPEF had slower TDI velocities, suggesting an inherent abnormality in LV relaxation independent of the influence of elevated filling pressure.15 We previously demonstrated that TDI velocities are markedly slowed with normal sedentary aging compared to young individuals.6 The present study suggests an additional slowing of myocardial relaxation in patients with HFPEF, which is not explainable by aging alone. TDI, therefore, may be a more-specific marker for the possible relaxation abnormalities in patients with HFPEF.
The Doppler and static LV compliance data from the present study, when examined by sex, further emphasize the heterogeneity in the pathophysiology underlying this disorder. Men with HFPEF appear to have more severe abnormalities in diastolic function than women with HFPEF, including higher left atrial pressures, higher static LV stiffness, and slower myocardial relaxation. By contrast, senior women with and without HFPEF appear to be more similar in terms of static LV compliance and myocardial relaxation properties, and both are similar to men with HFPEF. The influence of sex on ventricular diastolic function in this context remains poorly understood and requires larger studies powered to specifically address this issue.
Systolic and Ventricular-Vascular Function in HFPEF
Subtle impairments in systolic function have been postulated to play a role in the pathophysiology of HFPEF.36 In this study, the Frank-Starling curves and PRSW slopes were similar between the 2 groups, suggesting no substantial differences in LV contractile function. Moreover, despite larger pulse pressures, there was little evidence of increased arterial stiffening in the patients with HFPEF, such as total arterial compliance or effective arterial elastance, perhaps because these patients were well treated with antihypertensive medications and compensated at the time of study.
Study Strengths and Limitations
There are several key differences in patient selection and methodology in the present study compared with that in previous studies. First, we included patients with HFPEF who are most representative of the population described in large epidemiological studies, clinical trials, and registry data. Specifically, the patients were mostly women, older (all aged ≥65 years), hypertensive, and given a well-documented diagnosis of CHF. Second, the controls were healthy seniors of similar age who were rigorously screened for cardiovascular disease, allowing for direct comparison with sedentary aging. The methods used to derive the end-diastolic pressure-volume curves were comprehensive, including directly measured PCWP and near-simultaneous LVEDV across multiple levels of cardiac preload. Using this technique, we could generate a complete representation of static LV chamber compliance across a wide spectrum of clinically relevant filling pressures, including the evaluation of operational compliance during elevated filling pressures, which are associated with symptomatic decompensation. Given the strict selection criteria and invasive nature of the protocol, we evaluated a relatively small group of subjects. However, we also avoided studying patients who had alternative non-CHF-related etiologies for dyspnea, edema, and exercise tolerance, which may be one of the reasons for the high prevalence of HFPEF cases.37 Finally, although not powered for sex-based analysis, the disparate findings in men and women with HFPEF are novel and hypothesis generating.
Conclusions
Patients given a clear diagnosis of HFPEF compared with healthy, sedentary, age-matched individuals have an elevated left atrial pressure, even when clinically compensated, associated with reduced distensibility and increased diastolic wall stress; similar static LV compliance; no substantial differences in LV contractile function; and slower TDI velocities suggestive of impaired myocardial relaxation. These data highlight the need for additional study of this complex disease, particularly in a broader subset of patients, to improve the external validity of these results.
CLINICAL PERSPECTIVE.
Heart failure with preserved ejection fraction (HFPEF) has been described as an epidemic in the United States. Alterations of left ventricular relaxation and static chamber compliance have been invoked to explain the underlying pathophysiology of this disorder. However, the mechanistic basis for the syndrome of heart failure in these patients remains controversial. Our own studies have demonstrated marked abnormalities in static chamber compliance and Doppler measures of lusitropic function even in otherwise healthy sedentary seniors, suggesting that diastolic dysfunction alone is not pathognomonic for HFPEF. The primary purpose of the present study was to determine whether patients with HFPEF have clinically meaningful abnormalities of diastolic function. Therefore, we rigorously excluded patients who might have alternative reasons for the development of heart failure independent of diastolic function, including those with ischemia and atrial fibrillation. We obtained detailed hemodynamic data to perform a complete assessment of diastolic function. Our results demonstrated the following key points: (1) Compared with age-matched controls, increased static end-diastolic ventricular stiffness is not present in all patients with HFPEF; (2) patients with HFPEF have elevated filling pressures, reduced distensibility, and increased diastolic wall stress; and (3) ventricular relaxation as assessed by tissue Doppler variables appears impaired in patients with HFPEF. When taken in context with the published literature in the field, the present data suggest substantial heterogeneity in the pathophysiology of this complex disease.
Acknowledgments
Sources of Funding This study was supported by the National Institutes of Health (grant no. AG17479-02); The S. Finley Ewing Jr Chair for Wellness at Presbyterian Hospital, Dallas, Texas; and The Harry S. Moss Heart Foundation, Dallas, Texas.
Footnotes
Disclosures None.
References
- 1.Kitzman DW, Daniel KR. Diastolic heart failure in the elderly. Clin Geriatr Med. 2007;23:83–106. doi: 10.1016/j.cger.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Prasad A, Levine BD. Aging and diastolic heart failure. In: Klein A, Garcia M, editors. Diastology. Elsevier; Philadelphia: 2008. [Google Scholar]
- 4.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 5.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 6.Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol. 2007;99:1629–1636. doi: 10.1016/j.amjcard.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia EH, Perna ER, Farias EF, Obregon RO, Macin SM, Parras JI, Aguero MA, Moratorio DA, Pitzus AE, Tassano EA, Rodriguez L. Reduced systolic performance by tissue Doppler in patients with preserved and abnormal ejection fraction: new insights in chronic heart failure. Int J Cardiol. 2006;108:181–188. doi: 10.1016/j.ijcard.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 9.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: the cardiovascular health study. J Am Coll Cardiol. 2007;49:972–981. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 10.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 11.Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol. 1995;26:1565–1574. doi: 10.1016/0735-1097(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 12.Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and frank-starling relations in endurance athletes: implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- 13.Buckey JC, Jr, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Yancy CW, Jr, Meyer DM, Blomqvist CG. Central venous pressure in space. J Appl Physiol. 1996;81:19–25. doi: 10.1152/jappl.1996.81.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977;48:203–209. [PubMed] [Google Scholar]
- 15.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 16.Popovic ZB, Prasad A, Garcia MJ, Arbab-Zadeh A, Borowski A, Dijk E, Greenberg NL, Levine BD, Thomas JD. Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: impact of age and fitness. Am J Physiol Heart Circ Physiol. 2006;290:H1454–H1459. doi: 10.1152/ajpheart.00902.2005. [DOI] [PubMed] [Google Scholar]
- 17.Peshock RM, Willett DL, Sayad DE, Hundley WG, Chwialkowski MC, Clarke GD, Parkey RW. Quantitative MR imaging of the heart. Magn Reson Imaging Clin N Am. 1996;4:287–305. [PubMed] [Google Scholar]
- 18.Dauterman K, Pak PH, Maughan WL, Nussbacher A, Arie S, Liu CP, Kass DA. Contribution of external forces to left ventricular diastolic pressure: implications for the clinical use of the starling law. Ann Intern Med. 1995;122:737–742. doi: 10.7326/0003-4819-122-10-199505150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–H505. doi: 10.1152/ajpheart.1998.274.2.H500. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 21.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 22.Curran-Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society. Am J Physiol Regul Integr Comp Physiol. 2004;287:R247–R249. doi: 10.1152/ajpregu.00346.2004. [DOI] [PubMed] [Google Scholar]
- 23.Fung JW, Sanderson JE, Yip GW, Zhang Q, Yu CM. Impact of atrial fibrillation in heart failure with normal ejection fraction: a clinical and echocardiographic study. J Card Fail. 2007;13:649–655. doi: 10.1016/j.cardfail.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Aroesty JM, McKay RG, Heller GV, Royal HD, Als AV, Grossman W. Simultaneous assessment of left ventricular systolic and diastolic dysfunction during pacing-induced ischemia. Circulation. 1985;71:889–900. doi: 10.1161/01.cir.71.5.889. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg MA, Menegus MA. Ischemia-induced diastolic dysfunction: New observations, new questions. J Am Coll Cardiol. 1989;13:1071–1072. doi: 10.1016/0735-1097(89)90262-3. [DOI] [PubMed] [Google Scholar]
- 26.van Wezel HB, Koolen JJ, Visser CA, Schuurhuis A. Ischemia induced systolic and diastolic dysfunction in anesthetized patients undergoing percutaneous transluminal coronary angioplasty. J Cardiothorac Anesth. 1989;3:39. doi: 10.1016/0888-6296(89)90782-5. [DOI] [PubMed] [Google Scholar]
- 27.Tyberg JV, Smith ER. Ventricular diastole and the role of the pericardium. Herz. 1990;15:354–361. [PubMed] [Google Scholar]
- 28.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschope C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 29.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschope C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 30.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He KL, Burkhoff D, Leng WX, Liang ZR, Fan L, Wang J, Maurer MS. Comparison of ventricular structure and function in Chinese patients with heart failure and ejection fractions >55% versus 40% to 55% versus <40% Am J Cardiol. 2009;103:845–851. doi: 10.1016/j.amjcard.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47:500–506. doi: 10.1016/j.jacc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 33.Maurer MS, Spevack D, Burkhoff D, Kronzon I. Diastolic dysfunction: can it be diagnosed by Doppler echocardiography? J Am Coll Cardiol. 2004;44:1543–1549. doi: 10.1016/j.jacc.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 34.Maurer MS, King DL, El-Khoury Rumbarger L, Packer M, Burkhoff D. Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail. 2005;11:177–187. doi: 10.1016/j.cardfail.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 36.Steendijk P. Heart failure with preserved ejection fraction: diastolic dysfunction, subtle systolic dysfunction, systolic-ventricular and arterial stiffening, or misdiagnosis? Cardiovasc Res. 2004;64:9–11. doi: 10.1016/j.cardiores.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Ingle L, Cleland JG, Clark AL. Perception of symptoms is out of proportion to cardiac pathology in patients with “diastolic heart failure. Heart. 2008;94:748–753. doi: 10.1136/hrt.2007.131144. [DOI] [PubMed] [Google Scholar]