Abstract
Major well-defined medical problems that are, in part, the unfortunate outcome of a negative social environment may include specific addictive diseases and other mental health disorders, in particular the affective disorders of anxiety, depression, social phobia, and post-traumatic stress syndrome. This overview touches on the topic of extreme marginalization associated with addiction and other mental health disorders, along with arrest, imprisonment, and parole. All of these are characterized by lasting stigma that hauntingly continues to impact upon each person suffering from any of these problems.
Keywords: addiction, mental health disorders, stigma, imprisonment
Introduction
In this ARNMD symposium, the topic of focus was social neuroscience with considerations of the effects of genes (and specifically gene variants) interacting with the environment and that complex interacting with both the brain and the body. Stress, and the individual’s responsivity to stressors, both play an enormous role in the impact of environment upon the brain and body. Further, it is well recognized that many factors in the environment, including stress, may result in epigenetic changes that impact the function of genes for the life of the individual. A few very important functional gene variants have been identified that alter stress responsivity, responsivity that will be further impacted upon by environment and will affect the brain as well as other parts of the body, such as the pituitary, gastrointestinal tract, and immune systems, sometimes resulting in significant medical sequelae.1,2,3,4 Major well-defined medical problems that are, in part, the unfortunate outcome of the interplay between stress vulnerability and a negative social environment include addictive diseases and other mental health disorders, in particular the affective disorders of anxiety, depression, social phobia, and post-traumatic stress syndrome. This overview focuses on the topic of extreme marginalization associated with these disorders, many of which can result in arrest, imprisonment, and parole. The diseases themselves and their unfortunate legal sequelae are characterized by lasting stigma, and the stigma itself is a social stressor that can haunt each person suffering from any of these problems for the rest of their lives.
There are many marginalized populations. These certainly include the impoverished and the poorly educated, the elderly, and ethnic/cultural minority groups (whichever groups are not in the “majority” in any location). Also marginalized, most unfortunately, are those who are physically disabled, either by birth defects, by accidents, or, more recently, and also extremely evident and disturbing, by military conflict. Clearly, those who are mentally disabled, especially those who are not able to cope with many aspects of everyday life, are marginalized. Possibly most marginalized of all are those with specific addictive diseases, as well as those who are imprisoned for non-violent as well as violent crimes.
Of these marginalized populations, it is clearly evident that those who are mentally disabled, those with specific addictive diseases, and those who are classified as criminals are stigmatized and remain stigmatized even after entering into and remaining in effective treatment, or completing sentences and undergoing rehabilitation. Such stigma, which is often hard to define but not hard for those stigmatized to perceive, will further interact with gene variants and with the normal stresses of life to increase atypical responsivity.
For many years, we have conceptualized that the factors contributing to this vulnerability to develop a specific addictive disease fall into categories very similar to the topic of focus of this symposium: genes, environment, and the impact of drugs on the brain and the body, specifically drug-induced effects on mRNA levels, peptides, proteomics, neurochemistry, synaptogenesis, and resultant behaviors.5,6,7,8,9,10 Further, for many years, our group and others have focused on the fact that no single gene is responsible for the development of any specific addiction in the population. Rather, multiple variants of multiple genes acting in concert may increase vulnerability to develop, or protection from developing, specific diseases. Environmental factors certainly play a major role, and these include early environmental factors (which we now know may also bring about epigenetic changes), set and setting, peer pressure, and possibly most important of all, stressors and, in particular, the responsivity to those stressors.11, 12, 13
Investigating the stress response
In our animal model research, we can study stress responsivity in any part of the brain or pituitary, including the hypothalamic-pituitary-adrenal (HPA) axis.11, 12, 13 In our human studies, our focus must usually be more narrow, confined to that which we may directly or indirectly measure by peripheral biomarkers, such as blood levels of ACTH, beta-endorphin, cortisol, and related hormones from the HPA axis. Much of our work arose from the earliest days of our clinical research at The Rockefeller University Hospital, which led directly to the development of methadone maintenance treatment, with publications ensuing over the next several years to show the safety and efficacy of methadone in the treatment of long-term opiate addiction, especially heroin addiction, included studies of stress responsivity. We included in our prospective studies and our special studies the functionality of the stress-responsive HPA axis.14, 15
With the advent of more sophisticated technologies, these studies continue. In particular, in the bi-directional translational research at the Laboratory of the Biology of Addictive Diseases, we have been able to show that heroin, cocaine, and alcohol, three of the most commonly abused classes of drugs, impact upon gene expression, peptide and steroid content, not only a variety of gene products in the brain, but also in that part of the brain that, in primates, resides outside of the blood–brain barrier, the hypothalamus. The involvement of the hypothalamic releasing factors, arginine vasopressin and corticotropin-releasing factor from the hypothalamus; the alteration of processing and release of proopiomelanocortin, which yields both ACTH and beta-endorphin from the anterior pituitary; and the role of cortisol, released from the adrenal cortex upon stimulation by ACTH, have all been shown to be altered in levels and function by specific drugs of abuse––this, in addition to the role of tonic inhibition by activation of the mu opioid receptors, with beta-endorphin being the primary endogenous ligand acting at the hypothalamic and pituitary sites. The negative feedback regulation with the glucocorticoids pertains to humans and non-human primates, but with different glucocorticoids, cortisol in humans and corticosterone in rodent models. Recent studies have begun to question whether the kappa opioid receptor system may also play a role in the primate HPA axis, possibly by activating one or more components of this axis (Fig. 1).
Figure 1.
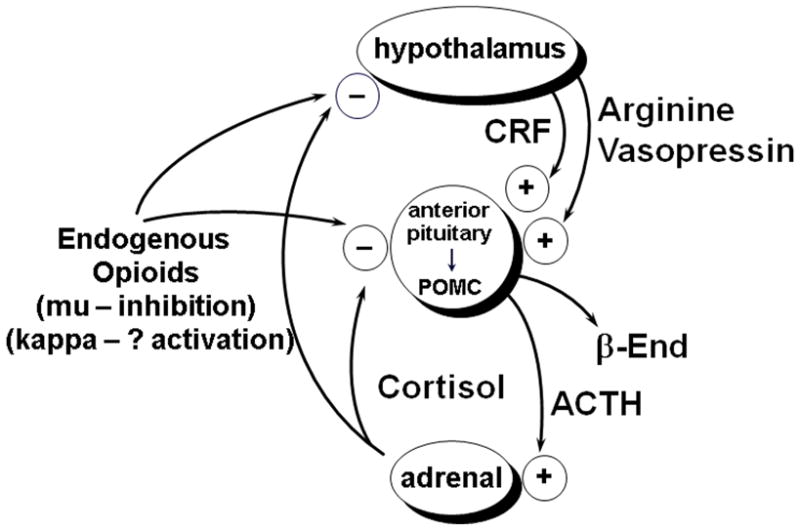
The hypothalamic-pituitary-adrenal stress responsive axis.
Learning from the mu opioid receptor
Our laboratory and others have found in both clinical and animal model studies that the mu opioid receptor is a central component of the rewarding effect of each of three major classes of drugs of abuse (opiates, cocaine and other stimulants, and alcohol). Further, heroin (actually its first metabolite, 6 acetyl-morphine, the active species in heroin) binds to the mu opioid receptor, as do both effective maintenance treatment agents for opiate addiction, i.e., methadone and buprenorphine.
Very soon after the cloning of the mu opioid receptor, Lei Yu and I formed a collaborative team to study the human molecular genetics of the mu opioid receptor. In 1995, when we initiated this work, techniques were still relatively primitive. We therefore performed classical Sanger sequencing in a forward and reverse direction and focused on a narrow area, the coding region of the mu opioid receptor, to determine whether there are any variants of potentially high enough allelic frequency in any ethnic/cultural group and with any changes in the amino acids in the receptor peptide that thus might alter response to all physiological as well as pharmacological actions that are modulated by the mu opioid receptor.
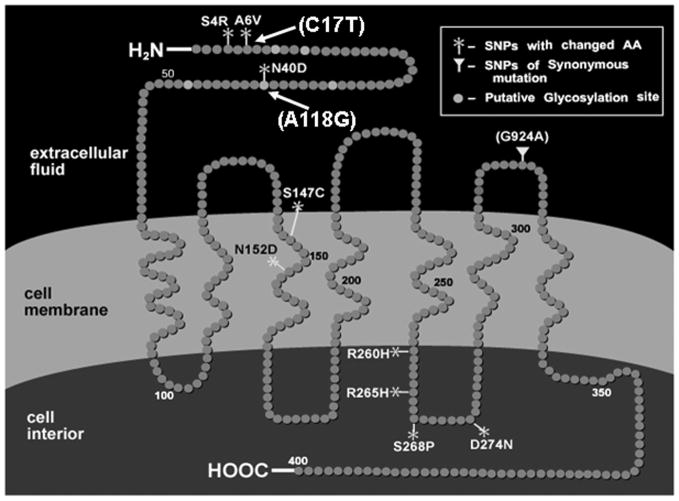
In 1998, we reported the finding of two extremely common allelic variants, both in the coding region, both located in the N-terminus of the mu opioid receptor protein, and both non-synonymous, that is, changing amino acid structure.1 One, the A118G variant, a single nucleotide polymorphism, was common (a minor allele frequency of 10–22%) in Caucasian populations, as originally and subsequently studied, much more common in Asians (40–50%), and very rare in African Americans. This particular variant was of particular interest since it resulted in a change of asparagine, which is probably very active, to aspartic acid, probably less active. The other common variant, C17T, we found to be extremely common in African American populations, which rarely have the A118G variant. This variant also results in a change in amino acid structure, but the effective change is less likely to lead to very important functional changes in activity (Fig. 2).
Figure 2.
The human mu opioid receptor: SNPs identified.
We proceeded to study the A118G variant and found in molecular and cellular construct studies that this was definitely a functional variant. Beta-endorphin, the longest (31 amino acids) and the longest-acting of the endogenous opioids that bind primarily at the mu opioid receptor, bound three times more tightly to the receptor resulting from the gene variant than to the prototype receptor. Further, in additional molecular cellular studies, we found that the Gprotein–coupled inwardly rectified potassium channels were three-fold greater activated when beta-endorphin bound to this variant receptor resulting from the A118G SNP than when it bound to the prototype receptor. However, no other endogenous or exogenous mu opioid receptor directed peptides or heterocyclic compounds bound differently. We predicted in our first paper in 1998 that we would find not only potential pharmacological differences but, more importantly, physiological differences, that is, changes in normal physiology in persons with one or two copies of the A118G variant. Since the physiological functions of the mu opioid receptor are diverse, we predicted that this would be extremely important, which has proven to be true. Specifically, we predicted that stress responsivity, which our group and others have shown is regulated in large part through the HPA axis by inhibition at the mu opioid receptor system, would be altered.
We further predicted that this variant might be associated both with heroin addiction, since heroin addicts avoid stress and stressors, and also with alcoholism, since our laboratory has shown in both animal models and humans that alcoholics seek activation of the stress-responsive HPA axis. We initiated our human physiological studies, as did at least three other groups. The first to document our prediction was the group of Wand at Johns Hopkins, which showed that healthy persons with one copy of the G variant would have a much greater activation of the stress-responsive HPA axis when a mu opioid receptor selective opioid antagonist, in their case naloxone, was administered in high doses. This has been corroborated by many other groups including, subsequently, their own group in a large study (reviewed in Ref. 4). Our group went on to show that healthy humans with one or two copies of this variant had higher basal levels of cortisol.16 Very recently, we have shown, using an objectively measured test of hypothalamic-pituitary reserve, that the metyrapone test, where the normal, circadian rhythm–driven negative feedback by glucocorticoids, cortisol in humans and other primates, acting at both the hypothalamic-pituitary and pituitary sites, led to the reduction of synthesis of cortisol and elevated release of CRF and POMC peptides. Just as we predicted, the tighter binding of beta-endorphin at mu opioid receptors, which acts to tonically inhibit the HPA axis, lessens the effect of blocking negative feedback at the hypothalamic-pituitary sites transiently over 4–8 hours.17
Physiogenomics: connection to heroin addiction and alcoholism
Although in our initial studies we found no association of this variant with opiate addiction in an admixed New York City population, we conducted further studies in Sweden, where the primary population is Swedish, with no admixture for over 500 years, and less than 20% are non-Swedes, with or without admixture.18 In these studies, we were able to show that the A118G variant is very strongly associated with heroin addiction, with the attributable risk to this single variant at over 18%.2 In further studies, also performed in collaboration with Heilig, then at the Karolinska Institute, we were able to show that this variant was associated with alcoholism, a disorder where alcoholics are seeking activation of the HPA axis, as shown by O’Malley in a clinical collaboration with my group.19,20 Numerous other groups have confirmed these findings.
We coined the term “physiogenetics” to parallel the long-standing term (even before molecular genetics studies existed) of “pharmacogenetics.”2 We also had predicted, since alcoholics like activation of the HPA axis, and since our group has shown in numerous studies that opioid antagonists such as naltrexone and nalmefene, as well as naloxone, activate this axis, that persons with one or two copies of this variant would be more likely to respond positively to treatment with a selective opioid antagonist. Studies performed on persons who had much earlier participated in clinical trials of alcoholism treatment with naltrexone, and who agreed to genotyping by the groups of O’Brien and Kranzler, show unequivocally that this is the case and that, in fact, essentially everyone who responded favorably to the naltrexone treatment had one or two copies of the G variant (reviewed in Ref. 4). These findings have subsequently been confirmed in two other studies. Studies by many other groups have shown that response to pain medications may be altered; further studies in non-human primate models, where a very similar variant has been identified, have shown similar molecular findings, as well as atypical responsivity to stress (reviewed in Refs. 3 and 4).
Therefore, one can say that the A118G variant contributes directly to stress responsivity and thus may contribute to the persistence and acquisition of specific addictive diseases. These kinds of findings must be taken into account when taking in the whole issue of social neuroscience and gene–environment interaction. Even more recently, when a large cohort of African American women with and without addiction became available to us for study, we found that the C17T variant of the mu opioid receptor, which has not been studied rigorously at the molecular cellular level but does result in a change of amino acids, when present as the homozygote, that is, the TT variant, was significantly associated with both alcohol and cocaine dependency.21 Thus, this gene variant similarly may contribute directly to the acquisition of addiction.
We have also identified significant epigenetic changes, increased methylation of DNA in the promoter region of the mu opioid receptor in long-term heroin addicts, stabilized in methadone treatment. The changes may have been present prior to exposure to heroin, caused by heroin use, or a result of successful treatment with methadone or more than one of the above.22, 23
At this point in time, in various epidemiological and survey studies done in the United States, we know that over 200 million persons have used alcohol at some time, and up to 19 million reach the criteria for alcoholism. About 35 million have tried cocaine, and three million meet the criteria for cocaine addiction. Around 3.7 million have used heroin at some time, and over 1 million meet the criteria for heroin addiction. Of great concern at this time, and for the past five to ten years, has been the increasing illicit use of prescription opiates. It is now estimated that over 34 million persons in the United States have illicitly used prescription opiates at some time, including 14% of the population over 12 years of age. However, the prevalence of actual addiction or dependence on opioid analgesics is unknown. It is of interest that approximately 1-in-8 to 1-in-15 who ever self-administer alcohol or cocaine will go on to develop an addiction to these substances. Much more striking are data by many different epidemiological groups by way of meta-analyses indicating that between 1-in-3 to 1-in-5 of persons who ever self-administer heroin become addicted.
Towards the goal of prevention as well as treatment
We know that the natural history of drug and alcohol dependence is such that primary prevention is most effective before any self-exposure. However, studies from our group and others, conducted in animal models, have shown that a very limited number of exposures to any of the drugs of abuse, even in the profound patterns used by humans, such as “binge” pattern cocaine or intermittent exposure of heroin, will not change the brain in ways that are permanent or persistent; only transient changes in gene expression, including primarily in a variety of early response genes, and similarly transient changes occur in neurochemistry and behavior. However, this has been well documented by many groups, including our own, in animal models as well as in observational studies in humans, when one begins to see profound brain changes. This may start as early as what we may describe as sporadic, or regular intermittent, use of a drug of abuse in humans. It is at that point that vaccines, as well as selected medications, including naltrexone to prevent any desired effects from prescription opiates or heroin, may be useful. Many users will proceed to regular use (multiple, daily use for opiates, daily or weekly use of “binge” cocaine, and daily or very frequent use of alcohol).
At this point, innumerable clinical research studies have shown that over 80% of persons reaching the criteria of addiction will relapse after achieving abstinence without selected and targeted pharmacotherapy, in addition to counseling, behavioral modification, and classical psychiatric and psychological therapy. Less than 20% of persons with any of these addictions are able to sustain abstinence without specific medications and only counseling and psychological and psychiatric support. Though devastating, these findings are what drive the continued vigorous research into the basic neurobiology to study mechanisms of specific addictions, as well as to develop specific medications for specific addictive diseases, to follow in the footsteps of the extremely effective methadone maintenance treatment long-acting mu opioid receptor full agonist, for heroin and other opiate addiction, and the subsequent development of buprenorphine, a partial, long-acting mu opioid agonist.
Abstinence-based treatment has been found in some studies to be effective in 30% and even up to 50% of long-term cocaine-dependent persons, a higher percentage than has been found for opiate dependence or for alcoholism. However, there is no treatment that has been proven to be effective for cocaine and other stimulant addiction. Some exciting preliminary studies from the group of Franck at the Karolinksa Institute in Stockholm indicate the possibility that naltrexone might be effective for amphetamine addiction.24 Studies from our own group have now shown in humans, as well as animal models, that the use of methadone might be effective in treatment of persons with cocaine addiction with some opiate use or with modest opiate dependence or even very limited to no opiate use.25, 26, 27, 28 Many studies have shown that an opioid antagonist, such as naltrexone or nalmefene, may be effective in 20–40% of unselected alcoholics, and the more recently developed sustained-release naltrexone seems to be similarly effective, at least in studies that have been conducted with at least six months of follow-up (reviewed in Ref. 4).
Treatments are therefore available and need to be offered, and offered without stigma, to those marginalized populations that are suffering from addictive diseases with or without concomitant mental diseases, primarily affective disorders of anxiety, phobic disorders, and post-traumatic stress disorder, as well as other mental disorders, such as schizophrenia and bipolar depression. However, treatment not only is needed, but also must be offered through the same appropriate medically oriented healthcare channels that provide treatment for all other medical and most other psychiatric conditions, and such treatment should be provided without stigma.
It has been shown by studies performed by the RAND Corporation and elsewhere that drug abuse treatment is cost-effective. As summarized by Mark Parrino, president of the organization, at an American Association for the Treatment of Opioid Dependence (AATOD) meeting in 2010, every dollar invested in treatment yields up to seven dollars in reduced crime-related costs, and savings can exceed costs by twelve-to-one when healthcare costs are included (personal communication). Further, there are reduced interpersonal conflicts, improved workplace productivity, and fewer drug-related accidents. Of importance for society, but also for this discussion of social neuroscience in marginalized populations, every study ever performed has shown that effective treatment for drug abuse and addiction reduces arrests and need for incarceration.
Marginalization: the tragedy of the stigmatized and disadvantaged
In data available from 2004, it was shown that over 7 million persons in the United States are under arraignment in federal or state jurisdictions, and over 2 million are in jails or prisons. It was further shown that in the United States, we have 724 prisoners per 100,000 persons, higher than any other nation of the world. Additionally, of interest in suggesting that there may be unevenness in the execution of the law, over 60% of the inmates are African American or Hispanic American. A very high proportion of those in jail or prisons have drug abuse or drug dependence, that is, addiction to one or more substances. It is therefore striking that whereas the prevalence of drug abuse and addiction, the root cause of a large portion of crimes leading to imprisonment, are similar in the three major ethnic/cultural groups (Caucasian, African American, and Hispanic), there is a disproportionate number of ethnic minorities in prison. A study has been conducted and reported that, since 2009, has been under the sponsorship of the Office of National Drug Control Policy.29 This study, Arrestee Drug Abuse Monitoring (ADAM II), covered 4700 arrestees from April 1, 2009 to September 30, 2009. Ten sites participated in the study. More than half of the male-booked arrestees tested positive for at least one addictive drug, ranging from 56% in North Carolina to 82% in Cook County, Illinois. Many of the arrestees tested positive for more than one illegal drug at the time of arrest. Among the arrestees who reported drug use in the last 12 months, only 1–10% were receiving any outpatient drug or alcohol treatment in the past year, and 2–10% reported receiving inpatient or residential treatment in the last year. Additionally, only 1–3% reported receiving any inpatient mental health or psychiatric treatment. Therefore, it is quite clear that the arrestees have a very high prevalence of drug abuse and addiction, yet very few are receiving any treatment.
The problem of poor access to treatment becomes more striking when one looks at jails or prisons, where the needs are enormous. One jail in the New York City area, the Rikers Island facility, has been noteworthy and quite exceptional for offering treatment of addiction for many, many years. This is a facility where inmates stay for 13 months or less. There is no other facility in New York City or New York State that offers such treatment. Therefore, when a person must move from Rikers Island to a more long-term facility, no treatment is available. However, when in Rikers, a program initiated many years ago, referred to as the Key Extended Entry Program (KEEP), is a specific program for treatment of narcotic addiction. This was introduced over 35 years ago for management of opiate addicts, and has been enhanced since 1987 as not only a program for narcotic addiction treatment, but also an AIDS prevention initiative, after our laboratory in collaboration with the Centers for Disease Control and Prevention found that the second risk group for developing AIDS is the parenteral drug abuser. This particular KEEP program at Rikers Island allows both the induction and maintenance treatment with methadone and, since 2000, also offers the alternative treatment of buprenorphine-naloxone.30 Thousands of patients are treated each year. Persons who are already in methadone maintenance or buprenorphine-naloxone maintenance treatment are kept in treatment during their jail time on Rikers Island. Induction at Rikers Island is also possible. However, this stay is short, so all of the inmates are connected while in jail with a program that will accept them for continuing treatment after leaving jail. It is strikingly positive that 76% of all inmate patients report to their assigned programs for continued pharmacological treatment of opiate addiction following release from jail. The inmates at Rikers Island who were treated with methadone maintenance or buprenorphine–naloxone maintenance treatment included 70% males and 30% females (10% of whom were pregnant). It would be wonderful to think that this type of program exists everyplace in the United States. However, this is not the case.
As suggested by the title, stigma supersedes our scientific and clinical research knowledge and our clinical care observations, and that stigma extends (and possibly is intensified) to those with addiction who are in prison. These are the ultimately marginalized populations. It is possible to put on one slide the various models of use of methadone or buprenorphine–naloxone treatment in prisons in the United States in 2010: in one prison in Pennsylvania, located in Philadelphia county, a program that continues persons in methadone treatment (CODAC); a similar program is available in Rhode Island for arrestees in treatment at time of incarceration; in Florida and in Maryland, methadone and/or buprenorphine studies as part of recidivism prevention with induction prior to release; other pilot projects are being or have been conducted in states like New Mexico and Washington. However, treatment of addiction, when diagnosed, is by no means a standard of care or component of rehabilitation within jails and prisons, despite the fact that the relapse rate to drug abuse and addiction is enormous after release and leads to the vicious circle of addiction, crime, and incarceration, often with the presence of other mental disorders.
Possibly the most important goal to be focused on within the fields related to social neuroscience is the eradication of stigma through an increase in our knowledge and sharing and teaching that knowledge to all. Such approaches are under consideration and study. For example, Dr. James Gilligan from New York University has stressed the importance of higher education as a means of reversing criminogenic effects of social adversity in violent prisoners,31 and Dr. Michelle Carsons from Johns Hopkins has described Experience Corps in addressing many of the problems of stigma in the elderly, as well as in the impoverished and poorly educated, and also especially those who are also members of underserved ethnic/cultural minority groups.32
Our basic scientific knowledge and clinical research knowledge in areas related to social neuroscience, including gene–environment and their interactions, have increased. However, we have not made striking progress in implementation of this knowledge. The time has come to address simultaneously the issue of biological vulnerability, environmental stressors, and the role of social stigma and marginalization in the expression and perpetuation of addictive and mental health disorders.
References
- 1.Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: Possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaForge KS, Yuferov V, Kreek MJ. Opioid receptor and peptide gene polymorphisms: potential implications for addictions. Eur J Pharmacol. 2000;410:249–268. doi: 10.1016/s0014-2999(00)00819-0. [DOI] [PubMed] [Google Scholar]
- 3.Kreek MJ, LaForge KS. Stress responsivity, addiction, and a functional variant of the human mu-opioid receptor gene. Molec Interv. 2007;7:74–78. doi: 10.1124/mi.7.2.7. [DOI] [PubMed] [Google Scholar]
- 4.Kreek MJ. Role of a functional human gene polymorphism in stress responsivity and addictions. Clin Pharmacol Ther. 2008;83:615–618. doi: 10.1038/clpt.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade. Arch Intern Med. 1966;118:304–309. [PubMed] [Google Scholar]
- 6.Kreek MJ. Methadone-related opioid agonist pharmacotherapy for heroin addiction: History, recent molecular and neurochemical research and the future in mainstream medicine. Ann N Y Acad Sci. 2000;909:186–216. doi: 10.1111/j.1749-6632.2000.tb06683.x. [DOI] [PubMed] [Google Scholar]
- 7.Kreek MJ. Molecular and cellular neurobiology and pathophysiology of opiate addiction. In: David KL, Charney D, Coyle JT, Nemerof C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams and Wilkins; Philadelphia: 2002. pp. 1491–1506. [Google Scholar]
- 8.Kreek MJ, LaForge KS, Butelman ER. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- 9.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk-taking, stress responsivity, and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 10.Kreek MJ. Endorphins, gene polymorphisms, stress responsivity, and special addictions: Selected topics. In: Madras B, Colvis CM, Pollock JD, et al., editors. Cell Biology of Addiction. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2006. pp. 63–92. [Google Scholar]
- 11.Kreek MJ, Koob GF. Drug dependence: Stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 12.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Proudnikov D, Yuferov V, Kreek MJ. Drug-induced and genetic alterations in stress-responsive systems: implications for specific addictive diseases. Brain Res. 2010;1314:235–252. doi: 10.1016/j.brainres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreek MJ. Medical safety and side effects of methadone in tolerant individuals. JAMA. 1973;223:665–668. [PubMed] [Google Scholar]
- 15.Cushman P, Kreek MJ. Some endocrinologic observations in narcotic addicts. In: Zimmerman E, George R, editors. Narcotics and the Hypothalamus. Raven Press; New York: 1974. pp. 161–173. [Google Scholar]
- 16.Bart G, LaForge KS, Borg L, Lilly C, Ho A, Kreek MJ. Altered levels of basal cortisol in healthy subjects with a 118G allele in exon 1 of the mu opioid receptor gene. Neuropsychopharmacology. 2006;31:2313–2317. doi: 10.1038/sj.npp.1301128. [DOI] [PubMed] [Google Scholar]
- 17.Ducat E, Ray B, Bart G, Umemura Y, Varon J, Ho A, Kreek MJ. Mu-opioid receptor A118G polymorphism in healthy volunteers affects HPA-axis ACTH stress response to metyrapone. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00313.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bart G, Heilig M, LaForge KS, Pollak L, Leal SM, Ott J, Kreek MJ. Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol Psychiatry. 2004;9:547–549. doi: 10.1038/sj.mp.4001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bart G, Kreek MJ, Ott J, LaForge KS, Proudnikov D, Pollak L, Heilig M. Increased attributable risk related to a functional mu-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology. 2005;30:417–422. doi: 10.1038/sj.npp.1300598. [DOI] [PubMed] [Google Scholar]
- 20.O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 21.Crystal H, Hamon S, Randesi M, Cook J, Anastos K, Lazar J, Liu C, Pearce L, Golub E, Valcour V, Weber K, Holman S, Ho A, Kreek MJ. A C17T polymorphism in the mu opiate receptor is associated with quantitative measures of drug use in African-American women. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2010.00265.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen DA, Yuferov V, Hamon S, Jackson C, Ho A, Ott J, Kreek MJ. Increased OPRM1 DNA methylation in lymphocytes of methadone-maintained former heroin addicts. Neuropsychopharmacology. 2009;34:867–873. doi: 10.1038/npp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen DA, Ji F, Yuferov V, Ho A, He C, Ott J, Kreek MJ. Genome-wide association study identifies genes that may contribute to risk for developing heroin addiction. Psychiatr Genet. 2010;20:207–214. doi: 10.1097/YPG.0b013e32833a2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayaram-Lindström N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Ppsych. 2008;165:1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- 25.Borg L, Broe DM, Ho A, Kreek MJ. Cocaine abuse sharply reduced in an effective methadone maintenance program. J Addict Dis. 1999;18:63–75. doi: 10.1300/J069v18n04_06. [DOI] [PubMed] [Google Scholar]
- 26.Peles E, Kreek MJ, Kellogg S, Adelson M. High methadone dose significantly reduces cocaine abuse in Methadone Maintenance Treatment (MMT) patients. J Addict Dis. 2006;25:43–50. doi: 10.1300/J069v25n01_07. [DOI] [PubMed] [Google Scholar]
- 27.Leri F, Zhou Y, Goddard B, Cummins E, Kreek EMJ. Effects of high dose methadone maintenance on cocaine place conditioning, cocaine self-administration, and mu-opioid receptor mRNA expression in the rat brain. Neuropsychopharmacology (Berl) 2006;31:1462–1474. doi: 10.1038/sj.npp.1300927. [DOI] [PubMed] [Google Scholar]
- 28.Leri F, Zhou Y, Goddard B, Levy AM, Jacklin D, Kreek MJ. Steady-state methadone blocks cocaine seeking and cocaine-induced gene expression alterations in the rat brain. Eur Neuropsychopharmacology. 2009;19:238–249. doi: 10.1016/j.euroneuro.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Office of National Drug Control Policy. ADAM II 2009 Annual Report: Arrestee Drug Abuse Monitoring Program II. Washington, DC: [accessed June 13, 2011]. http://www.whitehousedrugpolicy.gov/publications/pdf/adam2009.pdf. [Google Scholar]
- 30.Tomasino V, Swanson AJ, Nolan J, Shuman HI. The Key Extended Entry Program (KEEP): a methadone treatment program for opiate-dependent inmates. Mt Sinai J Med. 2001;68:14–20. [PubMed] [Google Scholar]
- 31.Devine J, Gilligan J, Miczek KA, Shaikh R, Pfaff D. Youth violence. Scientific approaches to prevention. Prologue. Ann N Y Acad Sci. 2004;1036:ix–xii. doi: 10.1196/annals.1330.035. [DOI] [PubMed] [Google Scholar]
- 32.Fried LP, Carlson MC, Freedman M, Frick KD, Glass TA, et al. A social model for health promotion for an aging population: Initial evidence on the experience corps model. J Urban Health: Bull NY Acad Med. 2004;81:64–78. doi: 10.1093/jurban/jth094. [DOI] [PMC free article] [PubMed] [Google Scholar]