Figure 7. Comparison of the extent of iPLA2β modification by (S)-BEL and (R)-BEL.
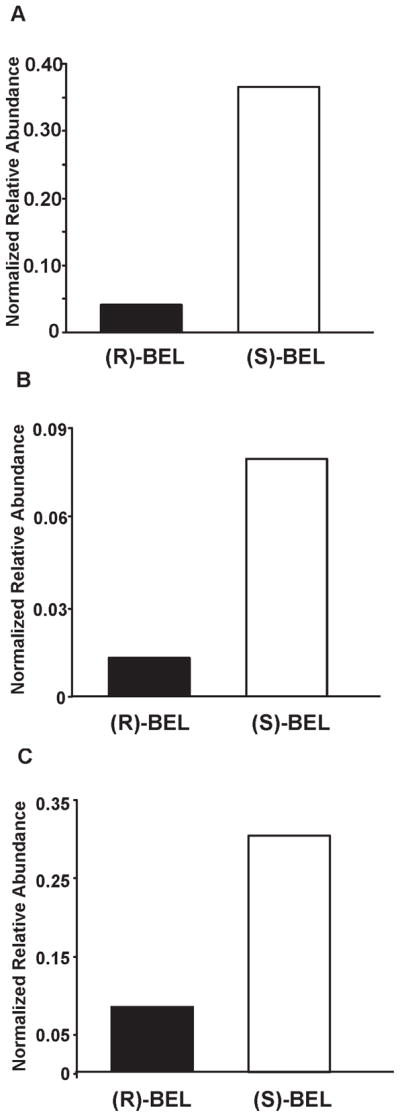
An equal amount of purified iPLA2β was incubated with either (S)-BEL or (R)-BEL at a molar ratio of 1:1 at 22°C for 3 min. The reaction was terminated by precipitating the protein with chloroform/methanol. The protein pellet was solubilized in buffer containing RapiGest™, digested with trypsin, and analyzed by LC/MS/MS as described in “Experimental Procedures”. The normalized relative abundance of a target peptide was calculated as described in “Experimental Procedures”. A, the normalized relative abundance of the BEL-modified S465-containing tryptic peptide (D456-K478) in (R)-BEL- or (S)-BEL-treated samples. B, the normalized relative abundance of the BEL modified C651-containing tryptic peptide (S644-K665) in (R)-BEL- or (S)-BEL-treated samples. C, the normalized relative abundance of the cross-linked peptide in (R)-BEL or (S)-BEL-treated samples. The results indicate that treatment with (S)-BEL results in a higher level of protein modification. By examining the changes in band intensity following SDS-PAGE in the time-course experiment and comparing the levels of modified S465 and C651 as determined by ESI-LC/MS/MS, we found that (S)-BEL is more efficient at covalent modification of iPLA2β than (R)-BEL. These observations are in good agreement with the fact that (S)-BEL is a selective inhibitor of iPLA2β, and demonstrate the enantioselectivity of (S)- vs. (R)-BEL in the kinetics of covalent modification of iPLA2β leading to inhibition of enzymic activity.
