Abstract
The nature of the relationship between affective disorders, bone mineral density (BMD), and bone metabolism is unresolved, although there is growing evidence that many medications used to treat affective disorders are associated with low BMD or alterations in neuroendocrine systems that influence bone turnover. The objective of this review is to describe the current evidence regarding the association of unipolar and bipolar depression with BMD and indicators of bone metabolism, and to explore potential mediating and confounding influences of those relationships. The majority of studies of unipolar depression and BMD indicate that depressive symptoms are associated with low BMD. In contrast, evidence regarding the relationship between bipolar depression and BMD is inconsistent. There is limited but suggestive evidence to support an association between affective disorders and some markers of bone turnover. Many medications used to treat affective disorders have effects on physiologic systems that influence bone metabolism, and these conditions are also associated with a range of health behaviors that can influence osteoporosis risk. Future research should focus on disentangling the pathways linking psychotropic medications and their clinical indications with BMD and fracture risk.
Keywords: Affective disorders, Depression, Bipolar disorder, Osteoporosis, Psychoneuroendocrinology, Aging, Psychotropic medications
Introduction
Osteoporosis and the increased risk of fracture associated with this condition is a growing public health issue in the United States, largely driven by the shift in the proportion of the population surviving into older age. The National Osteoporosis Foundation has projected that by 2020 47.5 million U.S. adults over the age of 50 will have osteopenia and another 13.9 million will have osteoporosis [1]. Osteoporosis is associated with fractures of the hip, vertebrae, and wrist, which incur a significant impact on functioning, disability and increased risk of mortality [2].
Psychiatric and medical health conditions often co-occur [3], and in the past three decades a growing body of research has indicated that psychiatric disorders often precede onset of medical conditions—generally by many years—suggesting that the causal nature of these relationships is bidirectional. For example, depression has been identified as a robust risk factor for several cardiometabolic conditions, including type 2 diabetes [4] and cardiovascular disease [5]. Several psychiatric conditions (i.e., schizophrenia) [6] are associated with hormonal disturbances and behaviors that may affect bone mineral density (BMD), and it has been suggested that unipolar depression (UPD) is an unrecognized risk factor for osteoporosis [7]. This review focuses on the relationship between BMD and two of the most common and costly—both in terms of health care expenses and disability-adjusted life years lost—psychiatric disorders: UPD and bipolar disorder (BPD) [8–10].
Unipolar Depression and Osteoporosis
Unipolar depression is one of the most prevalent psychiatric conditions, affecting approximately 8–16% of the U.S. adult population at some point over the lifespan [11]. Depression generally onsets in mid-life (median age of onset = 32 years) [11, 12], and characterized by an episodic course [13]. Episodes of UPD are characterized by cardinal symptoms of dysphoria and/or anhedonia and a clustering of associated symptoms, including appetite disturbances, problems sleeping, psychomotor changes, concentration problems, fatigue, feelings of guilt, and a preoccupation with death or dying [14].
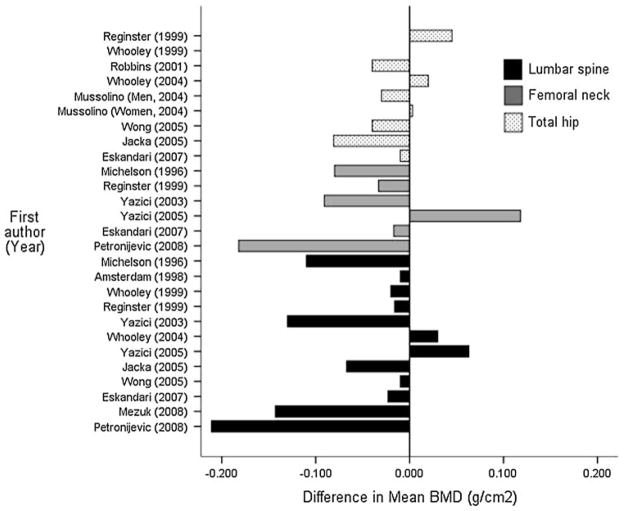
In 1994, Schwieger and colleagues published the first comparative study of depression and osteoporosis and reported that the depressed group had BMD values, on average, 15% lower than the control group [15]. The majority of subsequent studies have replicated this finding of an association between depression and low BMD, although differences in sample composition (particularly concerning age and sex distributions of the participants) and assessment of depression limit the strength of conclusions that can be drawn [16]. Figure 1 illustrates the difference in mean BMD (total hip, femoral neck, and lumbar spine, as indicated, ascertained via DEXA) between UPD and nondepressed comparison participants in those retrospective studies that provided sufficient data to estimate this effect [17–29]. As indicated by the figure, the finding of whether cases of UPD have lower BMD relative to controls, and the degree of that difference, varies substantially both across studies and across DEXA measurement sites. Even if UPD is associated with lower BMD, the clinical significance of this relationship remains to be established (e.g., whether the effect is large enough to warrant clinical intervention).
Fig. 1.
Difference in mean BMD in comparative samples of unipolar depression by DEXA measurement site. Values <0 indicate that the unipolar depression group had lower mean BMD values relative to the comparison group for each study. Note: Kavuncu et al. [84] and Altindag et al. [83] also reported mean DEXA BMD values. Kavuncu et al. [84] found no association between UPD and BMD, and Altindag et al. [83] reported significantly lower BMD among depressed cases relative to controls [83]. However, the BMD units reported from these studies were not comparable with the other reports and thus those values are not plotted in the figure
A handful of studies have examined the association between depressive symptoms at baseline and change in BMD at follow-up, with mixed results [17, 18, 30–33]. For example, Milliken et al. reported that depressive symptoms were associated with a reduction in BMD at the femoral neck among older women 3 years later [33], whereas Whooley et al. found no association between depressive symptoms and BMD change among older men [17]. Spangler et al. reported that the depressive symptoms were only associated with BMD change among the subset of the sample that was not using antidepressants [32].
The primary limitations of extant studies of UPD and BMD pertain to small sample sizes and differing sample composition, the relative lack of longitudinal analyses, and the risk of residual confounding from unaccounted variables, including psychotropic medication use (discussed below). Independent of the relationship between UPD and antidepressant use with BMD, there is substantial evidence that both are associated with risk of fracture, the primary clinical outcome of osteoporosis [16, 34, 35].
Bipolar Disorder and Osteoporosis
Bipolar disorder affects up to 6% of U.S. adults [36] and is characterized by episodes of mania, which are periods of increased energy, restlessness, irritability, racing thoughts, sleep disturbances, and euphoria, and the cycling between manic episodes, depressive episodes, and euthymic mood states [14]. Bipolar disorder is a chronic condition that often onsets early in the life course (median onset = 25 - years) [36] and, like UPD, is often extremely disabling in many functional domains [8].
In contrast to the evidence regarding the relationship between UPD and BMD, there has been relative little research on the association between BPD, or depressive symptoms among persons with BPD, and BMD despite the fact that most persons with BPD experience depressive episodes throughout their lifetime [37]. Studies of BPD and BMD thus far have relied on small (n<50) samples of BPD patients (or cases of both BPD and UPD) from clinics (Table 1). It is difficult to synthesize these results due to the differing study designs and methods used to assess bone mass (i.e., BMD versus BMC), but important limitations of these studies include: (1) heterogeneity in exposure (e.g., including both BPD and UPD cases), (2) limited sample size, and thus low statistical power to detect an effect, and (3) the notable lack of appropriate comparison groups. In addition, none of these reports examined whether characteristics of BPD (i.e., manic or depressive symptom severity or duration), as opposed to duration or type of medication treatment, were associated with bone mass. In sum, there is little evidence that either BPD or manic symptoms are associated with BMD, but this should be interpreted as an absence of evidence rather than evidence of the absence of such an association, particularly in light of the suggestive evidence that lithium, one of the primary medications used to treat this condition, is associated with lower fracture risk [35, 38] and may preserve bone mass [39] (discussed below).
Table 1.
Comparative studies of bipolar disorder (BPD) and bone mineral density (BMD)
| First author | Year | Study design | Sample source and composition | Exposurea | Outcome | Analytic comparison | Matching and/or statistical adjustment | Main findings |
|---|---|---|---|---|---|---|---|---|
| Plenge and Rafaelsen [130] | 1982 | Cross-sectional | In-patient psychiatric clinic Total N = 18 All had taken Li+ Composition not specified |
BPD | Right radius BMC PA | Initiating Li+ therapy versus Cessation of Li+ therapy | None specified | Li+ initiation associated with lower BMC and higher urinary calcium and potassium versus Li+ cessation |
| Nordenström et al. [131] | 1994 | Matched case–control | Mean age: 50.5 Male N = 12 All cases were currently taking Li+ (Avg duration = 15 years) Total N = 52 (Cases = 26) Out-patient psychiatric clinic |
BPD or UPD | Whole body, lumbar spine & femoral neck BMD DEXA |
UPD/BPD cases versus Healthy controls | Matched on age, sex, and BMI | No difference in BMD Cases had higher serum calcium and PTH versus Controls |
| Cohen et al. [132] | 1998 | Matched case–control | Mean age = 32.5 Male N = 5 All were currently taking Li+ Total N = 23 Out-patient psychiatric clinic |
BPD or UPD | Lumbar spine and femoral neck BMD DEXA |
Li+ therapy <1 year versus Li+ therapy >3 years | Matched on sex, weight, calcium intake, Li levels and smoking status | No difference in BMD or serum PTH |
| Gyulai et al. [133] | 1997 | Matched case–control | Out-patient psychiatric clinic Total N = 20 (Cases = 10) All cases were currently taking thyroxine for 1.5 years All women Mean age (Cases) = 40 |
BPD or UPD | Lumbar spine and femoral neck BMD DEXA |
BPD/UPD cases versus healthy controls | Matched on age and menopausal status | No difference in lumbar spine BMD Cases had significantly higher femoral neck BMD versus controls |
| Gyulai et al. [134] | 2001 | Cross-sectional design | Out-patient psychiatric clinic Total N = 26 All had were currently taking levothyroxine for 1+ years All women Mean age = 46.4 |
BPD or UPD | Lumbar spine and femoral neck BMD DEXA |
UPD/BPD cases versus LUNAR reference values | Stratified by menopausal status statistical adjustment | No difference in BMD of cases versus reference values |
| Bauer et al. [135] | 2004 | Prospective cohort | Out-patient psychiatric clinic Total N = 21 All were currently taking levothyroxine Male N = 5 Mean age = 47.3 Mean follow-up = 17.3 months |
BPD, UPD, or ScAD | Lumbar spine and femur BMD DEXA |
Percent change in BMD versus LUNAR BMD reference values | Adjusted for age | Clinical diagnosis not associated with percent BMD change Higher dose of levothyroxine therapy associated with greater BMD change versus reference values |
All diagnoses determined by clinical interview
BPD bipolar depression UPD unipolar depression, ScAD Schizoaffective disorder, BMC bone mineral content, BMD Bone mineral density, PA Photon absorption, DEXA Dual-energy X-ray absorptiometry, Li+ Lithium carbonate, PTH Parathyroid hormone
Potential Pathways Linking Mood Disorders and Osteoporosis
Physiology
Bone turnover is influenced by both local and peripheral hormonal systems. Many neuroendocrine hormones affect bone formation and/or bone resorption, indicating a potential link between psychiatric conditions and bone mass [40, 41]. Both UPD and BPD are associated with alterations in four key physiologic systems that influence bone metabolism: inflammatory processes; hypothalamic-pituitary-adrenal (HPA) axis activation and subsequent hypercortisolism; sympathetic nervous system (SNS) activation; and gonadal and adrenal steroids.
Inflammation
Levels of interleukin-6 (IL-6) and other inflammatory markers, such as C-reactive protein (CRP) and TNF-α, are elevated in depression and BPD [42–45]. These proinflammatory cytokines are associated with bone metabolism and BMD, both through direct and indirect pathways [43, 46, 47]. IL-1 and TNF-α, which are stimulated by IL-6 [43], stimulate bone resorption and inhibit bone formation [48]. IL-6 also stimulates corticotrophin releasing hormone (CRH) secretion and activates the HPA axis, further potentiating the effects of cortisol on bone metabolism [49]. In post-menopausal women the effects of IL-6 on bone metabolism may be more pronounced. Estrogen suppresses IL-6 both at the level of DNA transcription and receptor activation [43] and disease states characterized by a loss of gonadal hormones are associated with heightened levels of IL-6 [43]. Also, psychiatric symptoms including hostility, depression, and anxiety have been shown to influence T-cell activity [50–52], and T cells are key activators of receptor activator of nuclear factor KB ligand (RANKL), which is the primary regulator of osteoclast activity [46, 53]. It is important to note that the relationship between depressive symptoms and inflammation is clearly bi-directional [52], as many of the primary symptoms of depression, including appetite disturbances, sleep disturbances, and fatigue, are also consequences of proinflammatory cytokines.
Hypercortisolism
Hypercortisolism, a consequence of HPA axis activation, has potent effects on bone metabolism [54] and rate of bone loss [55, 56]. Both depressive symptoms and BPD have been associated with hypercortisolism [57, 58]. Cortisol directly alters bone formation by inhibiting production of type I collagen by osteoblasts [59], and indirectly influences bone metabolism by inhibiting absorption of calcium from the intestine [56]. Depressive symptoms and hypercortisolism are also associated with centralized deposition of fat and insulin resistance [60–62], which are thought to indirectly influence BMD through both inflammatory and steroidal pathways (discussed below). The links between hypercortisolism, depressive symptoms, insulin resistance, centralization of body fat, and low BMD are possibly best exemplified by the case of Cushings Syndrome, a state of hypercortisolism induced by a tumor of pituitary or adrenal glands [63, 64]. Long-term use of glucocorticoids can also induce a clustering of these signs and symptoms and secondary osteoporosis [65].
Sympathetic Activation
Depressive symptoms are associated with elevations in SNS activity as measured by catecholamine synthesis and hormone levels (i.e., tyrosine hydroxylase, norepinephrine) [66, 67], and elevated levels of these hormones are associated with reduced BMD [40]. Animal models suggest that exposure to chronic stress (an established risk factor for UPD) [68], and subsequent SNS activation, directly inhibits bone formation by reducing the number and activity of osteoblasts [69, 70]. While the influence of the SNS activation on BMD is thought to be modest, this system may also influence bone metabolism by moderating the response of bone to mechanical stimulation [70].
Gonadal and Adrenal Steroids
Gonadal and adrenal steroids decline with age, and the most rapid time of BMD loss occurs during and after menopause for older adults [1]. Both UPD and BPD are associated with decreased levels of the gonadal steroids estrogen [71] and testosterone [72], which are key regulators of bone formation [73, 74]. There is also suggestive evidence linking depressive symptoms to alterations in levels of the adrenal steroid dehydroepiandrosterone (DHEA) [75]. Elevated sex-hormone binding globulin (SHBG), the primary chaperone protein for gonadal hormones, has been associated with existing osteoporosis and markers of bone metabolism in men [76, 77]. Animal studies also suggest that the effect of pharmacologic agents on bone, including selective serotonin reuptake inhibitors (SSRIs), is moderated by sex hormones [78]. The adrenal steroid DHEA and its sulfate (DHEAS) have been associated with BMD, and synthesis of these adrenal steroids is influenced by circulating levels of cortisol [73]. Randomized controlled trials indicate that DHEA replacement in older adults is associated with increases in BMD [79], although this relationship appears to be mediated by estrogen [80].
Markers of Bone Turnover
Elevated bone turnover is thought to negatively influence BMD and indicate increased fracture risk [40, 81]. As discussed above, UPD and BPD are associated with hormones that increase bone resorption (i.e., cortisol); affective disorders are also associated with hormonal states and clinical conditions that potentiate bone formation (i.e., hyperinsulemia and type 2 diabetes, discussed below). However, only nine studies have directly examined the effect of depressive symptoms on markers of bone metabolism, with mixed results (Table 2). The findings regarding osteocalcin are contradictory [25, 47, 82, 83], and several investigations reported no difference in serum markers of bone turnover comparing depression cases to controls [21, 22, 26, 84]. Many additional studies have examined the relationship between UPD or BPD and neuroendocrine hormones that influence bone metabolism but are not indicators of bone turnover or calcium metabolism directly (i.e., prolactin, estrogen, cortisol, testosterone, IL-6, and leptin) [41, 82, 83, 85]. The primary limitations of existing studies of affective disorders and markers of bone turnover pertain to small sample sizes and the complete absence of studies in men. Finally, reflecting the dearth of investigations concerning the relationship between BPD and BMD, currently no studies have examined the influence of BPD or manic symptoms on markers of bone metabolism in a comparative manner.
Table 2.
Comparative studies of unipolar depression (UPD) and markers of bone turnover
| First author | Year | Study design | Sample source and composition | Measure of exposure | Indicators of bone metabolisma | Matching and/or statistical adjustment | Main findings |
|---|---|---|---|---|---|---|---|
| Michelson et al. [25] | 1996 | Matched case–control | Community volunteers and provider referrals Total N = 48 (Cases = 24) All women Mean age = 41.0 |
UPD Clinical diagnosis |
Osteocalcin Deoxypyridinoline-to-creatinine ratio N-telopeptide-to-creatinine ratio 25-OH VitD PTH |
Matched on age, race, body mass index, and menopausal status | Cases had lower levels of osteocalcin and lower ratios of deoxypyridinoline-to-creatinine versus controls No difference in N-telopeptide-to-creatinine ratio, PTH or 25-OH VitD |
| Herrán et al. [47] | 2000 | Case–control | Outpatient psychiatric clinic No previous psychiatric history Total N = 38 (Cases = 19) All women Mean age = 44.7 |
UPD Assessed via SCAN |
Osteocalcin C-terminal type I collagen propeptide BAP Telopeptide Crosslaps iPTH 25-OH VitD |
None Additional analyses stratified by menopausal status |
Cases had higher levels of osteocalcin, telopeptide, and crosslaps versus controls Cases had lower levels of iPTH versus Controls No difference in levels of type I collagen propeptide, BAP, or 25-OH VitD |
| Kavuncu et al. [84] | 2002 | Case–control | Outpatient psychiatric clinic Total N = 84 (Cases = 42) All women Men age (Cases) = 35.4 |
UPD Clinical diagnosis |
BAP Osteocalcin PTH Calcium Deoxypyridinoline-to-creatinine ratio |
Matched on age and body mass index | No difference in levels of BAP, osteocalcin, PTH or calcium Cases had higher deoxypyridinoline-to-creatinine ratio versus controls |
| Yazici et al. [22] | 2003 | Matched case–control | Outpatient psychiatric clinic Total N = 40 (Cases = 25) All women Mean age (Cases) = 31.8 |
UPD Assessed via SCAN |
Osteocalcin Deoxypyridinoline-to-creatinine ratio PTH 25-OH VitD |
Matched on age, body mass index, calcium intake, physical activity and social class | No difference in levels of osteocalcin, PTH or 25-OH VitD Cases had higher deoxypyridinoline-to-creatinine ratio versus controls |
| Yazici et al. [21] | 2005 | Case–control | Source unknown Total N = 65 (Cases = 35) All women Mean age (Cases) = 44.8 |
UPD Clinical diagnosis |
Osteocalcin C-telopeptide |
None Additional analyses stratified by severity of depressive symptoms |
No difference in levels of osteocalcin or telopeptide |
| Kahl et al. [82] | 2006 | Case–control | Source unknown Total N = 83 (Cases = 47) N = 24 UPD only N = 23 UPD + borderline PD N = 16 borderline PD only N = 20 controls All women Mean age (Cases) = 36.2 |
UPD Assessed via SCID |
Osteocalcin Crosslaps iPTH 25-OH VitD |
Adjusted for age; stratified by age | UPD only group had higher levels of crosslaps versus Controls UPD + borderline PD group had higher levels of osteocalcin versus Controls No difference in levels of 25-OH VitD or iPTH |
| Eskandari et al. [26] | 2007 | Case–control | Community volunteers Total N = 133 (Cases = 89) All women Mean age = 35 |
UPD Assessed via SCID |
BAP Osteocalcin N-telopeptide Total & ionized calcium 25-OH VitD iPTH |
None | Cases had higher levels of BAP and iPTH versus Controls Cases had lower levels of ionized calcium and 25-OH VitD versus Controls No difference in N-telopeptide, osteocalcin, or total calcium |
| Altindag et al. [83] | 2007 | Matched case–control | Outpatient clinic Total N = 77 (Cases = 36) All women Mean age (Cases) = 39.8 |
UPD Clinical diagnosis |
Osteocalcin C-telopeptide |
Matched on age and BMI | Cases had lower levels of osteocalcin and higher levels of C-telopeptide versus controls |
| Petronijević et al. [28] | 2008 | Matched case–control | Inpatient psychiatric clinic Total N = 120 (Cases = 73) All women Mean age (Cases) = 40.7 |
UPD Assessed via SCID |
BAP N-telopeptide PTH 5b-TRAP |
Matched on age, age of menarche, number of pregnancies & deliveries, BMI, smoking status & family history of osteoporosis | Cases had higher levels of N-terminal telopeptide and 5b-TRAP versus Controls No difference in levels of PTH or BAP |
Borderline PD borderline personality disorder, SCAN schedules for clinical assessment in neuropsychiatry, SCID structured clinical interview for DSM, iPTH intact parathyroid hormone; 25- OH VitD 25-hydroxy vitamin D, BAP bone-specific alkaline phosphatase, 5b-TRAP 5b-tartarate resistant acid phosphatase
Only markers that directly influence or indicate bone metabolism
Health Behaviors
Unipolar depression is associated with poor health behaviors, including smoking, decreased physical activity, and alcohol abuse, which are all associated with an increased risk for osteoporosis and fracture. The alternating manic and depressive symptoms that characterize BPD lead to sedentary behaviors, poor dietary intake (including calcium intake), increased alcohol intake, and nonadherence with medical regimens, which all negatively influence bone health [16]. These behaviors may have direct (i.e., physical activity, particularly resistance training, and directly potentiates bone formation) or indirect (via cascading physiologic changes) on bone strength.
Alcohol Use
Depression and BPD are associated with increased alcohol use and risk of alcohol dependence [86]. Chronic alcohol use is associated with low BMD via inhibition of osteoblast proliferation and function as well as decoupling the resorption/formation cycle of bone turnover [87, 88]. Chronic alcohol abuse is associated with elevated fracture risk [87], although evidence indicates that this risk is stronger for trauma-related rather than osteoporotic fractures [89]. However, the influence of moderate alcohol use on bone turnover is unresolved [87, 90], and there is suggestive evidence that the effect is sex-dependent, with moderate use being beneficial to BMD for women [91, 92].
Smoking
Bipolar disorder and depressive symptoms are associated with higher rates of smoking [93] and nicotine dependence [94]. Smoking is associated with lower BMD indirectly by interfering with estrogen activity and inhibiting calcium absorption by the intestines [95]. Smoking initiation usually begins before the second decade of life when peak bone mass is being reached [96]. There is evidence that the effects of smoking on bone mass are reversible, and smoking cessation has been associated with increases in BMD among post-menopausal women [97].
Weight Gain and Sedentary Lifestyle
Body weight is positively associated with BMD [98] and is thought to preserve bone mass by two mechanisms: (1) synthesis of estrogen in adipose cells [99], which in turn promotes bone formation [74], and (2) through providing physical resistance to skeletal movement, which stimulates osteoblast and osteoclast activity in vitro [100]. Studies have reported conflicting associations between UPD and BPD and overall obesity as indicated by body mass index [101], although there is relatively consistent evidence regarding the positive association between depressive symptoms and centralized obesity as measured by waist–hip-ratio [102]. The degree of adiposity is an important factor in the relation between affective disorders and BMD for two reasons: (1) abdominal obesity has been shown to be associated with higher urinary free cortisol [61, 62], a heightened cortisol response toCRHand adrenocorticotropin releasing hormone [61], and an abnormal response to the dexamethasone suppression test at doses below 1 mg, [62] and (2) obesity is associated with heightened levels of inflammatory markers and steroid hormones which influence bone metabolism, as discussed above [43]. Also, adipose tissue is a site of peripheral aromatization of estrone [103], and estrogens are a primary regulator of bone formation [104]. Thus, while higher BMI may be protective of BMD loss with age, centralized obesity may be detrimental to bone mass because it is symptomatic of HPA and SNS hyperactivation, which inhibits bone formation and promotes bone.
Depression and depressive episodes in BPD are associated with fatigue and physical inactivity [105, 106], and recent evidence suggests the relationship between depressive symptoms and physical activity is bidirectional, at least among young adults [107]. Physical activity, particularly resistance training, is associated with increased bone turnover and reduced fracture risk [108, 109]. Studies have demonstrated that even brief (12 weeks) periods of bed rest can produce alterations in markers of bone turnover consistent with increased bone resorption and BMD loss [110].
Confounding Influences
A confounder is a factor that is associated with the primary exposure of interest (e.g., affective disorders), is causally related to the outcome of interest (e.g., BMD or bone turnover), but is not a mediator in the exposure–outcome relationship. Failure to account for confounders can lead to spurious exposure–outcome relationships. Two key potential confounders that should be accounted for when studying the relationship between affective disorders and osteoporosis are psychotropic medications and comorbid medical conditions.
Psychotropic Medications
The relationship between psychotropic medications and BMD is complex and varies by pharmacologic class, each of which may impact bone metabolism in differing ways. The medications most commonly used to treat BPD are mood stabilizers (i.e., lithium carbonate), neuroleptics (i.e., haloperidol, clozapine), anticonvulsants (i.e., valproic acid, carbamazepine), and antidepressants (i.e., selective serotonin reuptake inhibitors, SSRIs) [111]. Recent evidence suggests that antidepressants, particularly SSRIs, may be associated with low BMD [29, 112–115], and there is consistent evidence that antidepressant use is associated with elevated risk of fracture [34, 35]. Anticonvulsant medications (used to treat epilepsy, but often used as a mood-stabilizer in BPD) interfere with metabolism of 25-hydroxy vitamin D and absorption of calcium from the intestines [116], and duration of use of these medications has been consistently associated with degree of BMD loss [117, 118]. Neuroleptic (also called antipsychotic) medications are often used to treat schizophrenia and other psychotic disorders, and their use is also common among patients with BPD. Conventional neuroleptic medications increase prolactin levels by interfering with the inhibitory functions of dopamine on the pituitary [118]. Elevated levels of prolactin are associated with hypogonadism and BMD loss [119]. Atypical neuroleptics appear to have less impact on prolactin metabolism, but have well-documented effects on weight gain and risk of glucose intolerance and type 2 diabetes [120]. Type 2 diabetes is associated with alterations in BMD and elevated fracture risk [121]. Mood-stabilizers such as lithium are associated with elevated parathyroid hormone (PTH) [73, 118]. Parathyroid hormone regulates the concentration of calcium in serum by influencing absorption of calcium from the intestines and bone turnover. Hyperparathyroidism with resultant hypercalcemia is associated with reduced BMD [73], although there is no consistent evidence that lithium use is associated with elevated bone turnover or BMD loss in BPD [35, 39, 122]. In sum, patients with affective disorders are often prescribed multiple medications to treat their psychiatric symptoms, and while these medications can improve functioning they have potential adverse iatrogenic effects on BMD. It is not uncommon for older UPD and BPD patients to have been taking psychotropic medications for many years [123], and the effect of such long-term use on bone histology, mass, and turnover is unknown.
It is important to emphasize that many factors, including pre-existing medical conditions, polypharmacy, and underlying frailty, likely moderate the relationship between psychotropic medication use and BMD. Subsequently, groups which have a higher burden of these factors (i.e., older residents in nursing homes and long-term care facilities) may be particularly prone to adverse effects of these medications [124].
Comorbid Medical Conditions and Associated Medications
Depressive symptoms commonly co-occur with medical conditions that influence bone strength, including type 2 diabetes and cardiovascular disease [125]. Both UPD and BPD are associated with the metabolic syndrome, a clustering of cardiovascular risk factors including adiposity, hyperlipidemia, hyperglycemia, hypercholesterolemia, and hypertension [60, 61]. The relationship between type 2 diabetes, BMD and fracture risk is complex, but this condition appears to elevate fracture risk despite being associated with normal or near-normal BMD [121, 126]. Finally, many medications used to treat cardiometabolic conditions, such as loop diuretics for hypertension and thiazolidinediones for diabetes, are associated with low BMD [127, 128].
Conclusion
The risk profile for developing osteoporosis has two peaks over the life course: (1) young adulthood, when there is a failure to reach peak bone mass; and (2) later adulthood, when bone mass is reabsorbed into the body at an accelerated rate due to the effects of aging and menopause [104]. Early or mid-life UPD or BPD episodes may exert influence on bone metabolism in one or both of these periods and subsequently heighten the risk for low BMD in later adulthood. Unipolar depression and BPD are associated with both physiological alterations and health behaviors that are known to influence bone turnover and mass. There is also suggestive evidence that many pharmacologic agents used to treat these conditions are associated with physiologic changes that may inhibit bone formation and/or promote bone loss. Many cardiometabolic conditions that are more common among BPD and UPD patients also influence BMD. Providers should be aware that patients receiving therapy for these psychiatric conditions should be monitored for BMD changes, and preventive actions (such as encouraging vitamin D and calcium supplementation and regular physical activity) should be integrated into treatment plans and guidelines [129] in order to protect against the increased potential for fracture.
References
- 1.U.S. Department of Health and Human Services. WE 225 B71259 2004 Bone health and osteoporosis: a report of the Surgeon General. 2004
- 2.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–7. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 3.Eaton WW, editor. Medical and psychiatric comorbidity over the lifecourse. Arlington, VA: American Psychiatric Publishing Inc; 2005. [Google Scholar]
- 4.Mezuk B, Eaton WW, Albrecht S, et al. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diab Care. 2008;31:2383–90. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Kooy K, van Hout H, Marwijk H, et al. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psych. 2007;22:613–26. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 6.Halbreich U. Osteoporosis, schizophrenia, and antipsychotics: the need for a comprehensive multifactorial evaluation. CNS Drugs. 2007;21:641–57. doi: 10.2165/00023210-200721080-00003. [DOI] [PubMed] [Google Scholar]
- 7.Cizza G, Ravn P, Chrousos G. Depression: a major, unrecognized risk factor for osteoporosis? Trend Endocrinol Metab. 2001;12:198–203. doi: 10.1016/s1043-2760(01)00407-6. [DOI] [PubMed] [Google Scholar]
- 8.Murray CJ, Lopez AD. Global burden, disability, and the contribution of risk factors: global burden of disease study. Lancet. 1997;349:1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg PE, Stiglin LE, Finkelstein SN, et al. The economic burden of depression in 1990. J Clin Psych. 1993;54:405–18. [PubMed] [Google Scholar]
- 10.Kleinman L, Lowin A, Flood E, et al. Costs of bipolar disorder. Pharmacoeconomics. 2003;21:601–22. doi: 10.2165/00019053-200321090-00001. [DOI] [PubMed] [Google Scholar]
- 11.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 12.Eaton WW, Kalaydjian A, Scharfstein DO, et al. Prevalence and incidence of depressive disorder: the Baltimore ECA follow-up, 1981–2004. Acta Psych Scand. 2007;116:182–8. doi: 10.1111/j.1600-0447.2007.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton WW, Shao H, Nestadt G, et al. Population-based study of first onset and chronicity in major depressive disorder. Arch Gen Psych. 2008;65:513–20. doi: 10.1001/archpsyc.65.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Assocation. Diagnostic and statistical manual of mental disorders—IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 15.Schweiger U, Deuschle M, Körner A, et al. Low lumbar bone mineral density in patients with major depression. Am J Psych. 1994;151:1691–3. doi: 10.1176/ajp.151.11.1691. [DOI] [PubMed] [Google Scholar]
- 16.Mezuk B, Eaton WW, Golden SH. Depression and osteoporosis: epidemiology and potential mediating pathways. Osteoporos Int. 2008;19:1–12. doi: 10.1007/s00198-007-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whooley MA, Cauley JA, Zmuda JM, et al. Depressive symptoms and bone mineral density in older men. J Geriatr Psych Neurol. 2004;17:88–92. doi: 10.1177/0891988704264537. [DOI] [PubMed] [Google Scholar]
- 18.Whooley MA, Kip KE, Cauley JA, et al. Depression, falls, and risk of fracture in older women. Arch Intern Med. 1999;159:484–90. doi: 10.1001/archinte.159.5.484. [DOI] [PubMed] [Google Scholar]
- 19.Reginster JY, Deroisy R, Paul I, et al. Depressive vulnerability is not an independent risk factor for osteoporosis in post-menopausal women. Maturitas. 1999;33:133–7. doi: 10.1016/s0378-5122(99)00057-2. [DOI] [PubMed] [Google Scholar]
- 20.Robbins J, Hirsch C, Whitmer R, et al. The association of bone mineral density and depression in an older population. J Am Geriatr Soc. 2001;49:732–6. doi: 10.1046/j.1532-5415.2001.49149.x. [DOI] [PubMed] [Google Scholar]
- 21.Yazici AE, Bagis S, Tot S, et al. Bone mineral density in premenopausal women with major depression. Joint Bone Spine. 2005;72:540–3. doi: 10.1016/j.jbspin.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Yazici K, Akinci A, Sütçü A, et al. Bone mineral density in premenopausal women with major depressive disorder. Psych Res. 2003;117:271–5. doi: 10.1016/s0165-1781(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 23.Jacka FN, Pasco JA, Henry MJ, et al. Depression and bone mineral density in a community sample of perimenopausal women: Geelong Osteoporosis study. Menopause. 2005;12:88– 91. doi: 10.1097/00042192-200512010-00015. [DOI] [PubMed] [Google Scholar]
- 24.Wong SY, Lau EM, Lynn H, et al. Depression and bone mineral density: is there a relationship in elderly Asian men? Osteoporos Int. 2005;16:610–5. doi: 10.1007/s00198-004-1730-2. [DOI] [PubMed] [Google Scholar]
- 25.Michelson D, Stratakis C, Hill L, et al. Bone mineral density in women with depression. NEJM. 1996;335:1176–81. doi: 10.1056/NEJM199610173351602. [DOI] [PubMed] [Google Scholar]
- 26.Eskandari F, Martinez PE, Torvik S, et al. Low bone mass in premenopausal women with depression. Arch Intern Med. 2007;167:2329–36. doi: 10.1001/archinte.167.21.2329. [DOI] [PubMed] [Google Scholar]
- 27.Amsterdam JD, Hooper MB. Bone density measurement in major depression. Prog Neuropsychopharmacol Biol Psych. 1998;22:267–77. doi: 10.1016/s0278-5846(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 28.Petronijević M, Petronijević N, Ivković M, et al. Low bone mineral density and high bone metabolism turnover in premenopausal women with unipolar depression. Bone. 2008;42:582–90. doi: 10.1016/j.bone.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Mezuk B, Eaton WW, Golden SH, et al. Depression, antidepressants, and bone mineral density in a population-based cohort. J Gerontol A Biol Sci Med Sci. 2008;63:1410–5. doi: 10.1093/gerona/63.12.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schweiger U, Weber B, Deuschle M, et al. Lumbar bone mineral density in patients with major depression: evidence of increased bone loss at follow-up. Am J Psych. 2000;157:118–20. doi: 10.1176/ajp.157.1.118. [DOI] [PubMed] [Google Scholar]
- 31.Søgaard AJ, Joakimsen RM, Tverdal A, et al. Long-term mental distress, bone mineral density, and non-vertebral fractures. Osteoporos Int. 2005;16:887–97. doi: 10.1007/s00198-004-1784-1. [DOI] [PubMed] [Google Scholar]
- 32.Spangler L, Scholes D, Brunner RL, et al. Depressive symptoms, bone loss, and fractures in postmenopausal women. J Gen Intern Med. 2008;23:567–74. doi: 10.1007/s11606-008-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milliken LA, Wilhelmy J, Martin CJ, et al. Depressive symptoms and changes in body weight exert independent and site-specific effects on bone in postmenopausal women exercising for 1 year. J Gerontol A Biol Sci Med Sci. 2006;61A:488–94. doi: 10.1093/gerona/61.5.488. [DOI] [PubMed] [Google Scholar]
- 34.Takkouche B, Montes-Martinez A, Gill S, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Safety. 2007;30:171–84. doi: 10.2165/00002018-200730020-00006. [DOI] [PubMed] [Google Scholar]
- 35.Vestergaard P. Fracture risks of antidepressants. Expert Rev Neurothera. 2009;9:137–41. doi: 10.1586/14737175.9.1.137. [DOI] [PubMed] [Google Scholar]
- 36.Kessler RC, Berglund P, Delmer O, et al. Lifetime prevalence and age of onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psych. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 37.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psych. 2002;59:530–7. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 38.Vestergaard P. Skeletal effects of central nervous system active drugs: anxiolytics, sedatives, antidepressants, lithium and neuroleptics. Curr Drug Saf. 2008;3:185–9. doi: 10.2174/157488608785699432. [DOI] [PubMed] [Google Scholar]
- 39.Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44:331–4. doi: 10.1016/j.bone.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Raisz L. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999;48:1353–8. [PubMed] [Google Scholar]
- 41.Williams LJ, Pasco JA, Jacka FN, et al. Depression and bone metabolism. Psychother Psychosom. 2009;78:16–25. doi: 10.1159/000162297. [DOI] [PubMed] [Google Scholar]
- 42.Licinio J, Wong M. The role of inflammatory mediators in the biology of major depression: Central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-response systems, and contribute to neurotoxicity and neuroprotection. Mol Psych. 1999;44:317–27. doi: 10.1038/sj.mp.4000586. [DOI] [PubMed] [Google Scholar]
- 43.Papanicolaou DA, Wilder RL, Manolagas SC, et al. The pathophysiologic roles of interleukin-6 in human disease. Ann Internal Med. 1998;128:127–37. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 44.De Berardis D, Campanella D, Gambi F, et al. The role of C-reactive protein in mood disorders. Int J Immunopathol Pharmacol. 2006;19:721–5. doi: 10.1177/039463200601900402. [DOI] [PubMed] [Google Scholar]
- 45.Taylor MJ, Goodwin GM. Long-term prophylaxis in bipolar disorder. CNS Drug. 2006;20:303–10. doi: 10.2165/00023210-200620040-00004. [DOI] [PubMed] [Google Scholar]
- 46.Rauner M, Sipos W, Pietschmann P. Osteoimmunology. Int Arch Allergy Immunol. 2007;143:31–48. doi: 10.1159/000098223. [DOI] [PubMed] [Google Scholar]
- 47.Herrán A, Amado JA, García-Unzueta MT, et al. Increased bone remodeling in first-episode major depressive disorder. Psychosom Med. 2000;62:779–82. doi: 10.1097/00006842-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Romas E, Martin T. Cytokines in the pathogenesis of osteoporosis. Osteoporos Int. 1997;7:S47–53. doi: 10.1007/BF03194342. [DOI] [PubMed] [Google Scholar]
- 49.Kiecolt-Glaser J, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–6. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 50.Mommersteeg PM, Vermetten E, Kavelaars A, et al. Hostility is related to clusters of T-cell cytokines and chemokines in healthy men. Psychoneuroendocrinology. 2008;33:1041–50. doi: 10.1016/j.psyneuen.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms in monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med. 2003;65:362–8. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- 52.Anisman H, Kokkinidis L, Merali Z. Further evidence for the depressive effects of cytokines: anhedonia and neurochemical changes. Brain Behav Immun. 2002;16:544–56. doi: 10.1016/s0889-1591(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 53.Galliera E, Locati M, Mantovani A, et al. Chemokines and bone remodeling. Int J Immunopathol Pharmacol. 2008;21:485–91. doi: 10.1177/039463200802100301. [DOI] [PubMed] [Google Scholar]
- 54.Kann P, Laudes M, Piepkorn B, et al. Suppressed levels of serum cortisol following high-dose oral dexamethasone administration differ between healthy postmenopausal females and patients with established primary vertebral osteoporosis. Clin Rheumatol. 2001;20(1):25–9. doi: 10.1007/s100670170099. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds RM, Dennison EM, Walker BR, et al. Cortisol secretion and rate of bone loss in a population-based cohort of elderly men and women. Calcif Tissue Int. 2005;77:134–8. doi: 10.1007/s00223-004-0270-2. [DOI] [PubMed] [Google Scholar]
- 56.Dennison E, Hindmarsh P, Fall C, et al. Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. J Clin Endocrinol Metab. 1999;84:3058–63. doi: 10.1210/jcem.84.9.5964. [DOI] [PubMed] [Google Scholar]
- 57.Carroll BJ, Curtis GC, Davies BM, et al. Urinary free cortisol excretion in depression. Psychol Med. 1976;6(1):43–50. doi: 10.1017/s0033291700007480. [DOI] [PubMed] [Google Scholar]
- 58.Watson S, Gallegher P, Ritchie J, et al. Hypothalamic–pituitary– adrenal axis function in patients with bipolar disorder. Br J Psych. 2004;184:496–502. doi: 10.1192/bjp.184.6.496. [DOI] [PubMed] [Google Scholar]
- 59.Delany AM, Gabbitas BY, Canalis E. Cortisol down-regulates osteoblast procollagen MRNA by transcriptional and post-transcriptional mechanisms. J Cell Biol. 1995;57:488–94. doi: 10.1002/jcb.240570314. [DOI] [PubMed] [Google Scholar]
- 60.Bjorntorp P, Rosmond R. The metabolic syndrome—a neuroendocrine disorder? Br J Nutr. 2000;83:S49–57. doi: 10.1017/s0007114500000957. [DOI] [PubMed] [Google Scholar]
- 61.Pasquali R, Cantobelli S, Casimirri F, et al. The hypothalamic– pituitary–adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab. 1993;77(2):341– 6. doi: 10.1210/jcem.77.2.8393881. [DOI] [PubMed] [Google Scholar]
- 62.Ljung T, Andersson B, Bengtsson B, et al. Inhibition of cortisol secretion by dexamethasone in relation to body fat distribution: a dose–response study. Obesity Res. 1996;4:277–82. doi: 10.1002/j.1550-8528.1996.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 63.Francucci CM, Pantanetti P, Garrapa GG, et al. Bone metabolism and bone mass in women with Cushing’s Syndrome and adrenal incidentaloma. Clin Endrocrinol. 2002;57:587–93. doi: 10.1046/j.1365-2265.2002.01602.x. [DOI] [PubMed] [Google Scholar]
- 64.Mancini T, Doga M, Mazziotti G, et al. Cushing’s syndrome and bone. Pituitary. 2004;7:249–52. doi: 10.1007/s11102-005-1051-2. [DOI] [PubMed] [Google Scholar]
- 65.De Nijs RN. Glucocorticoid-induced osteoporosis: a review on pathophysiology and treatment options. Minerva Med. 2008;99:23–43. [PubMed] [Google Scholar]
- 66.Lake CR, Pickar D, Ziegler MG, et al. High plasma norepinephrine levels in patients with major affective disorder. Am J Psych. 1982;139:1315–8. doi: 10.1176/ajp.139.10.1315. [DOI] [PubMed] [Google Scholar]
- 67.Maes M, Vandewoude M, Schotte C, et al. Positive relationship between the catecholaminergic turnover and the DST results in depression. Psychol Med. 1990;20(3):493–9. doi: 10.1017/s0033291700017001. [DOI] [PubMed] [Google Scholar]
- 68.Brown GW, Harris TO, Eales MJ. Aetiology of anxiety and depressive disorders in an inner-city population. Psychol Med. 1993;23:155–65. doi: 10.1017/s0033291700038940. [DOI] [PubMed] [Google Scholar]
- 69.Yirmiya R, Goshen I, Bajayo A, et al. Depression induces bone loss through stimulation of the sympathetic nervous system. Proc Natl Acad Sci USA. 2006;103:16876–81. doi: 10.1073/pnas.0604234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marenzana M, Chenu C. Sympathetic nervous system and bone adaptive response to its mechanical environment. J Musculoskelet Neuronal Interact. 2008;8:111–20. [PubMed] [Google Scholar]
- 71.Rehman H, Masson E. Neuroendocrinology of female aging. Gend Med. 2005;2:41–56. doi: 10.1016/s1550-8579(05)80008-7. [DOI] [PubMed] [Google Scholar]
- 72.Carnahan R, Perry P. Depression in aging men: the role of testosterone. Drug Aging. 2004;21:361–76. doi: 10.2165/00002512-200421060-00002. [DOI] [PubMed] [Google Scholar]
- 73.Shoback D, Marcus R, Bikle D. Chapter 8: metabolic bone disease. In: Greenspan F, Gardner D, editors. Basic and clinical endocrinology. New York: Lange Medical Books/McDraw-hill; 2004. pp. 295–361. [Google Scholar]
- 74.Khosla S, Melton LJ, III, Atkinson EJ, et al. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–74. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 75.Markopoulou K, Papadopoulos A, Juruena MF, et al. The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology. 2009;34:19–26. doi: 10.1016/j.psyneuen.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Legrand E, Hedde C, Gallois Y, et al. Osteoporosis in men: a potential role for the sex hormone binding globulin. Bone. 2001;29:90–5. doi: 10.1016/s8756-3282(01)00478-1. [DOI] [PubMed] [Google Scholar]
- 77.Lormeau C, Soudan B, d Herbomez M, et al. Sex hormone-binding globulin, estradiol, and bone turnover markers in male osteoporosis. Bone. 2004;34:933–9. doi: 10.1016/j.bone.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 78.Battaglino R, Vokes M, Schulze-Späte US. Fluoxetine treatment increases trabecular bone formation in mice. J Cell Biochem. 2007;100:1387–94. doi: 10.1002/jcb.21131. [DOI] [PubMed] [Google Scholar]
- 79.Jankowski CM, Gozanski WS, Schwartz RS, et al. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endrocrinol Metab. 2006;91:2986–93. doi: 10.1210/jc.2005-2484. [DOI] [PubMed] [Google Scholar]
- 80.Jankowski CM, Gozanski WS, Kittelson JM, et al. Increases in bone mineral density in response to oral dehydroandroepisterone replacement in older adults appear to be mediated by serum estrogens. J Clin Endrocrinol Metab. 2008;93:4767–73. doi: 10.1210/jc.2007-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melton LJ, III, Khosla S, Atkinson EJ, et al. Relationship of bone turnover to bone density and fractures. J Bone Miner Res. 1997;12:1083–91. doi: 10.1359/jbmr.1997.12.7.1083. [DOI] [PubMed] [Google Scholar]
- 82.Kahl KG, Greggersen W, Rudolf S, et al. Bone mineral density, bone turnover, and osteoprotegerin in depressed women with and without borderline personality disorder. Psychosom Med. 2006;68:669–74. doi: 10.1097/01.psy.0000237858.76880.3d. [DOI] [PubMed] [Google Scholar]
- 83.Altindag O, Altindag A, Asoglu M, et al. Relation of cortisol levels and bone mineral density among premenopausal women with major depression. Int J Clin Pract. 2007;61:416–20. doi: 10.1111/j.1742-1241.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 84.Kavuncu V, Kuloglu M, Kaya A, et al. Bone metabolism and bone mineral density in premenopausal women with mild depression. Yonsei Med J. 2002;43:101–8. doi: 10.3349/ymj.2002.43.1.101. [DOI] [PubMed] [Google Scholar]
- 85.Halbreich U, Rojansky N, Palter S, et al. Decreased bone mineral density in medicated psychiatric patients. Psychosom Med. 1995;57:485–91. doi: 10.1097/00006842-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 86.Grant B, Harford T. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39:197–220. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- 87.Turner RT. Skeletal response to alcohol. Alcohol Clin Exp Res. 2000;24:1693–701. [PubMed] [Google Scholar]
- 88.Chakkalakal DA. Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res. 2005;29:2077–90. doi: 10.1097/01.alc.0000192039.21305.55. [DOI] [PubMed] [Google Scholar]
- 89.Peris P, Guañabens N, Monegal A, et al. Aetiology and presenting symptoms in male osteoporosis. Br J Rheumatol. 1995;34:936–41. doi: 10.1093/rheumatology/34.10.936. [DOI] [PubMed] [Google Scholar]
- 90.Holbrook TL, Barrett-Connor E. A prospective study of alcohol consumption and bone mineral density. BMJ. 1993;306:1506–9. doi: 10.1136/bmj.306.6891.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Felson DT, Zhang Y, Hannan MT, et al. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am J Epidemiol. 1995;142:485–92. doi: 10.1093/oxfordjournals.aje.a117664. [DOI] [PubMed] [Google Scholar]
- 92.Feskanich D, Korrick SA, Greenspan SL, et al. Moderate alcohol consumption and bone density among postmenopausal women. J Womens Health. 1999;8:65–73. doi: 10.1089/jwh.1999.8.65. [DOI] [PubMed] [Google Scholar]
- 93.Anda RF, Williamson DF, Escobedo LG, Remington PL, et al. Depression and the dynamics of smoking: a national perspective. JAMA. 1990;264:1541–5. [PubMed] [Google Scholar]
- 94.Breslau N, Kilbey M, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Arch Gen Psych. 1991;48:1069–74. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- 95.Kapoor D, Jones T. Smoking and hormones in health and endocrine disorders. Eur J Endocrinol. 2005;152:491–9. doi: 10.1530/eje.1.01867. [DOI] [PubMed] [Google Scholar]
- 96.Centers for Disease Control and Prevention. Current trends differences in the age of smoking initiation between blacks and whites. MMWR Morb Mortal Wkly Rep. 1991;40:754–757. [PubMed] [Google Scholar]
- 97.Oncken C, Prestwood K, Kleppinger A, et al. Impact of smoking cessation on bone mineral density in postmenopausal women. J Womens Health. 2006;15:1141–50. doi: 10.1089/jwh.2006.15.1141. [DOI] [PubMed] [Google Scholar]
- 98.Dargent-Molina P, Poitiers F, Breart G. In elderly women weight is the best predictor of a very low bone mineral density: evidence from the EPIDOS study. Osteporos Int. 2000;11:881–8. doi: 10.1007/s001980070048. [DOI] [PubMed] [Google Scholar]
- 99.Blouin K, Nadeau M, Mailloux J, et al. Pathways of adipose tissues androgen metabolism in women: depot differences and modulation by adipogenesis. Am J Physiol Endocrinol Metab. 2008;296:244–55. doi: 10.1152/ajpendo.00039.2008. [DOI] [PubMed] [Google Scholar]
- 100.Kadow-Romacker A, Hoffmann JE, Duda G, et al. Effect of mechanical stimulation on osteoblast- and osteoclast-like cells in vitro. Cell Tissues Organs. 2008 doi: 10.1159/000178022. [DOI] [PubMed] [Google Scholar]
- 101.Lawlor DA, Hart CL, Hole DJ, et al. Body mass index in middle life and future risk of hospital admission for psychosis or depression: findings from the Renfrew/Paisley study. Psycholl Med. 2007;37:1151–61. doi: 10.1017/S0033291707000384. [DOI] [PubMed] [Google Scholar]
- 102.Ahlberg AC, Ljung T, Rosmond R, et al. Depression and anxiety symptoms in relation to anthropometry and metabolism in men. Psych Res. 2002;112:101–10. doi: 10.1016/s0165-1781(02)00192-0. [DOI] [PubMed] [Google Scholar]
- 103.Schiff I. Menopause. Philadelphia, PA: J.B. Lippincott Company; 1995. Principles and practice of endocrinology and metabolism; pp. 915–24. [Google Scholar]
- 104.WHO. Assessment of fracture risk and its application to screening for post-menopausal osteoporosis: Report of the WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–126. [PubMed] [Google Scholar]
- 105.Camancho T, Roberts R, Lazarus N, et al. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol. 1991;134(2):220–31. doi: 10.1093/oxfordjournals.aje.a116074. [DOI] [PubMed] [Google Scholar]
- 106.Kritz-Silverstein D, Barrett-Connor E, Corbeau C. Crosssectional and prospective study of exercise and depressed mood in the elderly: the Rancho Bernardo Study. Am J Epidemiol. 2001;153:596–603. doi: 10.1093/aje/153.6.596. [DOI] [PubMed] [Google Scholar]
- 107.McKercher CM, Schmidt MD, Sanderson KA, et al. Physical activity and depression in young adults. Am J Prev Med. 2009;36:161–4. doi: 10.1016/j.amepre.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 108.Adami S, Gatti D, Viapiana O, et al. Physical activity and bone turnover markers: a cross-sectional and a longitudinal study. Calcif Tissue Int. 2008;83:388–92. doi: 10.1007/s00223-008-9184-8. [DOI] [PubMed] [Google Scholar]
- 109.Korpelainen R, Korpelainen J, Heikkinen J, et al. Lifelong risk factors for osteoporosis and fractures in elderly women with low body mass index—a population-based study. Bone. 2006;39:385–91. doi: 10.1016/j.bone.2006.01.143. [DOI] [PubMed] [Google Scholar]
- 110.Zerwekh JE, Ruml LA, Gottschalk F, et al. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13:1594–601. doi: 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]
- 111.Bauer M, Unutzer J, Pincus HA, et al. Bipolar disorder. Ment Health Serv Res. 2002;4:225–9. doi: 10.1023/a:1020968616616. [DOI] [PubMed] [Google Scholar]
- 112.Haney EM, Chan BK, Diem SJ, et al. Association of low bone mineral density with selective serotonin reuptake inhibitors use by older men. Arch Intern Med. 2007;167:1246–51. doi: 10.1001/archinte.167.12.1246. [DOI] [PubMed] [Google Scholar]
- 113.Warden SJ, Haney EM. Skeletal effects of serotonin (5-hydroxytryptamine) transporter inhibition: Evidence from in vitro and animal-based studies. J Musculoskelet Neuronal Interact. 2008;8:121–32. [PMC free article] [PubMed] [Google Scholar]
- 114.Cauley JA, Fullman RL, Stone KL, et al. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteporos Int. 2005;16:1525–37. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 115.Diem SJ, Blackwell TL, Stone KL, et al. Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med. 2007;167:1240–5. doi: 10.1001/archinte.167.12.1240. [DOI] [PubMed] [Google Scholar]
- 116.Szulc P, Munoz F, Marchand F, et al. Role of vitamin D and parathyroid hormone in the regulation of bone turnover and bone mass in men; the MINOS study. Calcif Tissue Int. 2003;73:520–30. doi: 10.1007/s00223-002-2103-5. [DOI] [PubMed] [Google Scholar]
- 117.Petty SJ, O’Brien TJ, Wark JD. Anti-epileptic medication and bone health. Osteporos Int. 2007;18:129–42. doi: 10.1007/s00198-006-0185-z. [DOI] [PubMed] [Google Scholar]
- 118.Misra M, Papakostas G, Klibanksi A. Effect of psychiatric disorders and psychotropic medications on prolactin and bone metabolism. J Clin Psych. 2004;65:1607–18. doi: 10.4088/jcp.v65n1205. [DOI] [PubMed] [Google Scholar]
- 119.O’Keane V. Antipsychotic-induced hyperprolactinaemia, hypogonadism and osteoporosis in the treatment of schizophrenia. J Psychopharmacol. 2008;22:70–5. doi: 10.1177/0269881107088439. [DOI] [PubMed] [Google Scholar]
- 120.Scheen AJ, De Hert MA. Abnormal glucose metabolism is patients treated with antipsychotics. Diab Metab. 2007;33:169–75. doi: 10.1016/j.diabet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 121.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: a metaanalysis. Osteoporos Int. 2007;18:427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 122.Mak TW, Shek CC, Chow CC, Wing YK, Lee S. Effects of lithium therapy on bone mineral metabolism: a two-year prospective longitudinal study. J Clin Endocrinol Metab. 1998;83:3857–9. doi: 10.1210/jcem.83.11.5269. [DOI] [PubMed] [Google Scholar]
- 123.Depp C, Ojeda VD, Mastin W, et al. Trends in use of antipsychotics and mood stabilizers among Medicaid beneficiaries with bipolar disorder, 2001–2004. Psychiatr Serv. 2008;59:1169–74. doi: 10.1176/ps.2008.59.10.1169. [DOI] [PubMed] [Google Scholar]
- 124.Laroche ML, Charmes JP, Nouaille Y, et al. Is inappropriate medication use a major cause of adverse drug reactions in the elderly? Br J Clin Pharmacol. 2007;63:177–86. doi: 10.1111/j.1365-2125.2006.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Benton T, Staab J, Evans DL. Medical co-morbidity in depressive disorders. Ann Clin Psych. 2007;19:289–303. doi: 10.1080/10401230701653542. [DOI] [PubMed] [Google Scholar]
- 126.Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and type 2 diabetes. Calcif Tissue Int. 2009;84:45–55. doi: 10.1007/s00223-008-9195-5. [DOI] [PubMed] [Google Scholar]
- 127.Lim LS, Fink HA, Kuskowski MA, et al. Loop diuretic use and increased rates of hip bone loss in older men: the Osteoporosis Fractures in Men Study. Arch Intern Med. 2008;168:735–40. doi: 10.1001/archinte.168.7.735. [DOI] [PubMed] [Google Scholar]
- 128.McDonough AK, Rosenthal RS, Cao X, et al. The effect of thiazolidinediones on BMD and osteoporosis. Nat Clin Pract Endocrinol Metab. 2008;4:507–13. doi: 10.1038/ncpendmet0920. [DOI] [PubMed] [Google Scholar]
- 129.Javaid MK, Holt RI. Understanding osteoporosis. J Psychopharmacol. 2008;22:38–45. doi: 10.1177/0269881107087955. [DOI] [PubMed] [Google Scholar]
- 130.Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium, and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psych Scand. 1982;66:361–73. doi: 10.1111/j.1600-0447.1982.tb06718.x. [DOI] [PubMed] [Google Scholar]
- 131.Nordenström J, Elvius M, Bågedahl-Strindlund M, et al. Biochemical hyperparathyroidism and bone mineral status in patients treated with long-term lithium. Metabolism. 1994;43:1563–7. doi: 10.1016/0026-0495(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 132.Cohen O, Rais T, Lepkifker E, et al. Lithium carbonate therapy is not a risk factor for osteoporosis. Horm Metab Res. 1998;30:594–7. doi: 10.1055/s-2007-978939. [DOI] [PubMed] [Google Scholar]
- 133.Gyulai L, Jaggi J, Bauer MS, et al. Bone mineral density and L-thyroxine treatment in rapidly cycling bipolar disorder. Biol Psych. 1997;41:503–6. doi: 10.1016/S0006-3223(96)00472-6. [DOI] [PubMed] [Google Scholar]
- 134.Gyulai L, Bauer M, Garcia-Espana F, et al. Bone mineral density in pre- and post-menopausal women with affective disorder treated with long-term L-thyroxine augmentation. J Affect Disord. 2001;66:185–91. doi: 10.1016/s0165-0327(00)00306-2. [DOI] [PubMed] [Google Scholar]
- 135.Bauer M, Fairbanks L, Berghöfer A, et al. Bone mineral density during maintenance treatment with supraphysiological doses of levothyroxine in affective disorders: a longitudinal study. J Affect Disord. 2004;83:183–90. doi: 10.1016/j.jad.2004.08.011. [DOI] [PubMed] [Google Scholar]