Abstract
There is evidence that the kappa opioid system plays an important role in cocaine addiction and that chronic cocaine administration and withdrawal from chronic cocaine alter kappa opioid receptor (KOPr) density. The present study employed in situ [35S]GTPγS binding autoradiography to measure KOPr-stimulated activation of G-protein in the caudate putamen, nucleus accumbens core and shell, lateral hypothalamus, basolateral amygdala, substantia nigra compacta, substantia nigra reticulata and ventral tegmental area (VTA), in response to chronic cocaine administration or acute and chronic withdrawal from chronic cocaine. Male Fischer rats were injected i.p. with saline or cocaine three times daily at 1 hr intervals in an escalating-dose paradigm for 14 days (from 3 × 15 mg/kg/injection on days 1–3 up to 3 × 30 mg/kg/injection on days 10–14). Identically treated separate groups were withdrawn from cocaine or saline for 24 hr or 14 days. No significant change in KOPr agonist U-69593-stimulated [35S]GTPγS was found in the seven regions studied 30 minutes or 14 days after chronic 14 days escalating-dose binge cocaine administration. However there was an increase in KOPr -stimulated [35S]GTPγS binding in the VTA (P<0.01) of rats withdrawn for 24 hours from chronic cocaine. Our results show a cocaine withdrawal induced increase of KOPr signaling in the VTA, and suggest that the KOPr may play a role in acute withdrawal from cocaine.
Keywords: Autoradiography, G-protein, U-69593
Cocaine continues to be a widely abused drug in the United States. The rewarding properties of cocaine include an elevation of extracellular dopamine (DA) in dorsal striatum and nucleus accumbens (Di Chiara and Imperato, 1988; Maisonneuve and Kreek, 1994). There is strong evidence indicating that opioid systems are affected by cocaine treatment (see reviews in Kreek et al., 2002; Shippenberg et al., 2007). Cocaine administration in rodents, non-human primates and humans has been shown to cause a significant increase in preprodynorphin (ppDyn) mRNA levels in the striatum (Daunais et al., 1993; Hurd and Herkenam, 1993; Spangler et al., 1993; Turchan et al., 1998; Zhou et al. 2002; Fagergren et al., 2003; Schlussman et al., 2003). We have shown that escalating-dose binge administration of cocaine alters striatal ppDyn mRNA levels in rats (Schlussman et al., 2005). The increase of ppDyn mRNA levels has been observed in the lateral hypothalamus in acute (1 day) withdrawal from chronic escalating-dose binge cocaine administration (Zhou et al., 2008).
Opioid receptors are G-protein coupled, and activation of inhibitory G-proteins coupled to mu, kappa and delta opioid receptors inhibits adenylyl cyclase (Childers, 1991; Claye et al, 1996). Repeated cocaine administration has been found to induce changes in many G-protein coupled receptor systems in the brain, including the kappa opioid receptor (KOPr) system (Sharpe et al., 2000; Turchan et al., 1999; Bailey et al., 2005, 2007), although some reports found that chronic cocaine administration had no effect on dynorphin A1–17-stimulated [35S]GTPγS binding of KOPr in the caudate putamen, nucleus accumbens or cingulate cortex (Schroeder et al., 2003). Chronic steady-dose or chronic escalating-dose binge cocaine administration resulted in upregulation of KOPr binding in several mesolimbic areas of the rat brain (Unterwald et al., 1994; Bailey, et al., 2007). However, 14 days withdrawal from chronic escalating-dose binge cocaine administration led to a downregulation of KOPr binding in the basolateral amygdala and septum (Bailey et al., 2007) and an upregulation of mu opioid receptor binding in the frontal cortex, cingulate cortex and caudate putamen (Bailey et al., 2005). A recent pharmacological study has found that KOPr antagonists reduced cocaine intake in rats with extended access to cocaine self-administration (Wee et al., 2009). These studies, taken together, suggest that there are alterations in the KOPr function in the mesolimbic DA projection regions as a result of chronic cocaine administration.
However, relatively little is known about the effect of chronic cocaine and withdrawal on the functionality of G-protein of KOPr in the ventral tegmental area (VTA) and substantia nigra, DA cell body regions of mesolimbic and nigrostriatal systems, which play an important role in regulation of DA activity and reward. Therefore, using the specific KOPr agonist U-69593 for in situ [35S]GTPγS binding autoradiography, the current study examined the effect of acute (24 hours) and chronic (14 days) withdrawal from chronic escalating-dose binge cocaine administration, on the functionality of KOP-r G-protein in the VTA and substantia nigra, in contrast with the effect of chronic cocaine.
EXPERIMENTAL PROCEDURES
Animal and treatment
Forty-eight adult male Fischer rats (Charles River Laboratories, Kingston, NY; 210–240 g) were used in this study. Animals were housed individually in a temperature controlled room, with a 12h light/dark cycle (9:00 AM to 9:00 PM) and received food and water ad libitum. All animal were weighed daily and observed for 3 hours and 1 hour after each injection of cocaine or saline. After 5 days of acclimation, intraperitoneal (i.p.) injections of either saline (1ml/kg) or cocaine were administered in an escalating-dose “binge” paradigm to mimic a common pattern of self-administration in human cocaine abusers. Animals received 3 × 15 mg/kg/injection on days 1–3, 3 × 20 mg/kg/injection on days 4–6, 3 × 25 mg/kg/injection on days 7–9, and 3 × 30 mg/kg/injection on days 10–14. The three i.p. injections (1 hour apart) were administered daily for a final maximum daily total cocaine dose of 90 mg/kg, with the first injection 30 minutes after start of the light cycle. Animals were randomized into six groups. The first two groups received either saline (n=7) or cocaine (n=9) administration for 14 days, with ten animals in each group. The second two groups were withdrawn from this chronic saline or chronic cocaine administration for 1 day (24 hour). The third two groups were withdrawn for 14 days from chronic saline or chronic cocaine administration. Approximately 30% cocaine animals given cocaine showed splayed hind limbs following the last injection on the first day of the highest dose (90mg/kg/day, day 10) achieved in the escalating-dose injections. During exposure to the highest dose of cocaine, two animals of nine in each cocaine group died with seizures following the last cocaine injection on day 13 or day 14.
At three times (30 minutes, 24 hours or 14 days) after the last injection, animals were sacrificed by decapitation following brief exposure to CO2. The brains were quickly removed and frozen by immersion for 1 minutes in isopentane −35°C. Twenty micron cryostat sections of brain regions were thaw-mounted onto Plus Slide (Fisher Scientific, Fair Lawn NJ) and stored at −80°C to use for in situ [35S]GTPγS autoradiography. All animals were cared for in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) and with a protocol approved by the Rockefeller University Institutional Animal Care and Use Committee.
Drugs
[35S]GTPγS and nor-binaltorphimine dihydrochloride (nor-BNI) were purchased from Perkin- Elmer Life Science (Waltham, MA) and Tocris Bioscience (Ellisville, MO), respectively. Cocaine hydrochloride and U-69,593 were purchased from Sigma Aldrich (St. Louis, MO).
In situ [35S]GTPγS binding autoradiography
Agonist-stimulated [35S]GTPγS autoradiography was performed and adapted from earlier studies (Sim et al.,1995; Castelli et al., 2005; Bortolato et al., 2007). Brain sections were thawed at room temperature, followed by incubation in assay buffer (50 mM Tris HCl/3 mM MgCl2/0.2 mM EGTA/100 mM NaCl, pH 7.4) at 25°C for 10 min. Slides were then incubated with 2 mM GDP in assay buffer at 25°C for 15 min. Agonist-stimulated activity was determined by incubating [35S]GTPγS (0.04 nM) with 10 µM U-63593 and 2 mM GDP in assay buffer at 25°C for 2 hr. In each experiment, basal activity was assessed with GDP in the absence of agonist, and nonspecific binding was assessed in the presence of 10 µM unlabeled GTPγS.
Slides were then rinsed twice in ice-cold Tris buffer (50mM Tris HCl, pH 7.4) and rinsed once briefly in deionized water. Slides were dried under cold air and exposed to 35S sensitive film (Kodak Biomax MR film) for 24 hr. Films were digitized with an MCID image analyzer (Imaging Research, Canada). Quantification of images was obtained from densitometry analysis using 14C standards. Agonist-stimulated activity was calculated by subtracting the optical density of nonspecific sections from the optical density of both agonist-stimulated and basal binding section. Agonist-stimulated activity was expressed as a percentage of basal [35S]GTPγS binding.
To confirm that agonist-stimulated [35S]GTPγS of brain sections analyzed reflected the true neuroanatomical distribution of KOPr-coupled G-protein activity, we tested whether addition of appropriate specific antagonist (nor-BNI) could block labeling in those areas that are specifically stimulated by the KOPr agonist U-63593. Different concentrations of U-69593 (1, 5, 8, and 10 µM) and nor-BNI (0.04, 0.1, 0.5, 1, 2, 5, 10 µM) were determined by pilot autoradiography experiments. The concentration of antagonist nor-BNI at 1 µM was chosen since it completely blocked the stimulation produced by agonist U-69593 (10 µM).
Data analysis
Changes in body weight during the chronic escalating-dose binge cocaine phase were examined by three-way analysis of variance (ANOVA), Drug (cocaine, saline) X Group X Treatment Day, with repeated measures on the last variable. Changes in body weight across the 14 days of withdrawal were examined by two-way ANOVA, Drug X Withdrawal Day, with repeated measures on the last variable, followed by planned comparisons on the last day.
A preliminary two-way ANOVA of the levels of U-69593-stimulated [35S]GTPγS binding in all saline groups, Time (14 days chronic treatment, 1 day or 14 days withdrawal from chronic cocaine) X Brain Region showed that there was no significant main effect of Time, F(2,18) = 2.21, (showing no difference between Time groups), but there was an expected significant main effect of Brain Region, F(7,126) = 2.77, p<0.01, with no significant interaction, F(14,126) = 1.24. Therefore, levels of [35S]GTPγS binding autoradiography were examined separately in each brain region by two-way ANOVA, Drug X Time, followed by Newman-Keuls post hoc test or planned comparison. Comparison of basal [35S]GTPγS binding was carried out using both a three-way ANOVA, Region X Drug X Time, and a two-way ANOVA, Drug X Time, of each brain region. The accepted level of significance for all tests was p < 0.05. All statistical analyses were performed using Statistica (version 5.5, StatSoft Inc., Tulsa, OK).
RESULTS
Body weight
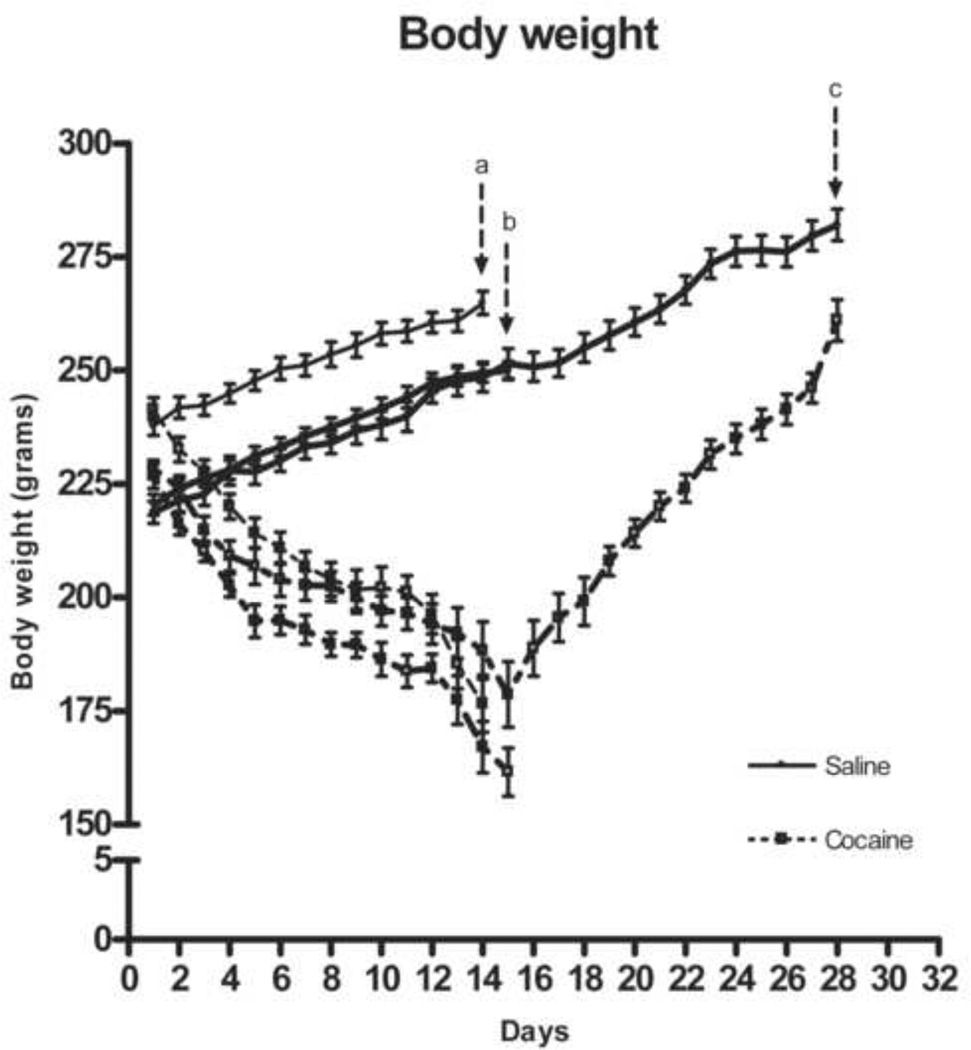
Body weight decreased progressively across the 14 days of chronic escalating-dose binge cocaine administration (see Fig. 1). ANOVA showed a significant effect of cocaine, F( 1,36) = 229.90, p<0.000001, a significant effect of Group, F(2,36) = 13.86, p<0.00005, (both cocaine treated and saline controls of one group, had higher body weights on arrival), and a significant effect of Treatment Day, F(13,486) =13.62, p<0.000001. Rats withdrawn for 1 day from chronic cocaine continued to lose body weight. ANOVA of the 14-day withdrawal groups showed that although the cocaine group increased in body weight across the days of withdrawal, Drug X Withdrawal Day interaction F(13, 156) = 32.85, p<0.000001, they were still significantly lower than saline controls after 14 days of withdrawal, F(1,12) = 18.06, p<0.002, (planned comparison).
Figure 1.
Effects of 14 days escalating-dose binge cocaine administration or saline (a), of 1 day withdrawal from 14 days escalating-dose binge cocaine administration or saline (b) and of 14 days withdrawal from 14 days escalating-dose binge cocaine administration or saline (c) on the body weight of rats. Data are expressed as mean ± SEM of seven rats.
In situ [35S]GTPγS binding autoradiography
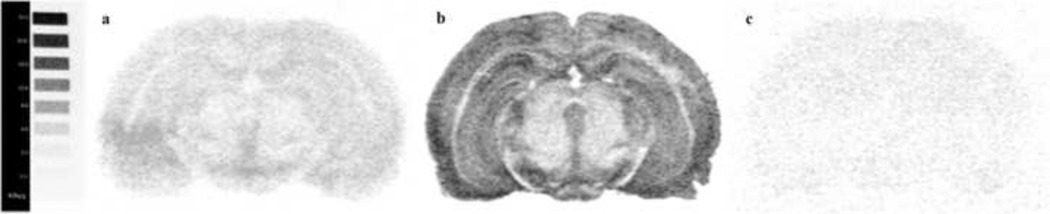
[35S]GTPγS autoradiography was used to evaluate KOPr activation of G-protein in the caudate putamen, nucleus accumbens core and shell, ventral tegmental area, lateral hypothalamus, basolateral amygdala, substantia nigra reticulata and substantia nigra compacta. A clear stimulation was observed in all regions incubated with KOPr agonist U-69593. Coronal brain sections assayed in the presence of nor-BNI (1 µM) and U-69593 (10 µM) showed that the antagonist nor-BNI completely blocked the agonist U-69593 stimulation of KOPr in areas analyzed (see Fig. 2).
Figure 2.
Autoradiography showing antagonist reversal of agonist [35S]GTPγS binding by kappa opioid receptor. The brain sections were incubated with 2 mM GDP before adding 0.04 nM radiolabeled [35S]GTPγS and 10 µM U-69,593 with (a) or without 1 µM nor-BNI (b) and in presence of 0.04 nM radiolabeled [35S]GTPγS with 10µM U-69593 and 10µM unlabeled GTPγS (c). (Bregma = −4.80). 14C scale bar on the left indicates lower levels of G protein activation correspond to 33.1 Kbq/g and higher levels of G protein activation correspond to 1.1 Kbq/g.
A three-way ANOVA, Drug X Region X Time, of the basal [35S]GTPγS data showed a significant difference among regions, F(7,252) = 21.6, p<0.001, but no significant main effect of Drug or Time, or their interactions. Two-way ANOVA, Drug X Times, of each brain region further showed no significant main effect or interaction on basal [35S]GTPγS binding.
Two-way ANOVA with repeated measures of U-69593 stimulated [35S]GTPγS binding of saline control animals across brain regions, Time X Region, showed a significant difference among regions, F(7,126) = 2.77, p<0.02, but no significant main effect of Time, nor a significant Time X Region interaction. Since there were significant differences in the levels of U-69593- stimulated [35S]GTPγS binding among brain regions, the effect of withdrawal was examined by two-way ANOVA in each brain region studied, Drug X Time.
The effect of 14 days chronic escalating-dose binge cocaine or saline, of 1 day, and of 14 days withdrawal from chronic escalating-dose binge cocaine or saline on U-69593-stimulated [35S]GTPγS binding in various brain regions is given in Table 1. Data are expressed in mean percent increase ± SEM from [35S]GTPγS under the basal condition.
Table 1.
U-69593-stimulate [35S]GTPγS autoradiography in the brains of rats following of rats given 14 days chronic escalating-dose binge cocaine or saline, 1 day or 14 days withdrawn from cocaine or saline. CPu caudate putamen; AcC, Accumbens core; AcS, Accumbens shell; LH, lateral hypothalamus; BLA basolateral amygdala; SNc, substantia nigra compacta; SNr, substatia nigra reticulata VTA, ventral tegmental area. The values represent means ± SEM of percent of activation from basal value (n=7).
| Stimulation [35S]GTPγS binding (%) | ||||||
|---|---|---|---|---|---|---|
| Regions | Chronic Saline |
Chronic Cocaine |
Saline 1 day withdrawal |
Cocaine 1 day withdrawal |
Saline 14 days withdrawal |
Cocaine 14 days withdrawal |
| CPu | 32.4 ± 1.3 | 35.6 ± 3.5 | 40.8 ± 2.3 | 42.3 ± 2.0 | 37.2 ± 2.6 | 32.1 ± 2.2 |
| AcC | 31.3 ± 1.8 | 38.3 ± 1.4 | 35.2 ± 1.9 | 44.4 ± 3.0 | 37.8 ± 1.6 | 36.3 ± 5.0 |
| AcS | 34.4 ± 1.4 | 42.0 ± 2.2 | 36.2 ± 3.3 | 35.8 ± 2.8 | 36.6 ± 2.6 | 38.9 ± 4.7 |
| LH | 37.8 ± 2.2 | 43.4 ± 2.9 | 38.5 ± 2.8 | 36.1 ± 3.2 | 35.9 ± 3.6 | 30.5 ± 2.1 |
| BLA | 35.4 ± 2.0 | 31.1 ± 1.4 | 38.2 ± 2.6 | 35.1 ± 3.1 | 30.0 ± 2.9 | 27.4 ± 1.7 |
| SNr | 32.4 ± 1.5 | 36.0 ± 1.9 | 37.7 ± 3.7 | 38.6 ± 4.8 | 36.2 ± 1.8 | 37.9 ± 2.3 |
| SNc | 28.5 ± 1.8 | 30.7 ± 1.4 | 31.9 ± 1.9 | 41.3 ± 5.5 | 37.8 ± 1.7 | 34.1 ± 3.4 |
| VTA | 28.0 ± 1.8 | 32.8 ± 2.2 | 27.5 ± 1.9 | 42.22 ± 4.1* | 32.8 ± 1.8 | 33.6 ± 1.4 |
P<0.01.
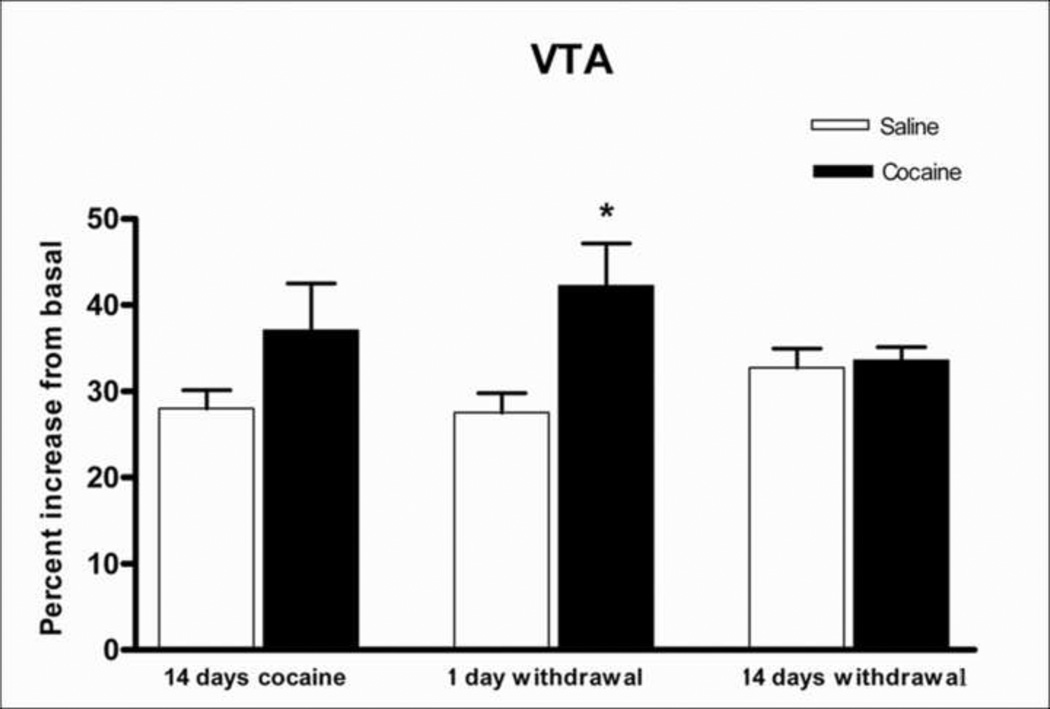
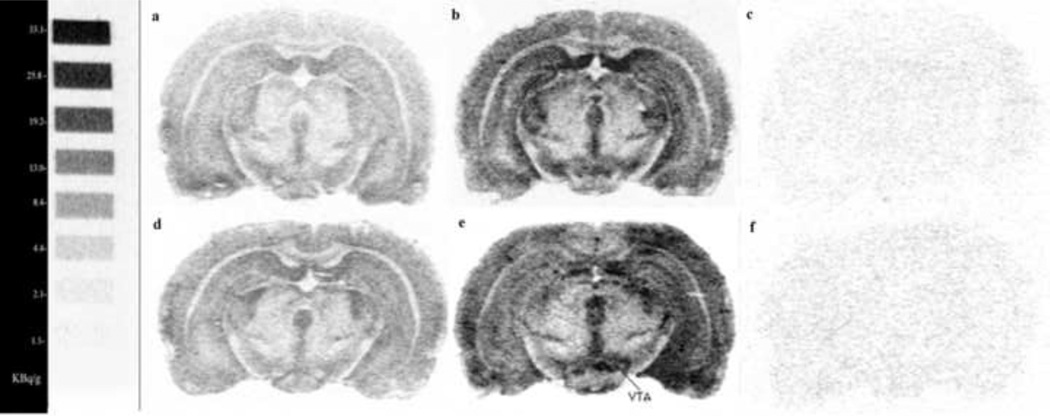
A two-way ANOVA, Drug X Time, showed a significant main effect of cocaine in the ventral tegmental area, F(1,36) = 8.65, p<0.01, and a Drug X Time interaction that just failed to reach significance, F(2,36) = 3.21, p=0.052. After 24 hours, U-69593-stimulated [35S]GTPγS binding was significantly higher in the animals withdrawn from chronic escalating-dose binge cocaine administration than in the saline controls (p<0.01, Newman Keuls post hoc test), as can be seen in Fig. 3. Representative digital images of brain sections containing the ventral tegmental area showing kappa receptor-stimulated [35S]GTPγS binding after 1-day withdrawal from saline or from chronic escalating-dose binge cocaine administration are shown in Fig. 4. Both basal and U-69593-stimulated slides are shown. No significant change in U-69593-stimulated [35S]GTPγS binding was observed in any other brain region studied.
Figure 3.
U-69593-stimulated [35S]GTPγS binding in the ventral tegmental area (VTA) of rats given 14 days cocaine or saline, 1 day and 14 days withdrawn from cocaine or saline. Data are expressed as percent of basal [35S]GTPγS binding + SEM of seven rats. *p<0.01.
Figure 4.
Digital images obtained from autoradiography of coronal brain sections showing the distribution of [35S]GTPγS binding stimulated by kappa opioid receptor. Autoradiograms represent brain sections incubated in presence of 0.04nM radiolabeled [35S]GTPγS without (basal) [a, d] or with 10µM U-69593 (specific binding) [b, e] and in presence of 0.04 nM radiolabeled [35S]GTPγS with 10µM U-69593 and 10µM unlabeled GTPγS (non specific binding) [c, f] in rat withdrawn for 1 day from 14 days of escalating-dose binge saline administration (a, b and c) and withdrawn for 1 day from 14 days of escalating-dose binge cocaine administration (d, e and f) (Bregma = −4.80).
14C scale bar on the left indicates lower levels of G protein activation correspond to 33.1 Kbq/g and higher levels of G protein activation correspond to 1.1 Kbq/g.
DISCUSSION
In situ receptor-stimulated GTPγS binding is an effective method for visualizing the distribution and density of receptor-activated G-proteins. In this study we use the highly selective KOPr agonist U-69593 to visualize G-protein coupled to KOPr after chronic cocaine administration and after 1 day or 14 days withdrawal from chronic cocaine in seven rat brain regions. Significant stimulation of [35S]GTPγS binding was observed in the nucleus accumbens core and shell, lateral hypothalamus, basolateral amygdala, substantia nigra compacta, substantia nigra reticulata and ventral tegmental area in rat brain sections. This observation extends the results of Park et al. (2000) who originally showed significant activation of G-protein by U- 69593 in the caudate putamen and prefrontal and cingulate cortex of wild-type mice in comparison with µ-opioid receptor knockout mice. In general, distribution of U-69593 stimulated G-protein is in agreement with previously published data on KOPr density measured using [3H]CI-977 in rats (Bailey et al. 2007) and KOPr mRNA expression in mice (Mansour et al. 1994).
We found that neither 14-day chronic escalating-dose cocaine administration nor its 14-day withdrawal alters the KOPr-stimulated [35S]GTPγs binding in any region analyzed. Using the same paradigm of cocaine treatment, our earlier study by Bailey et al (2007) showed no overall alteration of KOPr binding after 14-day chronic escalating-dose cocaine across most brain areas. However, a decrease in KOPr binding after 14 days of withdrawal was found in the basolateral amygdala when compared to the 14-day chronic escalating-dose cocaine group. Using chronic steady-dose (45 mg/kg/day) “binge” cocaine administration, Schroeder et al. (2003) found no significant change in G-protein coupling of KOPr in caudate putamen or nucleus accumbens core and shell. In another early study using chronic steady-dose “binge” cocaine, however, Unterwald et al. (1994) found that KOPr binding was significantly increased in the rostral cingulate cortex, rostral caudate putamen, caudal olfactory tubercle, and VTA. These apparently different results of KOPr binding among the above studies may depend on the dose and timing of cocaine administration. Further, as has been demonstrated by Bailey et al. (2008), chronic cocaine-induced change in the binding of dopamine D2-like receptors does not necessarily reflect the same direction of change in the D2 receptor functional coupling.
Of interest, we found that 1-day withdrawal from chronic escalating-dose cocaine administration resulted in significantly enhanced coupling of KOPr and G-protein in the VTA only. The increases in kappa opioid receptor-stimulated [35S]GTPγS binding in the VTA may be indicative of changes in the components of the KOPr signaling pathway. The actions of KOPr are mediated through activation of the Gi and Go family of guanine nucleotide-binding G-proteins (Childers, 1991; Law and Loh, 1999). A study by Nestler at al. (1990) demonstrated a decrease of Gi and Go alpha subunit protein levels in VTA, nucleus accumbens and locus coeruleus immediately (1-hour) after 14-day chronic cocaine administration (15 mg/kg, twice a day). Therefore, one might expect a decrease in both basal and KOPr-activated [35S]GTPγs binding 30 min after chronic escalating-dose cocaine administration in the VTA. However, we found that neither basal nor KOPr-stimulated [35S]GTPγs binding showed a significant difference from the saline control in any brain region examined, including the VTA. In contrast, we observed an enhanced KOPr-activated [35S]GTPγs binding after 1-day withdrawal from chronic escalating-dose cocaine.
Although the mechanism involved in the increase in KOPr coupling is not clear, it is possible that there is a rebound increase in the number of Gi and Go alpha subunit proteins induced by 1-day cocaine withdrawal. Alterations of other factors downstream of the receptor affecting Gi/o coupling to KOPr in response to 1-day cocaine withdrawal might also play an important role. The increase in KOPr coupling to G-protein in our study is unlikely to be due to decreased endogenous dynorphin release, since there is growing evidence that there is an activated dynorphin/KOPr system during early cocaine withdrawal, especially after chronic escalating-dose cocaine exposure (e.g., Zhou et al., 2008; Wee et al., 2009).
Receptor autoradiography using the selective KOPr-selective ligand, U-69593, have shown that KOPr binding is heterogeneously distributed in rostral portions of the VTA (Speciale et al., 1993). The VTA of the midbrain is an area rich in dopamine neurons which project to nucleus accumbens, amygdala, hippocampus, prefrontal cortex, ventral pallidum (Berendse et al., 1992; Everitt and Robbins, 2005; Heimer and Van Hoesen, 2006), whereas DA neurons arising in the substantia nigra innervate the dorsal striatum (Graybiel, 1990). This VTA dopamine pathway plays a key role in mediating the rewarding aspects of behavior (Wise, 1998; Ranaldi et al., 1999).
The KOPr agonists U-50488, U-69593 and TRK-820 have aversive effects as measured by the conditioned place preference procedure in rats (Bals-Kubik et al. 1989; Suzuki et al. 1992; Mori et al. 2002). Of particular interest, microinjection of the KOPr agonist U-50488 and the dynorphin derivative E-2078 into the VTA produce place aversion (Bals-Kubik et al., 1993). Although synthetic KOPr agonists (including U-50488 and U-69593) decrease DA levels in the nucleus accumbens and caudate putamen of rats and mice (Di Chiara and Imperato 1988; Spanagel et al. 1992; Devine et al. 1993; Zhang et al. 2004), microinjections of U-50488 or U- 69593 in the VTA fail to alter extracellular DA and DA metabolite concentration in the nucleus accumbens (Spanagel et al., 1992; Devine et al., 1993).
In contrast, it was shown that the selective activation of KOPr in the VTA inhibits VTA DA neurons projecting to the medial prefrontal cortex (Margiolis et al., 2006). In the same study, dual-probe microdialysis revealed a marked decrease in medial prefrontal cortical DA levels in response to intra-VTA KOPr agonist perfusion. VTA perfusion of a selective KOPr antagonist blocked the agonist-induced decrease DA levels in medial prefrontal cortex. During the early phase of cocaine withdrawal, in the medial prefrontal cortex, basal DA uptake is increase and cocaine-evoked dopamine levels are lower (Chefer et al., 2000). Decrease in extracellular DA levels in the medial prefrontal cortex is postulated to lead to the compulsive drug-seeking behavior (Schenk et al., 1991; McGregor et al., 1996; Kalivas et al., 2005).
The data presented here demonstrate that kappa opioid receptor-stimulated G- protein activation is enhanced in the VTA following 1-day withdrawal from chronic escalating binge cocaine administration. This result, in conjunction with previous research, adds further evidence that KOPr system could modulate and inhibit DA neurons projecting to the mesolimbic regions, in response to cocaine and especially to the early phase of withdrawal. This compensatory mechanism may be responsible for the decrease DA levels in the mesolimbic areas (including prefrontal cortex), and could be activated in different stages of drug exposure or withdrawal.
Acknowledgements
This work was supported by a Dorothea Dix Fellowship (APP) and NIH-NIDA Research Center Grant DA-P60-05130 (MJK). The authors would like to thank Dr. E.M. Unterwald for her advice on imaging analysis and protocols. We thank Susan Russo for proofreading the manuscript.
Abbreviations
- AcC
Accumbens core
- AcS
Accumbens shell
- BLA
basolateral amygdala
- CO2
carbon dioxide
- CPu
caudate putamen
- DA
dopamine
- G-protein
guanine nucleotide-binding protein
- GTPγS
guanosine 5'-O-[gamma-thio]triphosphate acid
- KOPr
kappa opioid receptor
- LH
lateral hypothalamus
- mRNA
messenger ribonucleic acid
- nor-BNI
nor-binaltorphimine dihydrochloride
- ppDyn
preprodynorphin
- SNc
substantia nigra compacta
- SNr
substatia nigra reticulata
- U-69,593
(+)-(5α,7α,8β)-N-Methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of interest
The authors declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest
REFERENCES
- Bailey A, Yuferov V, Bendor J, Schlussman SD, Zhou Y, Ho A, Kreek MJ. Immediate withdrawal from chronic "binge" cocaine administration increases mu-opioid receptor mRNA levels in rat frontal cortex. Brain Res Mol Brain Res. 2005;137:258–262. doi: 10.1016/j.molbrainres.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Bailey A, Gianotti R, Ho A, Kreek MJ. Downregulation of kappa-opioid receptors in basolateral amygdala and septum of rats withdrawn for 14 days from an escalating dose "binge" cocaine administration paradigm. Synapse. 2007;61:820–826. doi: 10.1002/syn.20436. [DOI] [PubMed] [Google Scholar]
- Bailey A, Metaxas A, Yoo JH, McGee T, Kitchen I. Decrease of D2 receptor binding but increase in D2-stimulated G-proteinactivation, dopamine transporter binding and behavioural sensitization in brains of mice treated with a chronic escalating dose 'binge' cocaine administration paradigm. Eur J Neurosci. 2008;28:759–770. doi: 10.1111/j.1460-9568.2008.06369.x. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Bals-Kubik R, Herz A, Shippenberg TS. Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl) 1989;98:203–206. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orrù M, Piras AP, Fà M, Tuveri A, Puligheddu M, Gessa GL, Castelli MP, Mereu G, Marrosu F. Activation of GABA(B) receptors reverses spontaneous gating deficits in juvenile DBA/2J mice. Psychopharmacology (Berl) 2007;194:361–369. doi: 10.1007/s00213-007-0845-5. [DOI] [PubMed] [Google Scholar]
- Castelli MP, Pibiri F, Piras AP, Carboni G, Orrù A, Gessa GL, Carai MA, Colombo G. Differential G-protein coupling to GABAB receptor in limbic areas of alcohol-preferring and -nonpreferring rats. Eur J Pharmacol. 2005;523:67–70. doi: 10.1016/j.ejphar.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Morón JA, Hope B, Rea W, Shippenberg TS. Kappa-opioid receptor activation prevents alterations in mesocortical dopamine neurotransmission that occur during abstinence from cocaine. Neuroscience. 2000;101:619–627. doi: 10.1016/s0306-4522(00)00417-6. [DOI] [PubMed] [Google Scholar]
- Childers SR. Opioid receptor-coupled second messenger systems. Life Sci. 1991;48:1991–2003. doi: 10.1016/0024-3205(91)90154-4. [DOI] [PubMed] [Google Scholar]
- Claye LH, Unterwald EM, Ho A, Kreek MJ. Both dynorphin A(1–17) and [Des-Tyr1]dynorphin A(2–17) inhibit adenylyl cyclase activity in rat caudate putamen. J Pharmacol Exp Ther. 1996;277:359–365. [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, McGinty JF. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266:1236–1246. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and the initiation of movement. Rev Neurol (Paris) 1990;146:570–574. [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- Law PY, Loh HH. Regulation of opioid receptor activities. J Pharmacol Exp Ther. 1999;289:607–624. [PubMed] [Google Scholar]
- Maisonneuve IM, Kreek MJ. Acute tolerance to the dopamine response induced by a binge pattern of cocaine administration in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1994;268:916–921. [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci USA. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Baker G, Roberts DC. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 1996;53:5–9. doi: 10.1016/0091-3057(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Mori T, Nomura M, Nagase H, Narita M, Suzuki T. Effects of a newly synthesized kappa-opioid receptor agonist, TRK-820, on the discriminative stimulus and rewarding effects of cocaine in rats. Psychopharmacology (Berl) 2002;161:17–22. doi: 10.1007/s00213-002-1028-z. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Terwilliger RZ, Walker JR, Sevarino KA, Duman RS. Chronic cocaine treatment decreases levels of the G protein subunits Gi alpha and Go alpha in discrete regions of rat brain. J Neurochem. 1990;55:1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Park Y, Ma T, Tanaka S, Jang C, Loh HH, Ko KH, Ho IK. Comparison of G-protein activation in the brain by mu-, delta-, and kappa-opioid receptor agonists in mu-opioid receptor knockout mice. Brain Res Bull. 2000;52:297–302. doi: 10.1016/s0361-9230(00)00265-3. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Pocock D, Zereik R, Wise RA. Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous D-amphetamine self-administration. J Neurosci. 1999;19:4102–4109. doi: 10.1523/JNEUROSCI.19-10-04102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe LG, Pilotte NS, Shippenberg TS, Goodman CB, London ED. Autoradiographic evidence that prolonged withdrawal from intermittent cocaine reduces mu-opioid receptor expression in limbic regions of the rat brain. Synapse. 2000;37:292–297. doi: 10.1002/1098-2396(20000915)37:4<292::AID-SYN6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Schenk S, Horger BA, Peltier R, Shelton K. Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Res. 1991;543:227–235. doi: 10.1016/0006-8993(91)90032-q. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Yuferov V, LaForge KS, Ho A, Kreek MJ. Acute 'binge' cocaine administration elevates dynorphin mRNA in the caudate putamen of C57BL/6J but not 129/J mice. Brain Res. 2003;974:249–253. doi: 10.1016/s0006-8993(03)02561-7. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhou Y, Bailey A, Ho A, Kreek MJ. Steady-dose and escalating-dose "binge" administration of cocaine alter expression of behavioral stereotypy and striatal preprodynorphin mRNA levels in rats. Brain Res Bull. 2005;67:169–175. doi: 10.1016/j.brainresbull.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Niculescu M, Unterwald EM. Cocaine alters mu but not delta or kappa opioid receptor-stimulated in situ [35S]GTPγS binding in rat brain. Synapse. 2003;47:26–32. doi: 10.1002/syn.10148. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5'-[gamma-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Unterwald EM, Kreek MJ. “Binge” cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res Mol Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- Speciale SG, Manaye KF, Sadeq M, German DC. Opioid receptors in midbrain dopaminergic regions of the rat. II. Kappa and delta receptor autoradiography. J Neural Transm Gen Sect. 1993;91:53–66. doi: 10.1007/BF01244918. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H. The role of mu- and kappaopioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- Turchan J, Przewłocka B, Lasoń W, Przewłocki R. Effects of repeated psychostimulant administration on the prodynorphin system activity and kappa opioid receptor density in the rat brain. Neuroscience. 1998;85:1051–1059. doi: 10.1016/s0306-4522(97)00639-8. [DOI] [PubMed] [Google Scholar]
- Turchan J, Przewłocka B, Toth G, Lasoń W, Borsodi A, Przewłocki R. The effect of repeated administration of morphine, cocaine and ethanol on mu and delta opioid receptor density in the nucleus accumbens and striatum of the rat. Neuroscience. 1999;91:971–977. doi: 10.1016/s0306-4522(98)00637-x. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology (Berl) 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the κ opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004;173:146–152. doi: 10.1007/s00213-003-1716-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Yuferov VP, Sora I, Ho A, Uhl GR, Kreek MJ. Effects of acute "binge" cocaine on preprodynorphin, preproenkephalin, proopiomelanocortin, and corticotropin-releasing hormone receptor mRNA levels in the striatum and hypothalamic-pituitary-adrenal axis of mu-opioid receptor knockout mice. Synapse. 2002;45:220–229. doi: 10.1002/syn.10101. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose "binge" pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]