Abstract
Objective:
We tested the hypothesis that brain pathology is associated with the rate of progression of physical frailty in older adults.
Methods:
A total of 791 older adults participating in the Religious Orders Study and Memory and Aging Project had annual clinical evaluations from which a previously established composite measure of physical frailty was derived and brain autopsy after death. A uniform neuropathologic examination included the assessment of macroinfarcts, microinfarcts, atherosclerosis, arteriolosclerosis, Alzheimer disease and Lewy body pathology, and nigral neuronal loss.
Results:
Mean follow-up before death was 6.4 years and age at death was 88.5 years. More than 95% of cases had evidence of one or more brain pathologies. In a linear mixed-effect model controlling for age, sex, and education, frailty increased at approximately 0.12 unit/year (estimate 0.117, SE 0.035, p < 0.001). The rate of progression of frailty was accelerated with increasing age (estimate 0.002, SE 0.001, p = 0.012). In separate models, the presence of macroinfarcts, Alzheimer disease and Lewy body pathology, and nigral neuronal loss was associated with a more rapid progression of frailty (all p values ≤0.010). When these 4 brain pathologies were considered together in a single model, Alzheimer disease pathology, macroinfarcts, and nigral neuronal loss showed independent associations with the rate of progression of frailty and accounted for more than 8% of the variance unexplained by demographic variables alone.
Conclusion:
The accumulation of common brain pathologies contributes to progressive physical frailty in old age.
Physical frailty in older adults is common and associated with a wide range of adverse health outcomes including mortality, disability, cognitive decline, mild cognitive impairment, and Alzheimer disease (AD).1–5 Frailty may occur in up to 50% or more of adults by the age of 85, making it essential to understand its underlying biology.6,7
We used data from 791 persons participating in 1 of 2 cohort studies of common chronic conditions of aging that include brain donation at death: the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP).8,9 Prior work in one of these cohorts showed that the level of frailty proximate to death was associated with postmortem indices of AD pathology even in individuals without dementia, suggesting that brain pathology may contribute to frailty.10 This study extends our prior work in 3 important ways. First, we increased the sample size to nearly 800 by including more than 300 persons from a second cohort study. Second, we investigated 6 neuropathologies in addition to AD pathology to assess a broad range of common diseases. Third, we linked pathology to progression of physical frailty in up to 14 annual assessments before death rather than relying on cross-sectional findings with frailty proximate to death.
METHODS
Participants.
Participants were from 2 ongoing studies of chronic conditions of aging. Both studies use common antemortem and postmortem data collection allowing analyses of data from the combined cohorts. More than 2,700 persons have enrolled since these studies began, and participation in the annual follow-up evaluations exceeds 90% of survivors and the autopsy rate exceeds 85%. To calculate the rate of change in frailty, only cases with 2 or more valid frailty measures were included. At the time of this study, postmortem indices had been collected from 879 brains. There were 4 cases missing clinical frailty measures (0.5%) and 84 cases (9.6%) that had only one clinical frailty measure before death, leaving 791 cases for these analyses (ROS: 458; MAP: 333).
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Institutional Review Board of Rush University Medical Center. Written informed consent and an anatomical gift act for brain donation at the time of death was obtained from all study participants.
Clinical evaluation and diagnoses.
An annual uniform structured clinical evaluation includes medical history, neurologic examination, and neuropsychological performance tests.8,9
Physical frailty.
Physical frailty was based on grip strength, timed walk, body composition, and fatigue and summarized in a previously established continuous composite measure.4 Grip strength was measured with the Jamar hydraulic hand dynamometer (Lafayette Instruments, Lafayette, IN). Gait was based on the time to walk 8 feet. Body composition was based on body mass index (BMI). As done in previous studies of frailty, we used 2 questions derived from a modified version of the Center for Epidemiologic Studies–Depression Scale.2–5,10 Higher frailty values indicate poorer performance and lower frailty values indicate better performance. Composite frailty is highly correlated with the categorical measure used by other investigators, and is associated with incident disability, mortality, mild cognitive impairment, AD, and cognitive decline.2–5,10
Comorbidities and other covariates.
Sex and years of education were recorded at the baseline interview. Age in years was computed from self-reported date of birth and clinical evaluation date. Dementia was diagnosed in a 3-step process. Nineteen cognitive tests were scored by a computer and reviewed by a neuropsychologist to diagnose cognitive impairment. Then participants were evaluated by a physician who used all cognitive and clinical data to diagnose dementia.8,9 Parkinson disease (PD) was based on a history of PD for which the participant received treatment with levodopa.11 Seven chronic diseases were documented at baseline based on self-report of hypertension, diabetes, myocardial infarction, cancer, thyroid disease, head trauma, and stroke. History of stroke was diagnosed at the clinical evaluation by a neurologist on the basis of a uniform structured examination and medical history.8,9 Disability was assessed at baseline with the 6-item Katz scale.12
Postmortem indices.
Brain removal, tissue sectioning and preservation, and a uniform gross and microscopic examination with quantification of postmortem indices followed a standard protocol that is detailed in previous publications.10,11,13,14 Postmortem indices included the presence of chronic macroinfarcts and microinfarcts as well as semiquantitative measures of cerebral atherosclerosis, arteriolosclerosis, and nigral neuronal loss, and a summary measure for AD and Lewy body disease pathology described in appendix e-1 on the Neurology® Web site at www.neurology.org.
Statistical analyses.
Pearson correlations were used to compare the relationship between baseline frailty and demographic variables; a t test was used to compare frailty between men and women. We used separate mixed-effect models15 to assess the relation of each brain pathology with the baseline level of frailty and its annual rate of change. The core model included terms for time in years since baseline, which indicates the average annual rate of change in frailty for a typical participant, as well as terms for brain pathology and a term for its interaction with time since baseline. All models included terms for age, sex, and education and their interactions with time. In subsequent models, we added interaction terms to determine whether the association of pathology and change in frailty varied by the presence of dementia during the course of the study, baseline level of disability, or chronic health conditions. The same approach described above for composite frailty was used to examine 3 of its 4 components. Because its fourth component, fatigue, involves only 3 levels, we modeled the odds of having higher vs lower fatigue levels using generalized linear mixed models with a random intercept and a cumulative logit link.15 Models were examined graphically and analytically and assumptions were judged to be adequately met. A priori level of statistical significance was 0.05. Programming was performed in SAS version 9.3 (SAS Institute Inc., Cary, NC).16
RESULTS
Clinical characteristics of participants at baseline and summary of postmortem indices.
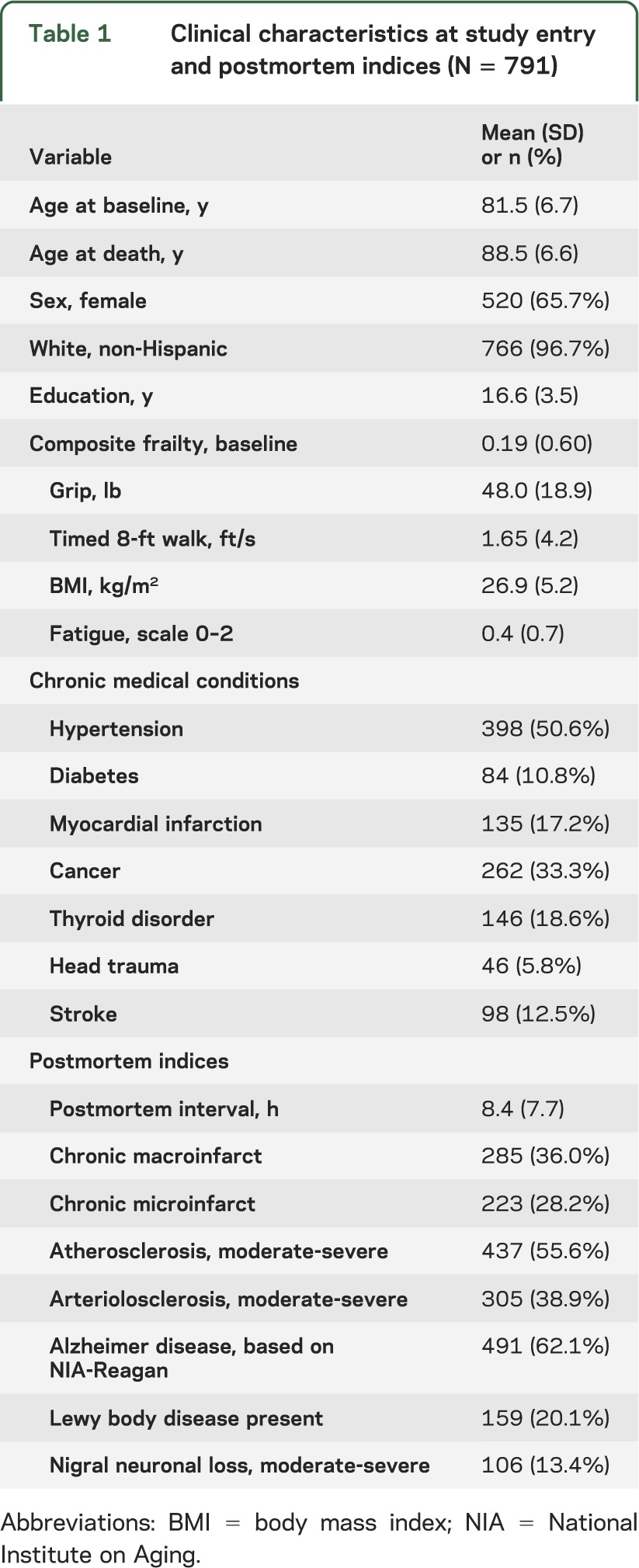
There were 791 participants (65.7% female) included in the primary analyses, and their clinical characteristics at baseline are included in table 1. Frailty at baseline ranged from −1.18 to 1.86, with a more positive value indicative of more severe frailty; on average, frailty was 0.19 (SD = 0.60) and interquartile range (Q1–Q3) = 0.77. Frailty was associated with age (ρ = 0.43, p < 0.001), sex (ρ = −0.15, p ≤ 0.001), and education (ρ −0.14, p < 0.001). A summary of the 7 brain pathologies measured in this study is included in table 1. More than 95% of cases (752/791) had evidence of one or more neuropathologies (1 = 22.1% [n = 175]; 2 = 23.1% [n = 183]; 3 = 25.4% [n = 201]; 4 = 15.8% [n = 125]; ≥5 = 8.6% [n = 68]).
Table 1.
Clinical characteristics at study entry and postmortem indices (N = 791)
Brain pathology and progression of frailty.
First, we examined person-specific differences in the annual rate of change in frailty using a mixed-effect model controlling for age, sex, and education and their interactions with time. Average follow-up was 6.4 years (SD = 2.80; range 2–14). The rate of change in frailty ranged from −0.04 to 0.27 unit/year, but on average, frailty increased by approximately 0.12 unit per year (estimate 0.117, SE 0.035, p < 0.001).
As illustrated in figure 1, nearly all of the cases showed increasing frailty (n = 788, 99.6%) during the course of this study. The rate of increasing frailty did not vary with sex but did vary with baseline age and education. Each additional year of age at baseline was associated with a 1.4% more rapid increase in frailty (time × age: estimate 0.002, SE 0.001, p = 0.012). By contrast, for each additional year of education, at baseline there was a 2.6% slower rate of increasing frailty (time × education: estimate −0.003, SE 0.001, p = 0.009).
Figure 1. Person-specific paths of progressive frailty.
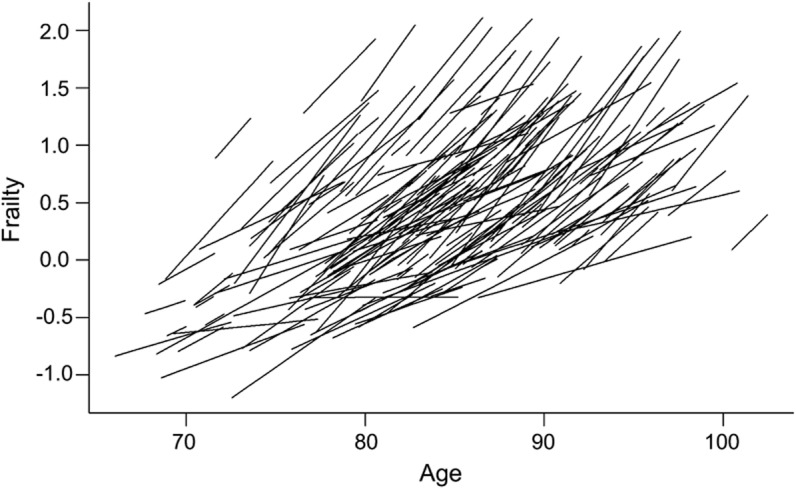
The figure is organized according to the age of the participant at each evaluation; the length of each line relative to the x-axis indicates the total years of observation for that individual. The figure is estimated for a 25% random sample of the cohort and shows smoothed person-specific paths estimated from a random-effects model with a term for time and controlled for age, sex, education, and their interaction with time.
Next, we added terms for each of the 7 pathologies to determine which pathologies were associated with rate of progression of frailty. The presence of macroinfarcts, AD, Lewy body disease pathology, and nigral neuronal loss was associated with a more rapid progression of frailty during the study, whereas the presence of microscopic infarcts, arteriolosclerosis, and atherosclerosis was not (table 2). Each of these pathologies alone explained up to 3.6% of the variance of progression of frailty unexplained by demographic variables alone.
Table 2.
Brain pathology and progression of frailty in old agea

In a final joint model including the 4 pathologies that were associated with progressive frailty, macroinfarcts, AD pathology, and nigral neuronal loss showed independent associations with the progression of frailty, whereas Lewy body pathology did not (data not shown). In a further analysis, we estimated the total variance of the rate of progressive frailty accounted for when these 3 pathologies were sequentially added to a single model. Together, these 3 pathologies accounted for more than 8% of the variance unexplained by demographic variables alone (table e-1).
Figure 2 illustrates the additive effects of these 3 pathologies on the rate of progression of frailty by showing the trajectory of progressive frailty for 4 average participants with increasing pathologies. The rate of increase in frailty for a participant with high levels of AD pathology (90th percentile), macroinfarcts, and severe nigral neuronal loss was almost 2.5 times more rapid than in an individual with only low levels of AD pathology (10th percentile).
Figure 2. Effect of more brain pathology on the predicted paths of frailty.
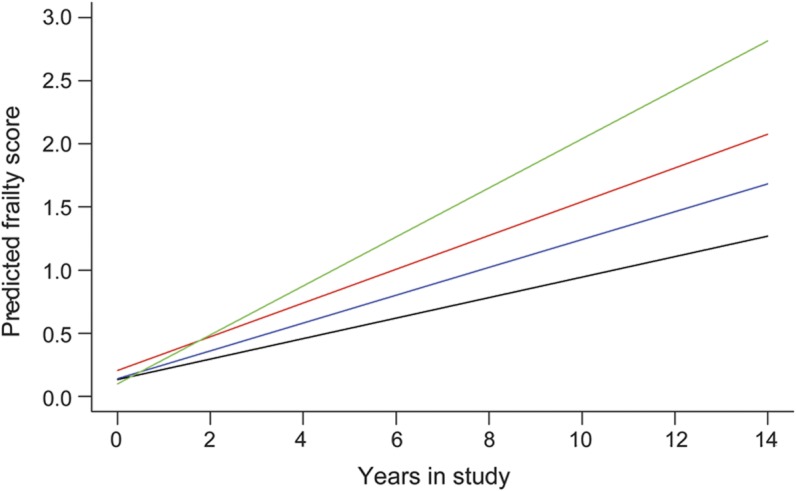
Predicted path of frailty for 4 participants with increasing brain pathology. 1) Predicted path of frailty for a participant with low level of Alzheimer disease (AD) pathology (10th percentile) (black line; slope = 0.081 unit/year). 2) Predicted path of frailty for a participant with a high level of AD pathology (90th percentile) (blue line: slope = 0.110 unit/year). 3) Predicted path of frailty for a participant with a high level of AD pathology (90th percentile) and macroinfarcts (red line: slope = 0.134 unit/year). 4) Predicted path of frailty for a participant with a high level of AD pathology (90th percentile), macroinfarcts, and severe nigral neuronal loss (green line: slope = 0.194 unit/year).
Brain pathology, other covariates, and progression of frailty.
To ensure that our findings linking AD pathology to the progression of frailty were not attributable to cases with dementia, we investigated whether the associations of brain pathology and the rate of change in frailty varied with the presence of dementia during the course of the study. We added terms to the models in table 2 to examine whether there was a 3-way interaction such that the association of brain pathology and the rate of change in frailty varied with dementia. The association of brain pathology and the progression of frailty did not vary between individuals with and without dementia except for nigral neuronal loss, which showed a more rapid progression of frailty in individuals with dementia (table e-2). Although at baseline ROS participants were on average younger (ROS, 80.3 years vs MAP, 83.2 years) and had more education (ROS, 18.1 years vs MAP, 14.5 years), the association of pathology and progression of frailty did not vary by study (table e-2). Using a similar approach, we found that the severity of baseline disability or the number of chronic health conditions did not affect the associations of brain pathology and the rate of change in frailty (table e-2).
In a final series of sensitivity analyses, we excluded 30 cases (3.8%) with a history of PD who had received levodopa and the associations of brain pathologies and the progression of frailty in table 2 were unchanged (results not shown).
Brain pathology and progression of the components of frailty.
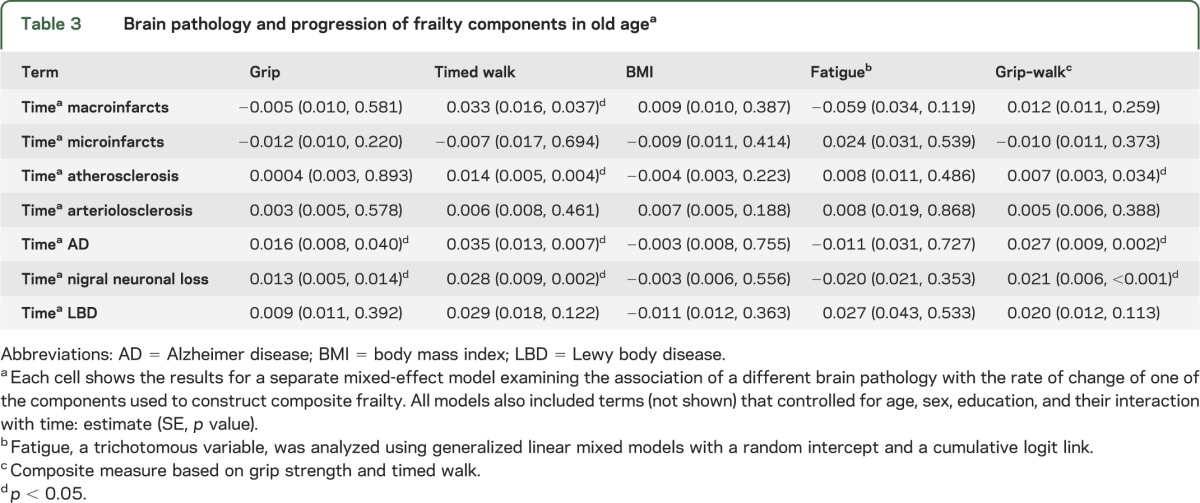
Because frailty is a multidimensional construct, it is possible that brain pathology may be associated with some of the individual components of frailty but not others. We repeated the analyses described above to determine the associations of brain pathology with the rate of change in grip strength, walking speed, and BMI. Levels of AD pathology and nigral neuronal loss were associated with the rate of decline in grip strength (table 3). Macroinfarcts, atherosclerosis, AD pathology, and nigral neuronal loss were associated with the rate of declining walking speed (table 3). Brain pathology was not associated with the rate of change in BMI or fatigue (table 3). Atherosclerosis, AD pathology, and nigral neuronal loss were associated with the rate of change in a composite measure based on grip strength and walking speed (table 3).
Table 3.
Brain pathology and progression of frailty components in old agea
DISCUSSION
In this clinical-pathologic cohort study of almost 800 older persons, nearly all the participants (>95%) showed progressive frailty during up to 14 years of follow-up. The rate of progression of frailty increased more rapidly with increasing age. Postmortem examination showed evidence of one or more brain pathologies in more than 95% of individuals. The presence of common brain pathologies including cerebrovascular disease, AD, and PD was associated with more rapid progression of frailty and in particular with more rapid decline of walking speed. These associations did not vary with the presence of clinical dementia, baseline level of disability, or the presence of chronic health conditions. These data suggest that the accumulation of diverse subclinical brain pathologies contributes to progressive frailty in older adults.
This study confirms that frailty is a progressive disorder in which rate of progression accelerates with increasing age.4,5 Although there is increasing recognition of the growing public health challenge of frailty in our aging population, its underlying pathology is unclear. In contrast to a prior study,10 the current, much larger study found that not only AD pathology but also several cerebrovascular pathologies, as well as nigral neuronal loss, a common finding in PD, were associated with the rate of progression of physical frailty in community-dwelling older adults. These associations were robust and unchanged after controlling for baseline chronic health conditions and disability and excluding cases with PD and did not vary by dementia status. Finally, the associations of these different pathologies with progressive frailty were additive (figure 2). Linking progressive frailty with common brain pathologies provides a host of potential new targets and pathways that may lead to interventions that prevent or ameliorate progressive frailty in older adults. Furthermore, it also suggests that there may be a much larger number of frail older adults who may benefit from treatments that have been developed for traditional neurologic diagnoses such as PD and stroke.
The basis for the association between brain pathology and frailty is uncertain and likely differs for each type of pathology. Frailty and brain pathology might be caused by a common underlying pathophysiology (e.g., inflammation, energy production, stress).17,18 Alternatively, analogous to pulmonary and cardiovascular pathology, subclinical brain pathology may manifest as frailty.19 Cross-sectional studies in the current cohorts as well as by other groups have suggested that several core components of frailty including strength, gait, and body composition are associated with AD, PD, and cardiovascular disease pathologies that were examined in the current study.10,11,14,20 The current analyses that link AD pathology and progressive frailty extend a prior study in one of the cohorts included in this study, which showed a cross-sectional link between AD pathology and frailty proximate to death. When examined alone, both Lewy body pathology and nigral neuronal loss, which characterize PD, were associated with progressive frailty (table 2). When both were considered together in a single model, Lewy body pathology was no longer associated with frailty (table e-2), consistent with prior studies suggesting that Lewy body's association with the severity of parkinsonism is mediated through nigral neuronal loss.11,21 Whereas several cardiovascular disease pathologies were related to progressive frailty when considered alone (table 2), when considered together only macroinfarcts remained associated with frailty, and its inclusion confounded the marginal association of arteriolosclerosis with progressive frailty. Furthermore, whereas strength and gait were associated with brain pathology, BMI and fatigue were not, suggesting that the brain pathologies examined in the current study may not contribute to all aspects of frailty to the same degree (table 3). Nonetheless, these results extend prior cross-sectional studies by showing that the subclinical accumulation of brain pathologies may lead to progressive physical frailty in older adults in whom clinical symptoms may not warrant traditional neurologic diagnoses such as dementia, stroke, or PD.
The current study has implications for identifying older individuals at risk of AD and PD. It has been suggested that frailty develops when age-associated degenerative processes overwhelm reserve capacity and reparative processes that maintain function of the nervous system and other physiologic systems.19 Our data suggest that this may not be a global process, but rather a multifactorial process whereby some conditions contribute to frailty whereas others do not. Our finding that subclinical AD and PD pathology contributes to frailty suggests that physical frailty may be a harbinger or preclinical manifestation of these diseases. For example, we now recognize that AD pathology accumulates over many years and is frequently observed in brains from older adults without dementia.22,23 In fact, in one of the cohorts, we previously showed that progression of frailty was associated with incident AD.5 However, the variance of progressive frailty explained by neuropathologies in the current study was modest. This raises the possibility that other pathologies that were not measured may have a more essential role in progressive frailty. An alternate explanation of the association of nigral neuron integrity (i.e., a semiquantitative assessment of neuron density) with progressive frailty is that the substantia nigra represents a structural component of neural reserve that contributes to brain reserve capacity.24 Thus, it will also be important to identify other factors in the brain that confer protective benefit (i.e., neural reserve or resilience) by reducing the deleterious effect of brain pathology on frailty in older adults.25
Our study also has some limitations. The findings are based on a selected cohort that differs in important ways from older persons in the general population regarding education, socioeconomic status, and lifestyle. It is important to investigate these findings in more diverse cohorts. Although accumulating brain pathology may cause progressive frailty, it is also possible that accumulating brain pathology and frailty share a common underlying pathophysiology, or more likely, that both occur. This study was large because on an individual level the effect sizes are small. Nonetheless, from a public policy perspective, given the extent of frailty in old age, even the modest effect sizes observed in the current study are likely to be important. Further studies are needed to more fully explicate the types and locations (i.e., brainstem, spinal cord, muscle, and nerve) of other pathologies that underlie physical frailty in old age.
Confidence in these findings is enhanced by several factors. Participants underwent detailed annual structured clinical examinations for up to 14 years, with more than 90% follow-up participation in survivors and a high autopsy rate. Uniform structured procedures were followed with masking of previously collected and postmortem data, reducing the potential for bias. Furthermore, analyses controlled for potentially confounding variables.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all the participants in the Rush Memory and Aging Project and Religious Order Study. The authors also thank Traci Colvin, RN, and Tracey Nowakowski, MS, for project coordination; John Gibbons, MS, for data management; Donna Esbjornson, MS, for statistical programming; and the staff of the Rush Alzheimer's Disease Center.
GLOSSARY
- AD
Alzheimer disease
- BMI
body mass index
- MAP
Memory and Aging Project
- PD
Parkinson disease
- ROS
Religious Orders Study
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Buchman had full access to all of the data in the study and takes responsibility for the integrity and accuracy of the data analysis. He was involved with obtaining funding for the study, study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. Dr. Yu provided statistical analyses and interpretation and critical revision of the manuscript for important intellectual content. Drs. Wilson and Schneider were involved in study design, acquisition of data, and critically revised the manuscript for important intellectual content. Dr. Bennett was involved with obtaining funding, study concept and design, acquisition of data, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content.
STUDY FUNDING
Supported by NIH grants R01AG17917, R01AG0433379, P30AG10161, AG31553, R01AG24480, R01NS078009, and AG040039, Illinois Department of Public Health, and the Borwell Endowment Fund.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr, Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc 2004;52:625–634 [DOI] [PubMed] [Google Scholar]
- 2.Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the Rush Memory and Aging Project, a community-based cohort study. BMC Med 2011;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc 2010;58:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman AS, Wilson RS, Bienias JL, Bennett DA. Change in frailty and risk of death in older persons. Exp Aging Res 2009;35:61–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer's disease and cognitive decline in the elderly. Psychosom Med 2007;69:483–489 [DOI] [PubMed] [Google Scholar]
- 6.Metzelthin S, Daniels R, van Rossum E, de Witte L, van den Heuvel W, Kempen G. The psychometric properties of three self-report screening instruments for identifying frail older people in the community. BMC Public Health 2010;10:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–1492 [DOI] [PubMed] [Google Scholar]
- 8.Bennett DA, Schneider JA, Buchman AS, et al. The Rush Memory and aging project. Curr Alzheimer Res 2012;9:646–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res 2012;9:628–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchman AS, Schneider JA, Leurgans S, Bennett DA. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology 2008;71:499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchman AS, Shulman JM, Nag S, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol 2012;71:258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv 1976;6:493–508 [DOI] [PubMed] [Google Scholar]
- 13.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology 2003;60:1082–1088 [DOI] [PubMed] [Google Scholar]
- 14.Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA. Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke 2011;42:3183–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988;44:1049–1060 [PubMed] [Google Scholar]
- 16.SAS/STAT® Software for Unix, version 9.18 [computer program]. Cary, NC: SAS Institute Inc.; 2002–2003 [Google Scholar]
- 17.Vassar R. Caspase-3 cleavage of GGA3 stabilizes BACE: implications for Alzheimer's disease. Neuron 2007;54:671–673 [DOI] [PubMed] [Google Scholar]
- 18.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008;57:178–201 [DOI] [PubMed] [Google Scholar]
- 19.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006;54:991–1001 [DOI] [PubMed] [Google Scholar]
- 20.Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology 2006;67:1949–1954 [DOI] [PubMed] [Google Scholar]
- 21.Luk KC, Kehm V, Carroll J, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012;338:949–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association Workgroup. Alzheimers Dement 2011;7:280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siderowf A, Lang AE. Premotor Parkinson's disease: concepts and definitions. Mov Disord 2012;27:608–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson RS, Nag S, Boyle PA, et al. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology 2013;80:1202–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle PA, Buchman AS, Wilson RS, Yu L, Schneider JA, Bennett DA. Effect of purpose in life on the relation between Alzheimer disease pathologic changes on cognitive function in advanced age. Arch Gen Psychiatry 2012;69:499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


