Abstract
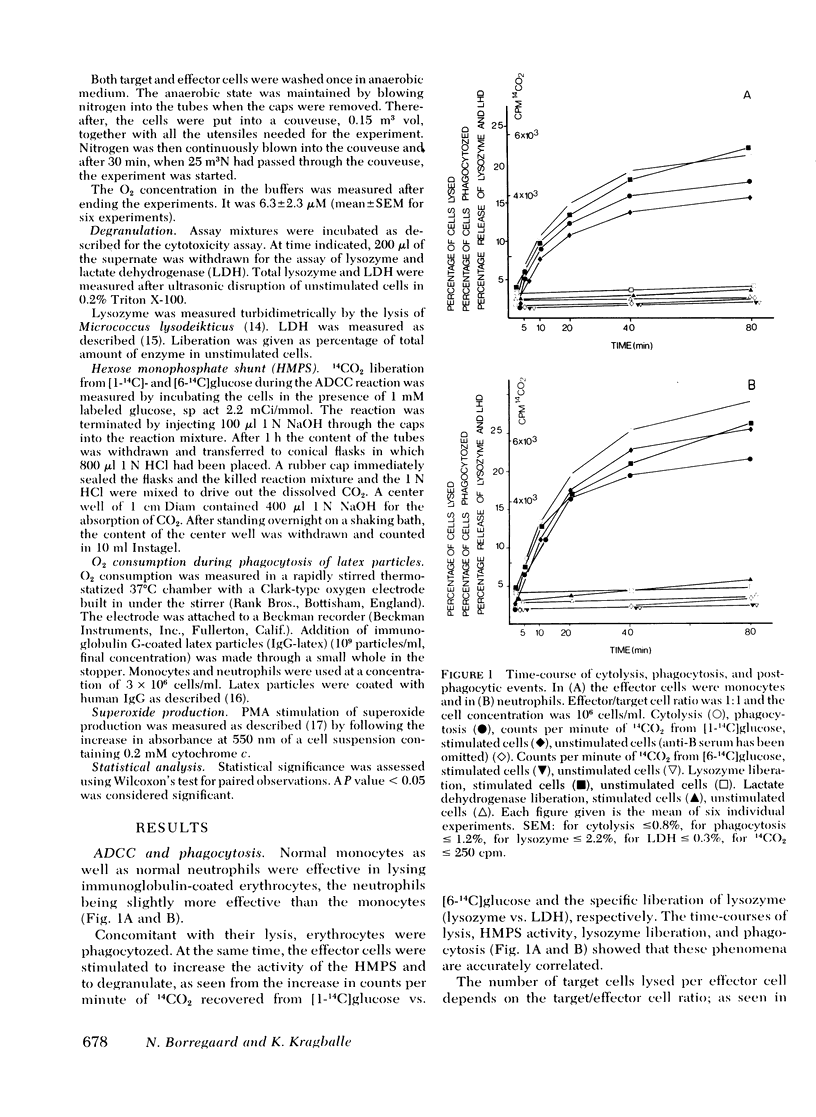
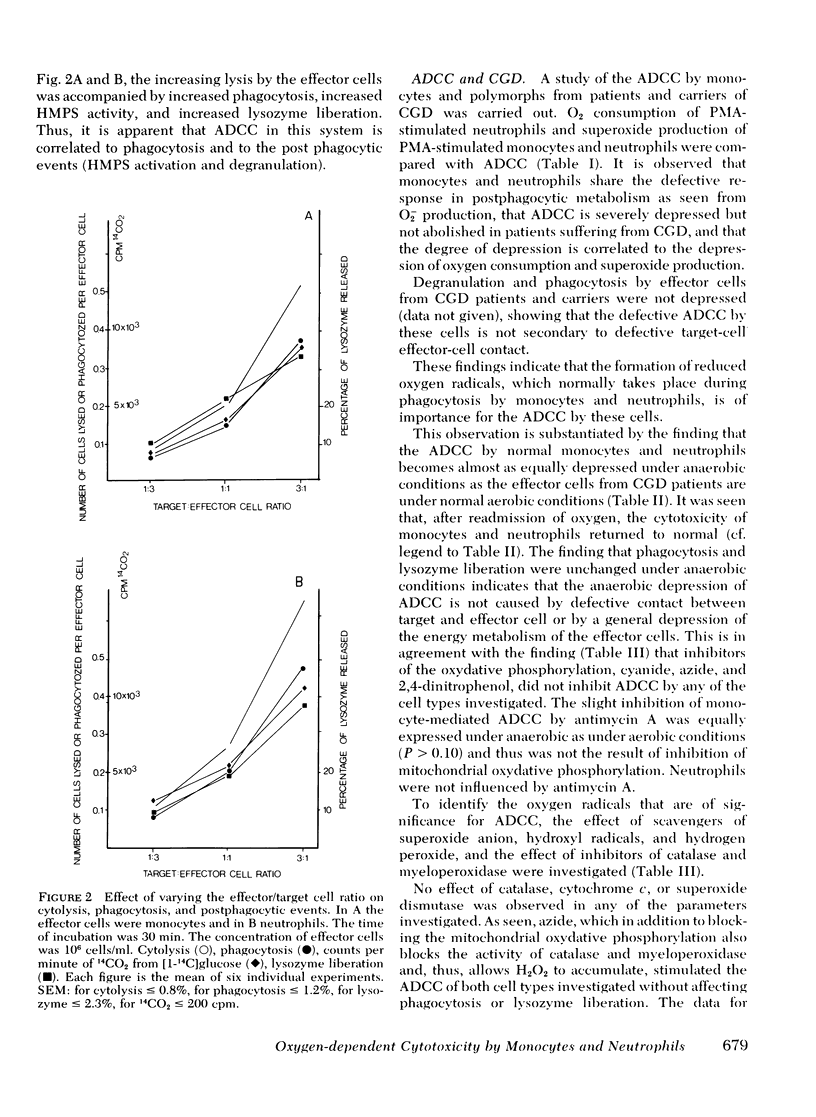
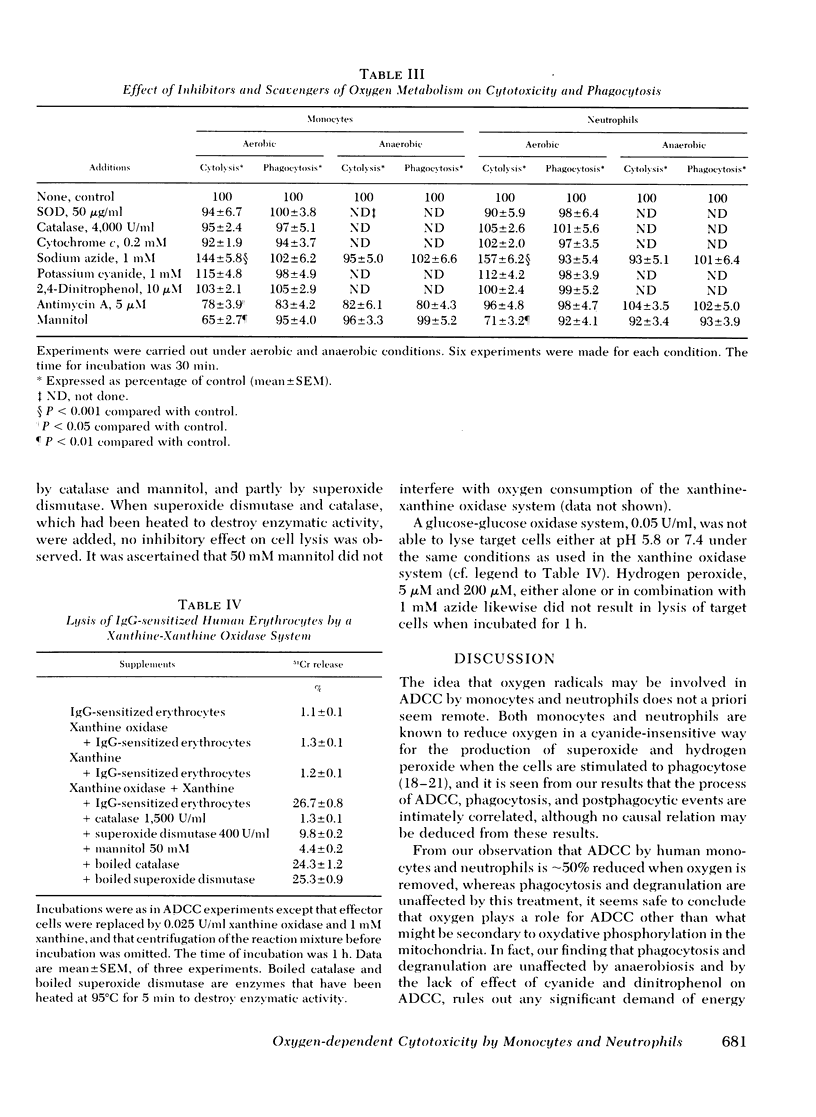
The antibody-dependent cell-mediated cytoxicity (ADCC) by human monocytes and neutrophils was investigated by measuring the release of 51chromate from prelabeled erythrocytes coated with immunoglobulin G. ADCC was found to be positively correlated to phagocytosis of 51Cr-labeled erythrocytes and to the postphagocytic events of the effector cells, activation of the hexose monophosphate shunt, and degranulation. Exclusion of oxygen from the incubation media halved the ADCC by both cell types without affectijg phagocytosis or degranulation. Likewise, ADCC by cells from patients suffering from chronic granulomatous disease (CGD) was only half the intensity of ADCC by cells from normals. Inhibitors of mitochondrial respiration were without depressing effect of ADCC. Azide, which in addition to its blocking action on oxydative phosphorylation also inhibits catalase and myeloperoxidase, resulted in a approximately equal to 40% stimulation of ADCC by cells from normals but was without effect of ADCC by cells from CGD patients. The hydroxyl radical scavenger, mannitol, significantly depressed ADCC by cells from normals (P < 0.01) but was without effect on cells from CGD patients. Azide and mannitol also were without effect on ADCC by normal cells when oxygen was excluded. In a xanthine-xanthine oxidase system, erythrocytes were effectively lysed. This lysis was inhibited by catalase, superoxide dismutase, and mannitol. When comparable concentrations of glucose oxidase were used no lysis was observed. H2O2 either alone or in combination with azide did not lyse erythrocytes. It is suggested that ADCC by both monocytes and neutrophils is partly dependent on the generation of hydroxyl radicals by the effector cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark R. A., Klebanoff S. J. Studies on the mechanism of antibody-dependent polymorphonuclear leukocyte-mediated cytotoxicity. J Immunol. 1977 Oct;119(4):1413–1418. [PubMed] [Google Scholar]
- Fleer A., Roos D., von dem Borne A. E., Engelfriet C. P. Cytotoxic activity of human monocytes towards sensitized red cells is not dependent on the generation of reactive oxygen species. Blood. 1979 Aug;54(2):407–411. [PubMed] [Google Scholar]
- Fleer A., van Schaik M. L., von dem Borne A. E., Engelfriet C. P. Destruction of sensitized erythrocytes by human monocytes in vitro: effects of cytochalasin B, hydrocortisone and colchicine. Scand J Immunol. 1978;8(6):515–524. doi: 10.1111/j.1365-3083.1978.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Green M. R., Hill H. A., Okolow-Zubkowska M. J., Segal A. W. The production of hydroxyl and superoxide radicals by stimulated human neutrophils- measurements by EPR spectroscopy. FEBS Lett. 1979 Apr 1;100(1):23–26. doi: 10.1016/0014-5793(79)81123-0. [DOI] [PubMed] [Google Scholar]
- Holm G. Lysis of antibody-treated human erythrocytes by human leukocytes and macrophages in tissue culture. Int Arch Allergy Appl Immunol. 1972;43(5):671–682. doi: 10.1159/000230883. [DOI] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P., Simone C. B., Henkart P. A., Fauci A. S. Mechanisms of antibody-dependent cellular cytotoxicity: the use of effector cells from chronic granulomatous disease patients as investigative probes. J Clin Invest. 1980 Jan;65(1):55–63. doi: 10.1172/JCI109660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass H. J., Hopkins J., Neale G., Peters T. J. The estimation of serum lysozyme: a comparison of four assay methods. Biochem Med. 1977 Aug;18(1):52–57. doi: 10.1016/0006-2944(77)90048-5. [DOI] [PubMed] [Google Scholar]
- Levy P. C., Shaw G. M., LoBuglio A. F. Human monocyte, lymphocyte, and granulocyte antibody-dependent cell-mediated cytotoxicity toward tumor cells. I. General characteristics of cytolysis. J Immunol. 1979 Aug;123(2):594–599. [PubMed] [Google Scholar]
- LoBuglio A. F., Cotran R. S., Jandl J. H. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967 Dec 22;158(3808):1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Bonnard G. D., Sordat B., Zawodnik S. A. Antibody-dependent cell-mediated cytotoxicity: heterogeneity of effector cells in human peripheral blood. Scand J Immunol. 1975 Sep;4(5-6):487–497. doi: 10.1111/j.1365-3083.1975.tb02654.x. [DOI] [PubMed] [Google Scholar]
- Penfold P. L., Greenberg A. H., Roitt I. M. Characteristics of the effector cells mediating cytotoxicity against antibody-coated target cells. III. Ultrastructural studies. Clin Exp Immunol. 1976 Jan;23(1):91–97. [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Sagone A. L., Jr, King G. W., Metz E. N. A comparison of the metabolic response to phagocytosis in human granulocytes and monocytes. J Clin Invest. 1976 May;57(5):1352–1358. doi: 10.1172/JCI108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. The subcellular distribution and some properties of the cytochrome b component of the microbicidal oxidase system of human neutrophils. Biochem J. 1979 Jul 15;182(1):181–188. doi: 10.1042/bj1820181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., King G. W., LoBuglio A. F. Evidence for hydroxyl radical generation by human Monocytes. J Clin Invest. 1977 Aug;60(2):370–373. doi: 10.1172/JCI108785. [DOI] [PMC free article] [PubMed] [Google Scholar]



