Abstract
Several recent efforts in radiation biology community worldwide have amassed records and archival tissues from animals exposed to different radionuclides and external beam irradiation. In most cases, these samples come from life-long studies on large animal populations conducted in national laboratories and equivalent institutions throughout Europe, North America, and Japan. While many of these tissues were used for histopathological analyses, much more information may still be obtained from these samples. A new technique suitable for imaging of these tissues is X-Ray Fluorescence Microscopy (XFM). Following development of third generation synchrotrons, XFM has emerged as an ideal technique for study of metal content, speciation, and localization in cells, tissues and organs. Here we review some of the recent XFM literature pertinent to tissue sample studies and present examples of XFM data obtained from tissue sections of beagle dog samples which show that the quality of archival tissues allows XFM investigation.
Keywords: x-ray fluorescence imaging, radiation exposure, radionuclides, tissue archive
Introduction
Normal tissue homeostasis requires correct execution of cellular processes which rely not only on RNA and proteins but also on inorganic elements that serve as their cofactors. Among these, metals fulfill two different essential roles: some such as calcium (Ca) serve for cellular signaling (Clapham, 2007), while others act as cofactors in enzymes and it is believed that one third of all proteins require a metal cofactor such as copper (Cu), iron (Fe) or zinc (Zn) (Tainer et al., 1991; Outten and O’Halloran, 2001). Disturbances of tissue homeostasis which follow tissue injury caused by exposure to irradiation can be expected, therefore, to be reflected in altered pattern of elemental distribution in cells and tissues. Moreover, tissue toxicities that are found as long term effects of irradiation such as development of neoplasias or fibrotic changes are associated with changes of proteins ratios and protein representation that are accompanied by changes in elemental distribution. Therefore, we can anticipate that such changes in protein populations will be accompanied by changes in metal distribution(s) and concentrations in tissues of animals that have been deposited into irradiated animals tissue archives.
Starting from 1950’s and ending in most cases in 1990’s different radiobiology mega studies have been conducted on model animals in order to evaluate radiation caused mortality and risk(s) for development of different morbidities. Parallel and often complementary research efforts were made in USA and in Europe, and studies on thousands of animals were conducted in an effort to extrapolate possible effects of different radiation exposures on humans. Most important research institutes in Russia were the Southern Urals Biophysics Institute (SUBI, Ozyorsk), Biophysics Institute (Moscow), and Urals Center of Radiation Medicine (Chelyabinsk). In the USA, several national laboratories, institutes and universities were active in this type of research as well; especially Argonne National Laboratory, Oakridge National Laboratory, Pacific Northwest National Laboratory, University of Davies California, Lovelace Respiratory Research Institute and others. Studies at Argonne National Laboratories (ANL) between 1969 and 1992, included nearly 700 beagles and 50,000 mice (Haley et al. 2011). These studies aimed to understand the effects of neutron and Cobalt-60 gamma-ray irradiation on lifespan and tumorigenesis across a wide range of dose and dosing patterns. In addition, beagle dogs were also used for inhalation and injection experiments partially at ANL but more at Pacific Northwest National Laboratory (PNNL), University of California Davies and Lovelace Respiratory Research Institute (LRRI). The radionuclides americium (241Am), cerium (144Ce), californium (249Cf, 252Cf), einsteinium (253Es), neptunium (237Np), plutonium (237, 238, 239Pu), radon (226Ra, 228Ra), strontium (90Sr), thorium (228Th), yttrium (90, 91Y) were delivered via inhalation or injection. In addition, different types of salt molecules or particulate formulations were used to deliver these radionuclides, which lead to different bioavailability and variable penetrance into targeted organs and tissues. The most relevant discoveries of the beagle dog studies from various institutions in the United States are available in a book published towards the end of the experimentation and sample collection period (Thompson, 1989). Many more sources detail the results of individual experiments. While concentrations and doses delivered were recorded in these experiments, accumulation of these elements has not been quantified and their distribution was monitored by autoradiography. Detailed pathological, clinical, treatment, and surgical notes were recorded for many of the animals in these studies and blood smear slides and paraffin embedded tissue samples were collected from tumors, lymph nodes as well as a standard set of internal organs. These samples were used (though in a more limited manner) for histopathological evaluation as well.
Work at SUBI, conducted between 1949 to 1996, included studies of alpha- (234,235U, 237Np, 238, 239Pu, 241Am) and beta- (3H, 90Sr, 137Cs, 144Ce) emitters delivered via different routes of administration, at different dose range, rates of intake and nuclides administration patterns; and studies of effects of external gamma and neutron irradiations. Experiments included rodents (mice, rats and rabbit) and other mammals (dog, pig, monkey) (Buldakov et al., 1968, 1969; Moskalyov et al., 1977, 1979; Muksinova et al., 1990; Mushkachyova et al 1994). Biological material was obtained from more than 23 thousand animals; much of it preserved in the SUBI Radiobiological Archive. At this time, efforts such as the European Commission (EU) funded STORE project, and others, aim to provide an infrastructure for the sharing of data and biomaterials such as tissues and histological specimens from radiobiological research institutes in Europe.
Many of large-scale studies of radiation effects on animals were performed in the past. Today, the scale, cost and ethical aspects make such mega studies unlikely to be repeated, thus, it is necessary to support work aimed at preserving collected unique experimental material and especially its usage. Since archival FFPE tissue samples are the most readily available material from large, different studies, they represent an inestimable source of biological information and pave the way for retrospective studies. Over the last decade advances in cytology and molecular biology techniques have made it possible to routinely work with archival materials.
New medical findings based on specimens from tissue and organ banks affiliated with hospitals and repositories have lead to important mechanistic discoveries, explaining human diseases in new ways. It can be expected that continual technical progress should provide us with new data on animal archival tissues as well; especially those produced from massive, thousands of animal experiments which are too expensive to be repeated in same volume any time in the future. One technique that is already available for a new type of histological imaging is hard X-ray fluorescence microscopy (XFM, also known as Synchrotron-based X-ray fluorescent microscopy or SXRF). XFM is a relatively non destructive technique which detects and quantifies metals and other elements present in tissue samples or single cells with very high sensitivity. XFM done with hard X-rays in particular is ideally suited for detection of trace elements, toxic heavy metals, and metals from therapeutic or diagnostic metal complexes (Petibois et al., 2008). Importantly, XFM can be done on different resolution scales loosing very little of elemental detection sensitivity. Thus, synchrotron based X-ray fluorescence microprobes perform imaging with resolution matching that of optical microscopes (scanning samples with hard X-ray beam sizes between several microns and 0.2 microns), while X-ray nanoprobes few of which now exist though still at developmental stage, and push resolution as far as 50nm. Moreover, due to a great depth of focus (10s to 100s of microns) XFM can also be used for tomographic elemental mapping (De Jonge and Vogt, 2010). Rapid growth of technical novelties associated with XFM has secured great interest in its application to study the structure and dynamics of metals in tissues, cells, cellular organelles, and metalloproteins (Cook et al., 2008).
X-ray fluorescence microscopy of biological samples
Development of metallomics, defined as a field of research activities aimed at the understanding of the molecular mechanisms of metal-dependent life processes has blossomed in recent years with development of new techniques for metal detection and quantification (Mounicou et al., 2009). Among them are sensitive mass spectrometric detection techniques e.g. inductively coupled plasma (ICP) as well as laser ablation ICP-MS which can be used for 2D imaging; secondary ion mass spectrometry (SIMS); use of metal detecting dyes for optical imaging; proton-induced X-ray emission microscopy and X-ray fluorescence microscopy (XFM) (Lobinski, et al., 2006). Among these techniques, XFM is gaining in predominance as very sensitive technique with big depth of focus, detecting complete spectra emitted from the sample in such a way that the data on each element can be quantified following rapid calibration with appropriate standards. Possibility to use XFM for samples that are relatively thick yet to do scanning at different resolutions allowed that the same technique and same instrument can be used to identify copper in subcellular compartments such as mitochondria and Golgi apparatus (Yang et al., 2005), as well as in liver tissue sections from a mouse model of Wilson disease (Ralle et al., 2010). While PIXE has similar penetration depth as XFM, it cannot be used to detect elemental speciation which can be done with X-rays, coupling XFM with micro X-ray Absorption Near Edge Structure (μ-XANES) (Ralle and Lutsenko 2009).
There are too many successful examples of XFM use relevant for study of “native” metal distribution in biological samples to mention them all, therefore only few of such examples will be touched upon. Similarly, metal and non-metal contaminants from the environment can also be detected and their speciation determined using XFM and μ-XANES. Again, only some choice examples of this type of research will be presented, keeping in mind that the goal of this document is to point out at XFM and μ-XANES as tools for investigation of archival samples from animals exposed to external beam radiation and radionuclides.
XFM is often used to support studies that look at the use of metal based drugs for different types of therapies. One of the main concerns when developing a new drug is its potential toxicity or the drug resistance that develops over time. It is easier to anticipate possibility of either after completing a study of the intercellular distribution and biological modification of these drugs in cells and tissues. Platinum anti-cancer therapeutics are among the major metal containing classes of drugs used in the treatment of cancer, although Ti, Mo, and Ru are used as well. Similarly, vanadium and chromium anti-inflammatory drugs and drugs for the treatment of diabetes are frequent as well. Most of these drugs are susceptible to biotransformations which are most often essential for fulfillment of their effects in the organism. Together XFM and μ-XANES were used to determine distributions and ascertain oxidation states of metals in metal containing drugs given to cells or animals. For example, the Hambley and Lay laboratories at the University of Sydney investigated distribution and speciation of platinum, ruthenium, chromium, arsenic and selenium etc. (Hall et al., 2006; Aitken et al., 2010, Liu et al., 2010; Harris et al 2005). Arsenic from medicinal arsenates was also detected in patients’ hair samples in a trivalent oxidation state using XRF and μ-XANES (Nicolis et al., 2009).
Contaminants from environment were also often detected and chemically characterized using XFM and XANES. In two most recent examples, silver and mercury in livers and other tissues of different marine organisms were quantified and their speciation determined (Nakazawa et al., 2011a, b). These in depth studies also suggested which elements from the organism are most likely to sequester these contaminants and protect the organisms from these metals.
As mentioned before, XFM is most often used to monitor concentration fluctuations during biological processes where one type of metal homeostasis is replaced by another in the process of cell differentiation or other types of changes. For example, human embryonic stem cells loose pluripotency in parallel with nuclear zinc accumulation (Wolford et al., 2010). During the process of angiogenesis, copper redistribution and removal from cells was found to be one of early events (Finney et al., 2007); while in neuronal cells copper distribution depends on calcium (Dodani et al., 2011). In all of these cases XFM was used as a key technique to uncover elemental distribution and concentration. In kidney tissue samples XFM allowed determination of the tissue topography of selenium (Malinouski et al., 2011). Same element was found to play a critical role in spermatogenesis (Kehr et al., 2009); while asymmetrical distribution of zinc was found to be crucial for normal development of oocytes (Kim et al., 2010). Zinc distribution was also found to have a discrete pattern in lactating mouse mammary gland (McCormick et al., 2010)
In the course of cancer progression elemental distributions in tissues are altered, and these changes can also be detected by XFM very well (Petibois et al., 2008). XFM was used to look at elemental distribution and concentrations in proteins from liver cancers (Liu et al., 2007; Gao et al., 2002). Distribution of zinc, sulfur, copper and iron in prostate cancer and hyperplasia samples was also found to be diseases dependent (Barrea et al., 2010); same was true for breast cancer samples as well (Pereira et al., 2008).
XFM work on archival samples
Previous studies have determined that formalin fixed paraffin embedded (FFPE) tissues preserve their morphology very well and can be used for XFM detection of elements firmly attached to proteins (Ralle and Lutsenko 2009). As mentioned before, copper, iron and zinc are among the tightly bound metal cofactors of proteins (Tainer et al., 1991; Outten and O’Halloran, 2001), and we have found that their distribution in FFPE tissues shows no significant difference in comparison to frozen, freeze dried samples (Bigio et al., unpublished data). Non-metal elements such as sulfur remain in the cell cytoplasm and nuclei; the same is true for phosphorus as well, though its concentration is highest in cell nuclei, which we noted previously (Paunesku et al., 2006). Thus, using distribution of stable elements which remain representative of the same cellular compartments, we are able to determine tissue cellularity and identify cell features.
To estimate whether XFM could be used to interrogate the archived tissues we looked at the distribution of Zn in prostate and testis tissues from archival beagle dog samples. The paraffin embedded tissues were cut at 5 μm and attached to a thin membrane (Ultralene®, 4μm) commonly used for XFM (Ralle and Lutsenko, 2009). The tissue sections were scanned at Advanced Photon Source Synchrotron, at the sector 8 bending magnet beamline with a several micron resolution in order to obtain a high throughput overview of distribution of elements between P and Zn in the periodic table. We tested this on several tissues, and were able to show that the elemental concentration and distribution remained as good as can be expected from paraffin embedded tissues. For example, while distribution of calcium shows only traces of the native distribution because this element is mostly in a diffuse/non-bound state inside cells (as it serves in cells a second messenger), zinc is mostly present in tissues firmly bound to the proteins that were immovable during the process of fixation and paraffin embedding. Especially in prostate cancer distribution of Zn is considered to be a valuable diagnostic marker. Zinc concentration differences between prostate and testis tissue were several-fold, as expected (Barrea et al., 2010, Haley et al., 2011). Additionally, in prostate it was possible to segregate the prostate gland tissue from connective tissue by comparing zinc concentrations and distribution patterns (Figure 1). Adjacent sections of the same tissues were stained with Hematoxylin and Eosin (H&E), and specific areas of interest identified by the XFM scans are shown. It is expected that radiation induced fibrotic changes may be expected to cause altered zinc distribution patterns.
Figure 1.
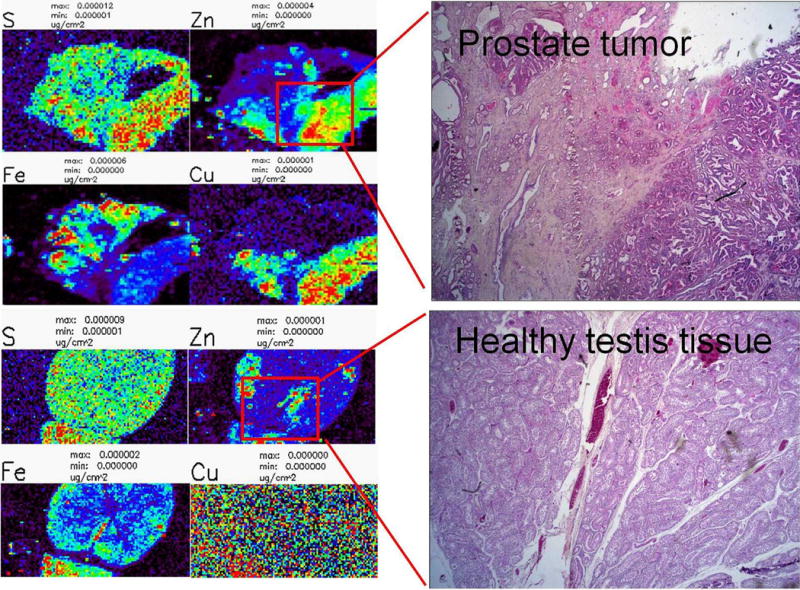
X-ray fluorescence microscopy of dog testis and prostate tissues. XFM analysis can be performed on sections of paraffin embedded dog tissues to show the distributions of various elements. In this example, both testis and prostate samples were sectioned at 5 μm and stained with H & E. The corresponding x-ray Fluorescence (XFM) scan shows the distribution of sulfur (S), zinc (Zn), iron (Fe) and copper (Cu) in both tissues.
To investigate if paraffin embedded tissues from SUBI have equally well preserved structure and elemental distribution, we scanned tissue sections from two spleen samples from CBA mice sacrificed 250 days after oral delivery of a total dose of 8.7 Gy 3HOH (370kBq/g body weight) over a period of 6 months (Figure 2). These samples showed excellent elemental preservation, and a detailed scan shown in Figure 2 shows the power of XFM to provide elemental content and 2D elemental distribution information sufficient to differentiate between different cell types (e.g. erythrocytes indicated by a white arrowhead and splenocytes indicated by a yellow arrowhead).
Figure 2.
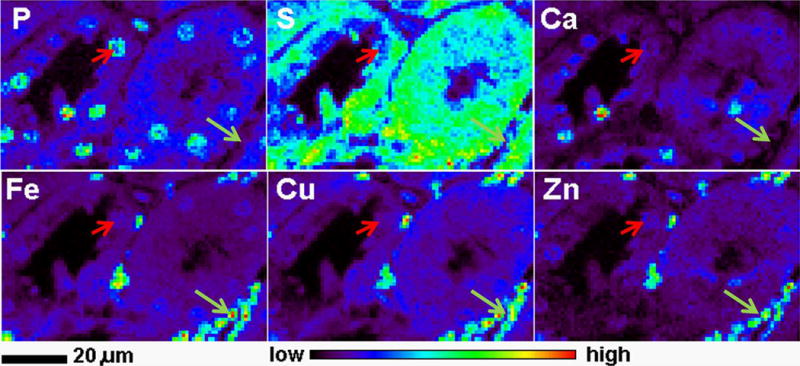
Detailed X-ray fluorescence microscopy gave detail rich elemental maps of a paraffin embedded spleen sample from a tritium exposed mouse. XFM maps show the distribution of phosphor (P), sulfur (S), calcium (Ca), iron (Fe) copper (Cu) and zinc (Zn). The white arrowhead indicates erythrocytes, while the yellow arrowhead points to a nucleus of a single splenocyte.
Conclusions
Hard X-rays XFM has micron scale focal depth and penetration in biological specimens coupled with submicron size lateral resolution. The advancement of third-generation synchrotrons enabled development of X-ray fluorescence microprobes and nanoprobes which will allow μ-XANES and XFM imaging at resolution as low as 50nm. XFM is also very sensitive and allows analysis of metal and non-metal elements present at low concentrations in the samples. Parallel development of cryo approaches will enable perhaps even better resolution of XFM in the future; and it has already enabled XFM imaging of hydrated samples close to their native state and in 3D.
We are interested in trying to use XFM for investigation of archival samples from animals exposed to external beam radiation and radionuclides. While histopathological examination was done on many of these tissues, using XFM to determine elemental concentrations and distribution in these tissues may provide novel and very informative data. On one hand, cancer development alters elemental distributions; likewise, fibrosis, inflammation and other types of non-cancer tissue toxicities in these tissues alter elemental distributions as well.. We tested paraffin embedded samples from beagle dogs exposed to external beam irradiation. After imaging prostate and testis by XFM we were able to conclude that these specimens do not show any signs of abnormal elemental distribution when compared to recently embedded samples.
It is also noteworthy that we have recently managed to investigate plutonium distribution and speciation in cultured rat cells (Aryal et al., 2011; Gorman-Lewis et al., 2011). Considering high sensitivity of XFM for plutonium, we anticipate that we will be able to detect remaining plutonium and its radioactive decay products in animal samples from the tissue archive. Similarly, we expect that investigation of distribution and speciation of other radionuclides and their products in archival animal samples should be possible as well. Possible XFM studies on radionuclide containing samples from SUBI at the APS or its European counterparts may provide a wealth of information that may enable very interesting comparative studies maximizing the knowledge that can be gained from archival samples.
Acknowledgments
This work was supported by the U.S. Department of Energy grant DE-SC0001271 and contract DE-AC02-06CH11357 awarded to Advanced Photon Source.
References
- Aitken JB, Levina A, Lay PA. Studies on the biotransformations and biodistributions of metal-containing drugs using X-ray absorption spectroscopy. Curr Top Med Chem. 2011;11(5):553–71. doi: 10.2174/156802611794785217. [DOI] [PubMed] [Google Scholar]
- Aryal BP, Gorman-Lewis D, Paunesku T, Wilson RE, Lai B, Vogt S, Woloschak GE, Jensen MP. Plutonium uptake and distribution in mammalian cells: Molecular vs. polymeric plutonium. Int J Radiat Biol. 2011;87(10):1023–32. doi: 10.3109/09553002.2011.584941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrea RA, Gore D, Kujala N, Karanfil C, Kozyrenko S, Heurich R, Vukonich M, Huang R, Paunesku T, Woloschak G, Irving TC. Fast-scanning high-flux microprobe for biological X-ray fluorescence microscopy and micro XAS. J Synchrotron Radiat. 2010;17(4):522–9. doi: 10.1107/S0909049510016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buldakov LA, Lubchansky ER, Moskalyov JI, Niphatov AP. Plutonium toxicology issues. M.: Atomizdat; 1969. p. 368. [Google Scholar]
- Buldakov LA, Moskalyov JI. Issues of distribution and experimental assessment of 137Cs, 90Sr and 106Ru allowed levels. M.: Atomizdat; 1968. p. 295. [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Cook JD, Penner-Hahn JE, Stemmler TL. Structure and dynamics of metalloproteins in live cells. Methods Cell Biol. 2008;90:199–216. doi: 10.1016/S0091-679X(08)00810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge MD, Vogt S. Hard X-ray fluorescence tomography--an emerging tool for structural visualization. Curr Opin Struct Biol. 2010;20(5):606–14. doi: 10.1016/j.sbi.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Dodani SC, Domaille DW, Nam CI, Miller EW, Finney LA, Vogt S, Chang CJ. Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy. Proc Natl Acad Sci U S A. 2011;108(15):5980–5. doi: 10.1073/pnas.1009932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney L, Mandava S, Ursos L, Zhang W, Rodi D, Vogt S, Legnini D, Maser J, Ikpatt F, Olopade OI, Glesne D. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc Natl Acad Sci U S A. 2007;104(7):2247–52. doi: 10.1073/pnas.0607238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Chen C, Chai Z, Zhao J, Liu J, Zhang P, Heb W, Huang Y. Detection of metalloproteins in human liver cytosol by synchrotron radiation X-ray fluorescence combined with gel filtration chromatography and isoelectric focusing separation. Analyst. 2002;127:1700–1704. doi: 10.1039/b208976a. [DOI] [PubMed] [Google Scholar]
- Gorman-Lewis D, Aryal BP, Paunesku T, Vogt S, Lai B, Woloschak GE, Jensen MP. Direct determination of the intracellular oxidation state of plutonium. Inorg Chem. 2011;50(16):7591–7. doi: 10.1021/ic200588p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley B, Wang Q, Wanzer B, Vogt S, Finney L, Yang PL, Paunesku T, Woloschak G. 2011 Past and Future Work on Radiobiology Mega-Studies: A Case Study at Argonne National Laboratory. Health Phys. 2011;100(6):613–621. doi: 10.1097/HP.0b013e3181febad3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MD, Alderden RA, Zhang M, Beale PJ, Cai Z, Lai B, Stampfl AP, Hambley TW. The fate of platinum(II) and platinum(IV) anti-cancer agents in cancer cells and tumours. J Struct Biol. 2006;155(1):38–44. doi: 10.1016/j.jsb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Harris HH, Levina A, Dillon CT, Mulyani I, Lai B, Cai Z, Lay PA. Time-dependent uptake, distribution and biotransformation of chromium(VI) in individual and bulk human lung cells: application of synchrotron radiation techniques. J Biol Inorg Chem. 2005;10:105–18. doi: 10.1007/s00775-004-0617-1. [DOI] [PubMed] [Google Scholar]
- Kehr S, Malinouski M, Finney L, Vogt S, Labunskyy VM, Kasaikina MV, Carlson BA, Zhou Y, Hatfield DL, Gladyshev VN. X-ray fluorescence microscopy reveals the role of selenium in spermatogenesis. J Mol Biol. 2009;389(5):808–18. doi: 10.1016/j.jmb.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, Vogt S, O’Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol. 2010;6(9):674–81. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Lim ZJ, Gwee YY, Levina A, Lay PA. Characterization of a ruthenium(III)/NAMI-A adduct with bovine serum albumin that exhibits a high anti-metastatic activity. Angew Chem Int Ed Engl. 2010;49(9):1661–4. doi: 10.1002/anie.200906079. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li L, Gao Y, Chen C, Li B, He W, Huang Y, Chai Z. Distribution of metalloproteins in hepatocellular carcinoma and surrounding tissues. Hepatogastroenterology. 2007;54:2291–96. [PubMed] [Google Scholar]
- Lobinski R, Moulin C, Ortega R. Imaging and speciation of trace elements in biological environment. Biochimie. 2006;88:1591–1604. doi: 10.1016/j.biochi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Malinouski M, Kehr S, Finney L, Vogt S, Carlson BA, Seravalli J, Jin R, Handy DE, Park TJ, Loscalzo J, Hatfield DL, Gladyshev VN. High-Resolution Imaging of Selenium in Kidneys: a Localized Selenium Pool Associated with Glutathione Peroxidase 3. Antioxid Redox Signal. 2012;16(3):185–92. doi: 10.1089/ars.2011.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]; McCormick N, Velasquez V, Finney L, Vogt S, Kelleher SL. X-ray fluorescence microscopy reveals accumulation and secretion of discrete intracellular zinc pools in the lactating mouse mammary gland. PLoS One. 2010;5(6):e11078. doi: 10.1371/journal.pone.0011078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalyov JI, Buldakov LA, Lubchansky ER, Kalmykova ZI, Niphatov AP, Rudnitskaya EI, Zalikin GA, Filippova LG. In: Issues of Am-241 radiobiology. Moskalyov JI, editor. M.: Atomizdat; 1977. p. 168. [Google Scholar]
- Moskalyov JI, Buldakov ЛA, Zhuravlyova AK, Zalikin GA, Karpova VN, Kreslov VV, Levdik TI, Lemberg VK, Lubchansky ER, Mushkachyova GS, Sevostyanova EP, Khalturin GV. In: Neptumium-237 toxicology and radiobiology. Moskalyov JI, editor. M.: Atomizdat; 1979. p. 96. [Google Scholar]
- Mounicou S, Szpunar J, Lobinski R. Metallomics: the concept and methodology. Chem Soc Rev. 2009;38:1119–1138. doi: 10.1039/b713633c. [DOI] [PubMed] [Google Scholar]
- Muksinova KN, Mushkachyova GS. In: Cellular and molecular foundations of blood formation alterations at protracted radiation exposure. Guslova AK, editor. M.: Energoatomizdat; 1990. p. 160. [Google Scholar]
- Mushkachyova GS, Rusinova GG, Muksinova KN, Lemberg VK, Shorokhova VB, Revina VS, Uryadnitskaya TI. In: Genetic organizations and tritium. Buldakov LA, editor. M.: Energoatomizdat; 1994. p. 192. [Google Scholar]
- Nakazawa E, Ikemoto T, Hokura A, Terada Y, Kunito T, Tanabe S, Nakai I. The presence of mercury selenide in various tissues of the striped dolphin: evidence from μ-XRF-XRD and XAFS analyses. Metallomics. 2011a;3(7):719–25. doi: 10.1039/c0mt00106f. [DOI] [PubMed] [Google Scholar]
- Nakazawa E, Ikemoto T, Hokura A, Terada Y, Kunito T, Yamamoto T, Yamada TK, Rosas FC, Fillmann G, Tanabe S, Nakai I. Silver speciation in liver of marine mammals by synchrotron X-ray absorption fine structure and X-ray fluorescence spectroscopies. J Environ Monit. 2011b;13(6):1678–86. doi: 10.1039/c1em10115c. [DOI] [PubMed] [Google Scholar]; Nicolis I, Curis E, Deschamps P, Bénazeth S. Arsenite medicinal use, metabolism, pharmacokinetics and monitoring in human hair. Biochimie. 2009;91(10):1260–7. doi: 10.1016/j.biochi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292(5526):2488–92. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- Paunesku T, Vogt S, Irving TC, Lai B, Barrea RA, Maser J, Woloschak GE. Biological applications of X-ray microprobes. Int J Radiat Biol. 2009;85(8):710–3. doi: 10.1080/09553000903009514. [DOI] [PubMed] [Google Scholar]
- Paunesku T, Vogt S, Maser J, Lai B, Woloschak G. X-ray fluorescence microprobe imaging in biology and medicine. J Cell Biochem. 2006;99(6):1489–502. doi: 10.1002/jcb.21047. [DOI] [PubMed] [Google Scholar]
- Pereira GR, Rocha HS, Anjos MJ, Farias PC, Pérez CA, Lopes RT. Elemental distribution mapping on breast tissue samples. Eur J Radiol. 2008;68(3 Suppl):S104–8. doi: 10.1016/j.ejrad.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Petibois C, Cestelli Guidi M. Bioimaging of cells and tissues using accelerator-based sources. Anal Bioanal Chem. 2008;391(5):1599–608. doi: 10.1007/s00216-008-2157-y. [DOI] [PubMed] [Google Scholar]
- Ralle M, Huster D, Vogt S, Schirrmeister W, Burkhead JL, Capps TR, Gray L, Lai B, Maryon E, Lutsenko S. Wilson disease at a single cell level: intracellular copper trafficking activates compartment-specific responses in hepatocytes. J Biol Chem. 2010;285(40):30875–83. doi: 10.1074/jbc.M110.114447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralle M, Lutsenko S. Quantitative imaging of metals in tissues. Biometals. 2009;22(1):197–205. doi: 10.1007/s10534-008-9200-5. [DOI] [PubMed] [Google Scholar]
- Tainer JA, Roberts VA, Getzoff ED. Metal-binding sites in proteins. Curr Opin Biotechnol. 1991;2(4):582–91. doi: 10.1016/0958-1669(91)90084-i. [DOI] [PubMed] [Google Scholar]
- Thompson R. Life Span effects of Ionizing Radiation in Beagle Dogs. 1. Pacific Northwest National Laboratory; Richland, WA: 1989. [Google Scholar]
- Wolford JL, Chishti Y, Jin Q, Ward J, Chen L, Vogt S, Finney L. Loss of pluripotency in human embryonic stem cells directly correlates with an increase in nuclear zinc. PLoS One. 2010;205(8):e12308. doi: 10.1371/journal.pone.0012308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, McRae R, Henary MM, Patel R, Lai B, Vogt S, Fahrni CJ. Imaging of the intracellular topography of copper with a fluorescent sensor and by synchrotron x-ray fluorescence microscopy. Proc Natl Acad Sci U S A. 2005;102:11179–11184. doi: 10.1073/pnas.0406547102. [DOI] [PMC free article] [PubMed] [Google Scholar]


