Summary
The intestinal mucus is an efficient system for protecting the epithelium from bacteria by promoting their clearance and separating them from the epithelial cells, thereby inhibiting inflammation and infection. The function of the colon inner mucus layer is especially important as this explains how we can harbor the large number of bacteria in our gut. The major component of this mucus system is the MUC2 mucin which organizes the mucus by its enormously large net-like polymers. Pathogenic microorganisms, in turn, have developed mechanisms for circumventing this well-organized mucus protective system.
Introduction
The gastrointestinal tract has a large surface exposed to the intestinal content and is as such a major entry point for pathogens. The organism’s defense system against this challenge can be viewed as consisting of several ‘levels’. The first is the stratified mucus layer which together with the glycocalyx of the epithelial cells provides physical protection. This first defense line is the focus of this review. The second is the single layer of epithelial cells that form a continuous cell sheet interconnected with tight junctions. Here, the goblet cells produce the mucus, and the other major cell type, the enterocytes, have key regulatory roles in mastering the interaction with the microbiota. Both these defense lines belong to the innate immune system together with the third level – resident macrophages and dendritic cells of the intestinal stroma. Finally, the adaptive immune system builds a fourth defense line both as master regulator and as an inducible system to remove microbiota that have sidestepped earlier defense lines.
The gastrointestinal tract
A digestive tract is required for all advanced multicellular organisms to provide the host with both sufficient energy and molecular building blocks. The organization of this into a stable system is a formidable challenge as it must degrade and absorb ingested food at the same time as it must handle the load of ingested microorganisms. As we understand it today, this task is performed using basically the same principle in all animals, but with slightly different molecular solutions. The one found in most species is based on mucus layers built around the mucins [1]. This conclusion is based on the observation that the gel-forming mucins appear already at the level of early metazoans during evolution [2]. An exception is the insects, where the intestine is protected by a chitin mesh that is reinforced by mucin type molecules anchored via their chitin binding domains [3]. Utilizing these two molecular solutions, all organisms have a proximal and distal part of their intestine that is protected with a physical barrier interrupted by a less protected central part that allows absorption of nutrients. Importantly, the use of highly glycosylated molecules which cannot be degraded by the host as the main building block is common to both insects and higher multicellular organisms.
Gastrointestinal mucus and mucins
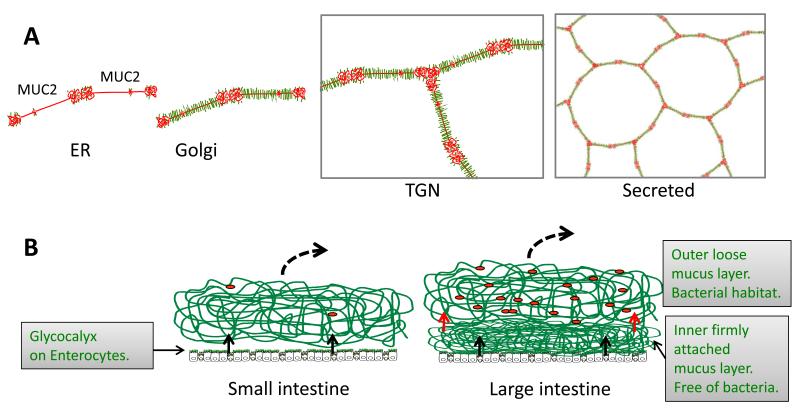
The gastrointestinal tract is covered by mucus, as revealed by detailed studies in rodents and humans [4;5]. The stomach and colon have a two-layered mucus with an inner mucus layer that is 50-200 μm thick and firmly attached to the epithelium (Fig. 1). The outer mucus layer is easily removed and has a less defined outer border. In contrast to colon, the small intestine has only one layer of mucus, which is possible to remove by aspiration. Normal mucus is totally transparent and microscopically invisible as it is made up of more than 98% water. This has made the mucus difficult to study, as it will collapse as soon as it is not well hydrated and lost when the tissue is fixed with formaldehyde. Recently, we learnt that the surface of mucus can be visualized with charcoal and that the mucus gel is relatively well preserved in Carnoy’s fixative [4;5]. Fig. 1B shows a schematic view of the mucus system of the intestine.
Fig.1.
Schematic outline of the MUC2 mucin and its formation of mucus in the small and large intestine. A. Assembly of the MUC2 mucin (protein core red) into dimeric forms in the endoplasmic reticulum (ER), O-glycosylation (green) in the Golgi apparatus, formation of trimeric forms in the trans Golgi network (TGN) and a schematic picture of the secreted MUC2 polymer. B. The MUC2 mucin is secreted from the goblet cells (black arrows) to form the mucus. Colon have a two layered mucus where the inner layer is converted (red arrows) to the outer mucus layer. The stomach has a mucus essentially as that of colon (not shown). Red dots symbolizes bacteria.
So far only the mucus of the mouse colon has been thoroughly studied for its proteome [5;6]. These studies have revealed a number of proteins which are likely to be important constituents for the formation of a stable mucus. However, it is already clear that the gel-forming mucins make up the mucus skeleton and provide most of the properties of the mucus. The major gel-forming mucin of the stomach is called MUC5AC and that of the intestine MUC2. These two mucins are more similar to each other than to the three other members found in the human genome. Although the MUC2 mucin is the most well-studied, most conclusions are probably valid for the MUC5AC mucin as well.
The MUC2 mucin encodes a protein of approximately 5,200 amino acids (an exact figure cannot be provided as the gene has not been fully sequenced yet) [7;8]. Its central part contains two so-called PTS domains [2] which are rich in the amino acids proline (P), threonine (T) and serine (S) [9]. PTS domains are often highly repetitive (as in the second MUC2 domain) but lack sequence conservation between species. The abundant hydroxy amino acids act as attachment sites for the O-glycans. Once the mucin apoprotein reaches the Golgi apparatus, it is densely decorated by consecutive additions of monosaccharides, a modification which turns these domains into long, stiff bottle brush-like rods where the glycans make up to more than 80% of the mass (Fig. 1A). These highly glycosylated domains are called mucin domains and characterize all mucins. The O-glycans make the mucin domains highly protease resistant [10] and give mucins their high water-binding capacity. Commensal bacteria use these glycans as an important energy source [1].
The MUC2 mucin forms an enormous net-like polymer. The C-termini of individual MUC2 molecules are linked by disulfide bonds into dimeric and the N-termini into trimeric complexes (Fig. 1A). Theoretically, this will lead to flat net-like polymers built around 6-cornered rings as shown in Fig. 1A, right [11;12]. This type of structures have the potential to spontaneously form sheets which in turn would give rise to stratified mucus layers. Such stratification has indeed been observed in the inner mucus layer of colon [5]. In addition to the disulfide bond-stabilized core of the MUC2 mucin, there are additional covalent cross-links that are less well studied [6;13;14]. Moreover, the MUC2 mucin, as well as most other gel-forming mucins, contain CysD domains [2]. In MUC2, one such domain is found on each side of the small mucin domain. CysD domains have been shown to engage in non-covalent dimeric interactions and by this they have the potential to stabilize the interaction of different MUC2 sheets in the stratified mucus layer [15]. Together, our current understanding of mucins suggests that most of the properties of mucus is built into the mucin macromolecule and the network formed upon its polymerization [9].
Commensal bacteria and the two mucus layers of the colon
The inner layer of the colonic mucus is attached to the epithelium, shows a compact and stratified appearance and ranges in thickness is from 50 μm (mouse) and up to several hundred micrometers in humans. Its most remarkable feature is that this inner mucus layer normally is devoid of bacteria. The mucus probably accomplishes this by acting as a filter where for example bacteria are too large to enter [5;9;16]. The inner mucus layer is renewed from below by secretion of the goblet cells and at its lumenal border it is converted to the outer mucus layer (red arrows in Fig. 1B). This outer layer is not attached to the epithelium and expands 4-5 fold in volume upon conversion due to endogenous protease activities acting on the MUC2 mucin. Expansion allows the endogenous bacteria to enter the outer mucus layer, creating a habitat for the intestinal microbiota [9].
The human commensal flora is dominated by Fermicutes and Bacteriodetes [17]. There is currently no deeper understanding of why humans have this flora and why other species have other types of bacteria. However, it is interesting to consider that the O-glycans found on the MUC2 mucin of sigmoid colon differ from those of the small intestine, stomach, and lungs by being almost identical between different individuals irrespective of their blood group status [18]. This could suggest that the mucin glycans are part of a mechanism for selecting our microbiota, maybe because the bacteria carries adhesins that are compatible with the host glycans.
The commensal bacteria have a high proportion of their genomes devoted to enzymes involved in glycan degradation [19;20]. These are typically exoglycosidase in nature, removing one monosaccharide at a time [21]. As the number of different glycans on a single MUC2 molecule is high, typically >100 different oligosaccharide species, many different enzymes would be required for their digestion [18]. This also suggests that the removal of all the glycans will take time, something that is probably important for the protection of colon. Dr. Lijun Xia et al. could recently show that mice lacking the Core 1 enzyme (which synthesizes the major type of extension of the GalNAc attached to the protein core) develop spontaneous colitis [22]. Although, the mechanism for this is not yet established, it is likely that the inner mucus layer of the mutant mice is less efficient in excluding bacteria and the mucins are probably degraded quicker. Preliminary estimates of the MUC2 mucin turnover in the distal colon suggest a half-life of only a few hours, implying that a continuous renewal of the inner mucus layer is crucially important.
The released mucin monosaccharides are used by the commensal bacteria as an energy source in addition to undigested carbohydrates from the food [1]. The relative importance of these different carbohydrate sources is not well studied, but would of course vary dependent on the food intake. In any case, the released monosaccharides are converted into short fatty acids by bacterial metabolism and these can diffuse through the inner mucus layer. By this route, the carbohydrates provide a major energy source to the epithelium and the host. As a large amount of energy is spent on building the quickly turned over mucins and mucus, recovery of most of this energy with the help of the commensal bacteria is probably important for the host.
The mucus layer of the small intestine
The small intestine is covered by a single layer of mucus built around the MUC2 mucin [4;7;8]. This mucus is not attached to the epithelium and is also more permeable to bacteria (Fig. 1B). The mucins are normally released at the crypt openings and the mucus will cover the villi. The small intestine has a low bacterial density compared to colon, due to the fast transit and its efficient trapping and distal transport of bacteria [23]. Hooper et al have recently shown that the antibacterial protein RegIIIγ secreted by the enterocytes limits the contact of the gram-positive bacteria with the epithelial cells, but does not lower the number of bacteria in the intestinal lumen [24]. In the absence of this protein, bacteria come in contact with the epithelial cells. RegIIIγ is retained in the mucus after its secretion and by this generates a physical separation between the epithelium and bacteria [23]. The antibacterial peptides produced by the Paneth cells keep the crypts free of bacteria and probably also contribute, together with secreted IgA, to further limiting bacterial growth in the small intestine [25].
Bacteria can be bound to the mucus in several ways. First, the bacteria can be trapped in the pores of the MUC2 network although the small intestinal mucus has larger pores than in colon. Secondly, although the mucins are largely hydrophilic due to their glycan decoration, they also have hydrophobic properties probably conferred by their CysD domains [15]. These domains are suggested to be a major reason of the well-known stickiness of mucus. Thirdly, the polymorphic and variable mucin glycans will bind bacteria carrying adhesins that have specificities for these glycan structures.
Mechanisms for microorganisms to circumvent the mucus layers
The major function of the mucus is to limit bacterial contact with the epithelium and transport bacteria distally [26]. However, this system is not perfect and especially pathogenic bacteria have developed methods to circumvent it. As we still lack some understanding of how mucin and mucus function, we only have limited knowledge of the mechanisms for how pathogenic bacteria avoid this primary protection system. A few aspects and examples will be discussed here.
Small intestine
Pathogens infecting the small intestine have to avoid being trapped by the mucus. It is important for these pathogens to move efficiently towards the epithelium and against the flow caused by secretion and renewal of the mucus. This is reflected in the observation that many intestinal pathogenic bacteria have flagella [27;28]. Bacteria lacking adhesins specific for the mucin glycans of the specific host are probably better off in this environment as they will not be as efficiently trapped. Interestingly, it was noticed several years ago that bacterial adhesins often bind to glycosphingolipids which carry several glycan substructures that are not found among the mucin glycans [29;30]. As glycosphingolipids are abundant in the apical membranes of the enterocytes, these could act as anchoring sites for the bacteria once they have reached the epithelial cells. This and other similar mechanisms will allow specific bacteria to reside below the mucus layer.
Salmonella is an invasive bacterium which needs to circumvent the innate immune system to reach the epithelium [27]. In order to come in contact with the epithelium and swim against the mucus flow, its flagella must be able to propel the bacteria towards the epithelial cells directed by chemotaxis [28]. It has also recently been suggested that the IFNγ receptor mediated signaling triggered by Salmonella infection can affect the goblet cells in a for the bacteria beneficial way, something that could further facilitate bacterial penetration [31].
Stomach
Helicobacter pylori – The stomach has a two layered mucus system similar to the one found in colon (Fig. 1B) [4]. The attached inner mucus layer maintains a pH gradient with a pH of 2 at the lumen and 7 at the epithelial surface [32]. Few bacteria have developed mechanisms to infect this organ, but H. pylori is a specialist which can cause both gastric ulcer and cancer. An important virulence factor is the adhesin BabA which binds the blood group antigen Lewis b (Leb) and related antigens [33]. As mucins of the stomach also carries Leb antigens, modulation of attachment is probably dynamic during the primary infection when H. pylori penetrate the mucus [34]. In fact, recent observations suggest modulation of BabA expression during colonization [35]. It is also possible that H. pylori mucus interactions can be altered by the mucus pH gradient. The inner mucus layer of the stomach can drastically change its properties as it opens pores in the mucus, probably in a pH-dependent manner, to allow the secretion of hydrochloric acid from the glands [36]. It has also been suggested that H. pylori can alter the mucus viscosity while moving through the mucus gel [37]. Lindén et al. could recently show that also the non-gelforming transmembrane MUC1 mucin limits H. pylori infection both by steric hindrance and by acting as a releasable decoy which washes away the bacteria [38]. The mechanism by which H. pylori penetrates the mucus and infects the host is however complex and far from understood [34].
Colon
The parasite Entamoeba histolytica has a Gal-binding lectin that anchors the parasite to the colon mucus, probably to the attached inner mucus layer. This allows the parasite to reside in the colon [39]. The specificity for Gal/GalNAc of this lectin is in line with E. histolytica binding to the glycans on the MUC2 mucin [18]. Once bound, the parasite turns on a program which induces the expression of a cysteine protease necessary for invasion [40]. This enzyme is able to degrade the MUC2 mucin by cleavage at a very specific site localized N-terminally of the first Cys amino acid in the MUC2 C-terminus [41]. The cysteines in this part of MUC2 form numerous disulfide bonds which stabilize the protein and make it resistant to many proteases. A second E. histolytica cleavage site further C-terminally is surrounded by disulfide bonds and a cleavage here will not disrupt the polymer. Cleavage with the E. histolytica cysteine protease at the first site will dissolve the inner mucus layer of colon and thus allow the parasite to reach the epithelium. E. histolytica can then attach to the epithelium and penetrate the intestine to cause the systemic infection typical of this disease. The amino acid sequence containing the E. histolytica cleavage site in the human MUC2 polymer is absent in rodents, something that may explain why rodents are not infected with E. histolytica [41]. It should also be noted that only a portion of humans infected with E. histolytica have invasive disease, a finding that might be related to variable MUC2 glycosylation which may affect cleavage at this site.
Simple experiments have shown that fecal bacteria can secrete proteolytic activities that are capable of cleaving the protein core of the MUC2 mucin [42]. This suggests the existence of bacteria that can cleave MUC2 and by this maybe also disrupt the MUC2 network. Interestingly, several commensal bacteria belonging to the Lactobacilli and Bacteroidetes species do not secrete such proteases [42]. This observation suggests, as expected, that commensal bacteria are not able to disrupt the inner mucus of colon. However, there are other bacteria which can dissolve the inner mucus and if these bacteria are efficient, these and other bacteria can reach the epithelial cells. This situation is similar to the one observed in mice lacking the MUC2 mucin or having defects in MUC2 glycosylation, where severe colitis develops [22;43;44].
The robustness of the inner mucus layer in maintaining a bacteria-free shield is challenging for pathogens affecting the colon [26]. Little is known of this, but in a mouse colon infection model (Citrobacter rodentium), the bacteria reside below the inner mucus layer by a mechanism similar to one described for H. pylori [45].
Inflammation
In the absence of MUC2, as in the Muc2-null mice, the bacteria are in direct contact with the epithelial cells. The bacteria penetrate down into the otherwise sterile crypts and are also found inside the enterocytes [5;43]. These mice develop, depending on the animal housing and genetic background, a more or less severe colon inflammation with infiltration of both neutrophils and lymphocytes, diarrhea, rectal prolapses and failure to thrive [5;46]. The Muc2-null mice also develop cancer after several months, further suggesting that the phenotype of these mice resembles the human disease ulcerative colitis [43]. A further illustration of the importance of the inner mucus layer for protection of colon is shown by the observation that the most commonly used model for colitis in rodents, dextran sulfate treatment, causes the inner mucus layer to become permeable to bacteria several days before any inflammation is observed [16].
Concluding remarks
The importance of the intestinal mucus has recently been rediscovered, something that is timely with the characterization of the human intestinal microbiota by novel sequencing methods. The organization of the inner mucus layer of colon is very important for homeostasis as defects in or loss of this allows bacteria to reach the epithelium and trigger inflammation. An understanding of the formation of the mucus layer and how the properties are altered, as triggered by intestinal microbiota and the host immune system, will provide new insight into colon inflammation and the poorly understood disease ulcerative colitis.
Acknowledgments
This work was supported by the Swedish Research Council (no. 7461, 21027, and 342-2004-4434), The Swedish Cancer Foundation, The Knut and Alice Wallenberg Foundation (KAW2007.0118), IngaBritt and Arne Lundberg Foundation, Sahlgren’s University Hospital (LUA-ALF), EU-FP7 IBDase (no. 200931), Wilhelm and Martina Lundgren’s Foundation, Torsten och Ragnar Söderbergs Stiftelser, The Sahlgrenska Academy, and The Swedish Foundation for Strategic Research - The Mucus-Bacteria-Colitis Center (MBC) of the Innate Immunity Program (2010-2014). Dr. Dan Baeckström is acknowledged for editing the text.
References
- 1.Kim YS, HO SB. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr.Gastroenterol.Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •2.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc.Natl.Acad.Sci.USA. 2007;104:16209–16214. doi: 10.1073/pnas.0705984104. Describes the evolution of mucins and especially that the typical domain arrangement in the gelforming mucins is found already among metazoans.
- 3.Syed ZA, Hard T, Uv A, van Dijk-Hard IF. A Potential Role for Drosophila Mucins in Development and Physiology. PLoS ONE. 2008;3:e3041. doi: 10.1371/journal.pone.0003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••4.Atuma C, Strugula V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am.J.Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. The mucus thickness throughout the gastrointestinal tract was measured on live, anaestesized rats.
- ••5.Johansson MEV, Phillipson M, Petersson J, Holm L, Velcich A, Hansson GC. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc.Natl.Acad.Sci.USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. This show that the inner mucus layer is separating the colon bacteria from the epithelial cells. It also shows that lack of this mucus layer allow bacteria to contact the epithelia, enter the crypts and the the epithelial cells, something that triggers inflammation.
- •6.Johansson MEV, Thomsson KA, Hansson GC. Proteomic Analyses of the Two Mucus Layers of the Colon Barrier Reveal That Their Main Component, the Muc2 Mucin, Is Strongly Bound to the Fcgbp Protein. J.Proteome Res. 2009;8:3549–3557. doi: 10.1021/pr9002504. This shows the mucus proteome of the colon mucus and that the Fcgbp protein is covalently attached to the Muc2 mucin.
- 7.Hansson GC, Baeckstrom D, Carlstedt I, Klinga-Levan K. Molecular Cloning of a cDNA Coding for a Region of an Apoprotein from the Insoluble Mucin Complex of Rat Small Intestine. Biochem.Biophys.Res.Commun. 1994;198:181–190. doi: 10.1006/bbrc.1994.1026. [DOI] [PubMed] [Google Scholar]
- 8.Gum JR, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J.Biol.Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 9.Johansson MEV, Holmen Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc.Natl.Acad.Sci.USA. 2011;108:4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlstedt I, Herrmann A, Karlsson H, Sheehan JK, Fransson L, Hansson GC. Characterization of two different glycosylated domains from the insoluble mucin complex of rat small intestine. J.Biol.Chem. 1993;268:18771–18781. [PubMed] [Google Scholar]
- 11.Asker N, Axelsson MAB, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J.Biol.Chem. 1998;273:18857–18863. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- •12.Godl K, Johansson MEV, Karlsson H, Morgelin M, Lidell ME, Olson FJ, Gum JR, Kim YS, Hansson GC. The N-termini of the MUC2 mucin form trimers that are held together within a trypsin-resistant core fragment. J.Biol.Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. The N-termial part of the MUC2 mucin forms trimeric complexes in contrast to the at the time assumed dimeric complexes. This suggested for the first time that the MUC2 mucin forms large net-like polymers.
- 13.Axelsson MAB, Asker N, Hansson GC. O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J.Biol.Chem. 1998;273:18864–18870. doi: 10.1074/jbc.273.30.18864. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann A, Davies JR, Lindell G, Martensson S, Packer NH, Swallow DM, Carlstedt I. Studies on the “Insoluble” glycoprotein complex from human colon. J.Biol.Chem. 1999;274:15828–15836. doi: 10.1074/jbc.274.22.15828. [DOI] [PubMed] [Google Scholar]
- 15.Ambort D, van der Post S, Johansson MEV, MacKenzie J, Thomsson E, Krengel U, Hansson GC. Function of the CysD domain of the gel-forming MUC2 mucin. Biochem.J. 2011;436:61–70. doi: 10.1042/BJ20102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson MEV, Gustafsson JK, Sjoberg KE, Pettersson J, Holm L, Sjovall H, Hansson GC. Bacteria penetrate the inner mucus layer before inflammation in the Dextran sulfate colitis model. PLoS ONE. 2010;5:e12238. doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-Bacterial Mutualism in the Human Intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- ••18.Holmen Larsson JM, Karlsson H, Sjovall H, Hansson GC. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology. 2009;19:756–766. doi: 10.1093/glycob/cwp048. Studies of sigmoid biopsies from 50 human indiviguals show that the O-glycans of the MUC2 mucin is almost identical in contrast to the mucins from other organs. This suggested for the first time that mucin glycosylation could be part of selective mechanisms for the commensal flora.
- •19.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. This describes the first deep sequencing of the human microbiota.
- 20.van Passel MWJ, Kant R, Zoetendal EG, Plugge CM, Derrien M, Malfatti SA, Chain PSG, Woyke T, Palva A, de Vos WM, Smidt H. The Genome of Akkermansia muciniphila, a Dedicated Intestinal Mucin Degrader, and Its Use in Exploring Intestinal Metagenomes. PLoS ONE. 2011;6:e16876. doi: 10.1371/journal.pone.0016876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Png CW, Linden SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin THJ. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa Augment In Vitro Utilization of Mucin by Other Bacteria. Am J Gastroenterol. 2010 doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- ••22.Fu J, Wei B, Wen T, Johansson MEV, Xiaowei Liu X, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, McDaniel M, Sferra TJ, Turner J, Chen H, Hansson GC, Braun J, Xia L. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis. J.Clin.Invest. 2011;121:1657–1666. doi: 10.1172/JCI45538. Truncation of the O-glycans by removing the Core 1 enzyme give colitis. This is the first time that it has been shown that the mucin glycans are very important for protecting colon.
- 23.Johansson MEV, Hansson GC. Keeping Bacteria at a Distance. Science. 2011;334:182–183. doi: 10.1126/science.1213909. [DOI] [PubMed] [Google Scholar]
- ••24.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The Antibacterial Lectin RegIII+| Promotes the Spatial Segregation of Microbiota and Host in the Intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. This describes for the first time that bacteria are kept at a distance from the epithelial cells also in the small intestine.
- 25.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA Response to Symbiotic Bacteria as a Mediator of Gut Homeostasis. Cell Host & Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 26.McGuckin MA, Lind+⌝n SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Micro. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 27.Hornef MW, Wick MJ, Rhen M, Normark S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nature Immunology. 2002;3:1033–1040. doi: 10.1038/ni1102-1033. [DOI] [PubMed] [Google Scholar]
- 28.Stecher B, Hapfelmeier S, Muller C, Kremer M, Stallmach T, Hardt WD. Flagella and Chemotaxis Are Required for Efficient Induction of Salmonella enterica Serovar Typhimurium Colitis in Streptomycin-Pretreated Mice. Infection and Immunity. 2004;72:4138–4150. doi: 10.1128/IAI.72.7.4138-4150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansson GC, Karlsson KA, Larson G, Stromberg N, Thurin J. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: A rationalized approach to the study of host cell glycolipid receptors. Analytical Biochemistry. 1985;146:158–163. doi: 10.1016/0003-2697(85)90410-5. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson KA. Animal glycosphingolipids as membrane attachment sites for bacteria. Ann.Rev.Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- 31.Songhet P, Berthel M, Stecher B, Muller AJ, Kremer M, Hansson GC, Hardt WD. Stromal IFN-gR-Signaling Modulates Goblet Cell Function During Salmonella Typhimurium Infection. PlosONE. 2011;6:e22459. doi: 10.1371/journal.pone.0022459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schade C, Flemstrom G, Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology. 1994;107:180–188. doi: 10.1016/0016-5085(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 33.Boren T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to Human Gastric Epithelium Mediated by Blood Group Antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- •34.Moore ME, Boren T, Solnick JV. Life at the margins: Modulation of attachment proteins in Helicobacter pylori. Gut Microbes. 2011;2:42–46. doi: 10.4161/gmic.2.1.14626. This review discusses the complex and intricate control that Helicobacter pylori has developed to circumvent the mucus and other protective system of the stomach.
- 35.Styer CM, Hansen LM, Cooke CL, Gundersen AM, Choi SS, Berg DE, Benghezal M, Marshall BJ, Peek RM, Jr., Boren T, Solnick JV. Expression of the BabA Adhesin during Experimental Infection with Helicobacter pylori. Infection and Immunity. 2010;78:1593–1600. doi: 10.1128/IAI.01297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson M, Synnerstad I, Holm L. Acid transport through channels in the mucous layer of rat stomach. Gastroenterology. 2000;119:1297–1304. doi: 10.1053/gast.2000.19455. [DOI] [PubMed] [Google Scholar]
- 37.Celli JP, Turner BS, Afdhal NH, Keates S, Ghiran I, Kelly CP, Ewoldt RH, McKinley GH, So P, Erramilli S, Bansil R. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. PNAS. 2009;106:14321–14326. doi: 10.1073/pnas.0903438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linden SK, Sheng YH, Every AL, Miles KM, Skoog EC, Florin THJ, Sutton P, McGuckin MA. MUC1 Limits Helicobacter pylori Infection both by Steric Hindrance and by Acting as a Releasable Decoy. PLoS Pathog. 2009;5:e1000617. doi: 10.1371/journal.ppat.1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petri WA, Haque R, Mann BJ. The Bittersweet Interface of Parasite and Host: Lectin- Carbohydrate Interactions During Human Invasion by the Parasite Entamoeba histolytica. Annu.Rev.Microbiol. 2002;56:39–64. doi: 10.1146/annurev.micro.56.012302.160959. [DOI] [PubMed] [Google Scholar]
- 40.Moncada D, Keller K, Chadee K. Entamoeba histolytica cysteine proteinases disrupt the polymeric structure of colonic mucin and alter its protective function. Infec.Immunity. 2003;71:838–844. doi: 10.1128/IAI.71.2.838-844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •41.Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal part and dissolves the protective colonic mucus gel. Proc.Nat.Acad.Sci.Usa. 2006;103:9298–9393. doi: 10.1073/pnas.0600623103. A specific protease secreted by a microorganism cleave MUC2 mucin at a specific site and by this dissolves the mucin polymers. As later understood, it is the inner firmly attached mucus layer that is dissolved, something this is necessary for the parasite to reach the epithelial cells.
- 42.Subramani DB, Johansson MEV, Dahlén G, Hansson GC. Lactobacillus and Bifidobacterium species do not secrete proteases that can cleave the MUC2 mucin protein core which organizes the colon mucus layers. Beneficial Microbes. 2010;1:343–350. doi: 10.3920/BM2010.0039. [DOI] [PubMed] [Google Scholar]
- ••43.Velcich A, Yang WC, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal Cancer in Mice Genetically Deficient in the Mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. This show that mice lacking the Muc2 mucin develop spontaneous colitis and later colon cancer.
- 44.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, Adams R, Kato M, Nelms KA, Hong NA, Florin THJ, Goodnow CC, McGuckin MA. Aberrant Mucin Assembly in Mice Causes Endoplasmic Reticulum Stress and Spontaneous Inflammation Resembling Ulcerative Colitis. PLoS Medicine. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergstrom KSB, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA. Muc2 Protects against Lethal Infectious Colitis by Disassociating Pathogenic and Commensal Bacteria from the Colonic Mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van der Sluis M, De Koning BAE, De Bruijn ACJM, Velcich A, Meijerink JPP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AWC. Muc2- Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]