Abstract
Study objective
We determine whether imaging with cardiac magnetic resonance imaging (MRI) in an observation unit would reduce medical costs among patients with emergent non-low-risk chest pain who otherwise would be managed with an inpatient care strategy.
Methods
Emergency department patients (n=110) at intermediate or high probability for acute coronary syndrome without electrocardiographic or biomarker evidence of a myocardial infarction provided consent and were randomized to stress cardiac MRI in an observation unit versus standard inpatient care. The primary outcome was direct hospital cost calculated as the sum of hospital and provider costs. Estimated median cost differences (Hodges-Lehmann) and distribution-free 95% confidence intervals (Moses) were used to compare groups.
Results
There were 110 participants with 53 randomized to cardiac MRI and 57 to inpatient care; 8 of 110 (7%) experienced acute coronary syndrome. In the MRI pathway, 49 of 53 underwent stress cardiac MRI, 11 of 53 were admitted, 1 left against medical advice, 41 were discharged, and 2 had acute coronary syndrome. In the inpatient care pathway, 39 of 57 patients initially received stress testing, 54 of 57 were admitted, 3 left against medical advice, and 6 had acute coronary syndrome. At 30 days, no subjects in either group experienced acute coronary syndrome after discharge. The cardiac MRI group had a reduced median hospitalization cost (Hodges-Lehmann estimate $588; 95% confidence interval $336 to $811); 79% were managed without hospital admission.
Conclusion
Compared with inpatient care, an observation unit strategy involving stress cardiac MRI reduced incident cost without any cases of missed acute coronary syndrome in patients with emergent chest pain.
INTRODUCTION
Background
Current guidelines from the American College of Cardiology and the American Heart Association1 suggest that patients being evaluated for acute coronary syndrome without diagnostic ECGs or biomarkers may be further evaluated in an observation unit. In practice, patients at intermediate or high probability of experiencing acute coronary syndrome, such as patients with previous myocardial infarction, diabetes, or advanced age, are commonly admitted to the hospital. After admission, these patients frequently receive highly variable and aggressive care, with cardiac catheterization rates as high as 37% to 56%.2-4
Cardiac magnetic resonance imaging (MRI) is an established stress-testing modality, with sensitivity and specificity superior to that of stress echocardiography5 and without the radiation exposure or radioisotope-related time delays associated with nuclear testing. It has proven to be highly accurate for identifying acute coronary syndrome in patients presenting with emergent chest pain in a research setting.6-8 Cardiac MRI testing is appealing because it can detect recent infarction before biomarker increase,8 identify inducible myocardial ischemia, and differentiate between new and old myocardial infarctions.7,9 The comprehensive information provided by stress cardiac MRI makes it appropriate for most non-low-risk patients with emergent chest pain, including those with known coronary artery disease.10 These attributes also make it well suited for protocol-driven rapid care units.
Importance
Improving the efficiency of chest pain evaluations through the use of observation units could reduce the risk of such evaluations. The strengths of cardiac MRI testing and its high accuracy suggest that cardiac MRI is an ideal test for integration into an observation unit care pathway. However, implementing a cardiac MRI testing program requires equipment purchases and personnel training; for these reasons, it is associated with “up-front” expenses. For this to be a worthwhile investment, a cardiac MRI strategy would have to represent an improvement over existing models of care delivery. Therefore, the cost associated with a cardiac MRI care strategy should be examined.
Goals of This Investigation
The purpose of this trial was to integrate cardiac MRI testing with observation unit care among patients with emergent non-low-risk chest pain and compare direct medical cost of this care pathway with that of conventional inpatient care.
MATERIALS AND METHODS
Study Design
A single-center randomized clinical trial was conducted from January 2008 to March 2009.
Setting
Participants were recruited from the emergency department (ED) at Wake Forest University Baptist Medical Center, a tertiary care hospital in Winston-Salem, NC, with a total annual volume of approximately 96,000 visits per year (including 29% pediatrics and 15% urgent care). The observation unit is under direction of the ED. The observation unit provides care to approximately 600 to 700 patients annually with low-risk chest pain, defined jointly by the care provider’s clinical impression and a Thrombolysis in Myocardial Infarction risk score11 less than or equal to 1. For this trial, all study participants gave written informed consent. The study protocol was Health Insurance Portability and Accountability Act compliant, approved by the institutional review board of the sponsoring institution, and registered at clinicaltrials.gov (NCT00678639). Participants were responsible for all costs associated with their medical care.
Selection of Participants
Participants were recruited by study personnel from 8 am to 11 pm Monday through Thursday and from 8 am to 11 am on Friday and when the cardiac MRI scanner had capacity for examinations within approximately 24 hours. Initial participant screening was conducted by using chief complaints or by discussion with care providers. Review of records or interviews were then conducted to determine eligibility. To be eligible, participants were required to have intermediate or high probability for experiencing acute coronary syndrome. Intermediate or high probability was defined by either the ED care provider’s clinical impression or a Thrombolysis in Myocardial Infarction risk score greater than or equal to 2. Providers were encouraged to use the American College of Cardiology/American Heart Association framework to formulate the clinical impression.1 The Thrombolysis in Myocardial Infarction risk score correlates with the likelihood of an ED patient’s experiencing acute coronary syndrome during the index visit or the subsequent 30 days.12 Additional inclusion criteria were aged 18 years or older, symptoms of possible acute coronary syndrome, care provider impression that inpatient evaluation was required, and ability to be discharged if cardiac disease was excluded. Patients were excluded for an initial increased troponin I level, new ST-segment elevation (≥1 mV) or depression (≥2 mV), inability to lie flat, systolic blood pressure less than 90 mm Hg, contraindications to MRI, refusal of follow-up procedures, terminal diagnosis with less than 3 months to live, pregnancy, renal insufficiency, chronic liver disease, or a history of heart, liver, or kidney transplant.
Interventions
Participants were randomized to one of 2 study groups with a stratified blocked randomization scheme. Stratification was conducted according to the presence of known coronary artery disease and the time of presentation (6 am to 3 pm or 3 pm to 6 am) to ensure equal accrual within each of the 4 stratification levels. Time of presentation was chosen to allow equal access to testing available during daytime hours and thus equal likelihood of having a same-day evaluation. In trials with moderate sample size, it has been established that stratified randomization should have a negligible effect on power.13 Treatment assignments were generated by study staff and placed in opaque, sealed, sequentially numbered envelopes in containers corresponding to strata for the study team to access.14 Study groups are defined below.
Observation unit–cardiac MRI participants received care in an ED observation unit staffed by nurse practitioners or physician assistants and supervised by a board-certified/board-prepared emergency physician. Orders included cardiac biomarkers at 4 and 8 hours from the initial blood draw and a stress cardiac MRI examination. Stress cardiac MRI was available from 8 am to 5 pm Monday through Friday, times similar to those of other testing modalities in the study institution. If the 4-hour troponin I level was less than 1.0 ng/mL, patients could receive the stress cardiac MRI examination at the first available period. Examinations were scheduled by MRI staff, following customary practices for triage of clinical cases. After the randomization to the care pathway and initial placement of orders, the care provided was at the discretion of the care providers. Care providers could change imaging and testing strategies, order additional tests, and obtain consultations if necessary to optimize patient care. Interpretation of cardiac MRI reports, ordering additional testing, and patient disposition were determined by the care providers. Initially, the care pathway recommended that participants also receive a cardiology consultation after imaging. This provision was deemed unnecessary by the care providers and was subsequently removed through a protocol amendment after enrollment of 22 participants.
Cardiac MRI in observation unit participants was similar to current imaging protocols used for clinical stress testing at the primary institution. Imaging was performed with a 1.5-T Siemens Magnetom Avanto system with Total Imaging Matrix technology (Siemens Medical Solutions, Munich, Germany). Initial orders were placed for an adenosine cardiac MRI that included assessments of resting wall motion, T2 dark blood for myocardial edema, stress perfusion, rest perfusion, and delayed enhancement (typical imaging parameters are detailed in Table E1, available online at http://www.annemergmed.com). To replicate clinical practice, the imaging team was permitted to modify imaging parameters according to patient characteristics; dobutamine stress cardiac MRI was available as an alternative strategy if patients exhibited reactive airway disease or another contraindication to the receipt of adenosine. Cardiac MRI images were interpreted by a clinical reading pool consisting of 8 board-certified radiology or cardiology faculty with at least level II training in cardiac MRI.15 Reports included wall motion abnormalities, perfusion abnormalities, delayed enhancement, and a summary interpretation. Results were posted to the electronic medical record. Classification of image results from the reports for this analysis was performed by an investigator (C.D.M.).
Inpatient care participants were evaluated by a consulting physician in the ED for the intent of admission, following usual procedures. Patients with established cardiology care or higher-risk profiles were generally admitted to the cardiology service. Others were admitted to hospital-based services and cared for by internists or family medicine physicians. Care patterns in this group were determined by the care providers unaffected by the study protocol. Cardiac MRI was available to these participants.
Methods of Measurement
Sources of data were determined before study initiation. Data collection templates were used to prospectively capture data that were unreliable or unavailable in the medical record. Initial ECG interpretation was conducted after enrollment by a study investigator (C.D.M.) blinded to study group allocation. Other information was gathered from the electronic medical record or billing records by using a structured review.
To determine cardiac events since discharge, participants were contacted by telephone at 30 days, with a modification of a previously described scripted follow-up dialogue.16 Hospitalization records were obtained for patients reporting hospitalizations at any facility since discharge. The occurrence of acute coronary syndrome within 30 days of enrollment was determined by adjudication with a consensus of 2 board-certified emergency physicians from outside institutions, blinded to treatment assignment and method of stress testing. Adjudication was conducted with deidentified summary data from each participant, with additional information available on request.
Acute coronary syndrome was defined as one of the following: (1) acute myocardial infarction, (2) ischemia symptoms leading to revascularization, (3) death likely related to cardiac ischemia, or (4) discharge diagnosis of definite/probable unstable angina with evidence of coronary stenosis greater than 70% or inducible ischemia on cardiac stress testing.
Acute myocardial infarction was defined as a troponin I level greater than 1.0 ng/mL in the presence of ischemic symptoms. Troponin I was measured in the central laboratory with either the TnI-Ultra assay using the ADVIA Centaur platform (Siemens Healthcare Diagnostics Inc., Deerfield, IL) or the Access AccuTnI Troponin I Assay using the dxi800s platform (Beckman Coulter, Fullerton, CA).
Outcome Measures
The primary outcome was direct medical cost of the index hospital visit, calculated as the sum of hospital and provider cost and measured from the hospital perspective. For hospital cost, itemized patient charges were converted to cost, with 2008 departmental-specific cost:charge ratios used to file cost reports with the Centers for Medicare & Medicaid Services annually. Provider cost was determined by using current procedural terminology codes from each charged service, converting to physician work relative value units with the Centers for Medicare & Medicaid Services physician fee schedule and subsequently converting to dollars with the Medicare conversion factor.17
According to preliminary cost data from the study institution, we estimated an approximate $2,000 difference in direct cost of the index hospital visit ($8,000 versus $6,000), favoring the intervention group, with an SD=$3,400. To detect a difference in means of $2,000, 47 participants per arm were required to provide 0.8 power with a 2-sided α=.05. Estimating a 15% attrition rate, sample size was set at 110. Data were reviewed after enrollment of 40 and 80 participants with the safety monitor, and an interim analysis was presented in abstract form on the first 50 participants. These analyses were not intended or used to determine study termination or adjust sample size, and therefore no adjustment for multiple comparisons was made.
Statistical Analysis
The primary analysis was conducted according to intention to treat. Cost was found to be non-normally distributed. Transformations were explored without adequate correction of the data’s right skewness, and therefore nonparametric comparisons were implemented. The median cost difference was estimated with the Hodges-Lehmann approach, with a distribution-free 95% confidence interval (CI) calculated with the method of Moses.18(p75-82),19 To determine the mechanism of observed cost differences, comparative histograms were created from itemized costs for the following categories: ED facility cost, laboratory cost, pharmacy cost, catheterization and revascularization cost, noninvasive imaging cost, inpatient facility cost, and provider cost. To determine whether the observed cost difference was the result of unbalanced revascularization rates, a post hoc comparison was conducted after removing patients undergoing revascularization from both groups. Analysis of covariance with the rank transformation20 was performed to adjust for covariates (Appendix E1, available online at http://www.annemergmed.com). To examine the effect of incomplete cost data caused by participants leaving against medical advice, cost data were further analyzed, giving the 3 censored subjects in the inpatient care group the lowest cost ranks and the one censored subject in the observation unit–cardiac MRI group the highest rank, with p<0.05 considered significant. Statistical analysis was conducted with SAS Enterprise Guide version 4.2 and SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Between January 7, 2008, and March 4, 2009, 967 patients were screened and recorded, 178 approached, and 110 enrolled, with 57 participants randomized to inpatient care and 53 randomized to observation unit–cardiac MRI (Figure 1). Baseline demographic information and medical history are displayed in Table I. Established coronary disease and previous revascularization were present in 28% and 26% of inpatient care participants compared with 21% and 17% of observation unit–cardiac MRI participants, respectively. Most participants presented with a chief complaint of chest pain (92%), had multiple episodes of symptoms (59%), and had chest pain present on arrival to the ED (69%) (Table 2). Most participants had a Thrombolysis in Myocardial Infarction risk score of 2 (36%) or 3 (30%) (Table 3).
Figure 1.
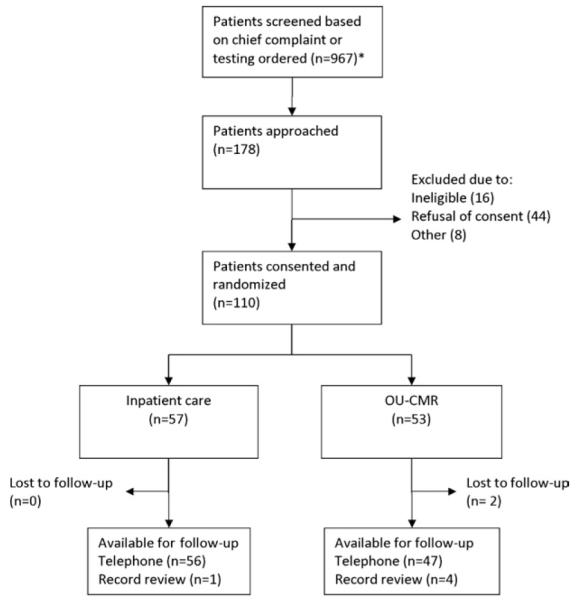
Screening, enrollment, randomization, and followup of patients in the trial.
Table 1.
Participant demographics and medical history.
| Patient Characteristics | Inpatient Care, n/N (%) |
OU-CMR, n/N (%) |
|---|---|---|
| Age, y* | 57 (47, 64) | 55 (48, 61) |
| Age ≥65 y | 10/57 (18) | 8/53 (15) |
| Female sex | 27/57 (47) | 28/53 (53) |
| White race | 40/57 (70) | 35/53 (66) |
| Hypertension | 43/57 (75) | 36/53 (68) |
| Diabetes mellitus | 23/57 (40) | 20/53 (38) |
| Current smoking | 18/57 (32) | 18/53 (34) |
| Hyperlipidemia | 44/57 (77) | 39/53 (74) |
| Previous heart failure, confirmed | 3/57 (5) | 2/53 (4) |
| Established CAD | 16/57 (28) | 11/53 (21) |
| Previous MI (%) | 15/57 (26) | 8/53 (15) |
| Previous revascularization, confirmed | 15/57 (26) | 9/53 (17) |
| Previous CABG | 3/57 (5) | 2/53 (4) |
CAD, Coronary artery disease; MI, myocardial infarction; CABG, coronary artery bypass graft.
Data are presented as median (first quartile, third quartile).
Table 2.
Presenting characteristics and physical examination findings.
| Presenting characteristics and exam findings |
Inpatient Care, n/N (%) |
OU-CMR, n/N (%) |
|---|---|---|
| Presenting characteristics | ||
| Chest pain chief complaint | 52/57 (91) | 49/53 (92) |
| Chest pain at rest | 49/57 (86) | 43/53 (81) |
| Multiple episodes of
symptoms within 24 h |
32/57 (56) | 33/53 (62) |
| Chest pain present on arrival
to the ED |
45/57 (79) | 31/53 (58) |
| Chest pain pleuritic | 7/55 (13) | 4/52 (8) |
| Time from onset of last
episode to arrival, h* |
2.5 (1.0, 8.0) | 3.5 (1.0, 10.0) |
| Duration of last episode, h* | 2.0 (0.75, 5.5) | 1.0 (0.25, 5.0) |
| Physical examination | ||
| Pulse rate, beats/min* | 75 (70, 91) | 77 (68, 86) |
| Systolic blood pressure, mm Hg* | 141 (128, 159) | 139 (124, 155) |
| Murmur | 0/57 (0) | 0/53 (0) |
| Rales | 0/57 (0) | 1/53 (2) |
| JVD | 0/57 (0) | 0/53 (0) |
| Chest pain reproducible | 4/56 (7) | 4/53 (8) |
JVD, Jugular venous distention.
Data presented as median (first quartile, third quartile).
Table 3.
ED evaluation results.
| ECG and risk
stratification characteristics |
Inpatient Care, n/N (%) |
OU-CMR, n/N (%) |
|---|---|---|
| ECG findings | ||
| ST-segment depression | 0/57 (0) | 1/53 (2) |
| Known to be old | 0/57 (0) | 1/53 (2) |
| ST-segment elevation | 0/57 (0) | 0/53 (0) |
| T-wave inversion | 8/57 (14) | 6/53 (11) |
| <2 mm, old | 4/57 (7) | 3/53 (6) |
| <2 mm, not known to be old | 1/57 (2) | 0/53 (0) |
| >2 mm, old | 3/57 (5) | 1/53 (2) |
| >2 mm, not known to be old | 0/57 (0) | 2/53 (4) |
| Left bundle branch block | 0 (0) | 0 (0) |
| Right bundle branch block | 0/57 (0) | 2/53 (4) |
| Pathological Q waves | 6/57 (11) | 6/53 (11) |
| Known to be old | 5/57 (9) | 3/53 (6) |
| Overall ECG classification | ||
| Normal | 24 (42) | 25 (47) |
| Nonspecific ST–T-wave changes | 22 (39) | 17 (32) |
| Early repolarization only | 1 (2) | 0 (0) |
| Abnormal but not diagnostic
of ischemia |
3 (5) | 4 (8) |
| Infarction or ischemia known to be old | 3 (5) | 3 (6) |
| Infarction or ischemia not known to
be old |
4 (7) | 4 (8) |
| Suggestive of acute MI | 0 (0) | 0 (0) |
| Risk stratification | ||
| Emergency physician assessment of
% likelihood of ACS within 30 days* |
15 (10, 25) | 10 (7.8, 20) |
|
Emergency physician overall
impression |
||
| Acute MI | 0/57 (0) | 0/50 (0) |
| Unstable angina | 25/57 (44) | 19/50 (38) |
| Atypical | 27/57 (47) | 26/50 (52) |
| Nonischemic | 5/57 (9) | 5/50 (10) |
| TIMI risk score | ||
| 0 | 1/57 (2) | 1/53 (2) |
| 1 | 10/57 (18) | 8/53 (15) |
| 2 | 18/57 (32) | 22/53 (42) |
| 3 | 17/57 (30) | 16/53 (30) |
| 4 | 11/57 (19) | 5/53 (9) |
| 5 | 0/57 (0) | 1/53 (2) |
MI, Myocardial infarction; ACS, acute coronary syndrome; TIMI, Thrombolysis in Myocardial Infarction.
Data presented as median (first quartile, third quartile).
Inpatient care participants most commonly underwent stress echocardiography testing (54%). Observation unit–cardiac MRI participants most commonly underwent stress cardiac MRI testing (92%), with testing occurring during a median 53 minutes (first quartile (Q1) 44, third quartile (Q3) 58) (Table 4). No patients experienced ventricular arrhythmias, hypotension, cardiac arrest, persistent ST-segment elevation, or death during cardiac MRI testing. Four subjects had cardiac MRI ordered but not completed because of leaving against medical advice, troponin level increase, ventricular tachycardia before testing, and care provider discretion. For subjects who started cardiac MRI testing, 3 of 49 examinations were stopped early because of vomiting, patient request, and tachycardia with adenosine infusion. Two of these 3 examinations contained adequate information for disposition without additional testing; one participant was admitted for cardiac catheterization.
Table 4.
Cardiac testing and clinical outcomes during index hospital visit or observation unit care.
| Diagnostic testing, clinical outcomes, and
cardiac-related health care utilization |
Inpatient Care, n/N (%) | OU-CMR, n/N (%) |
|---|---|---|
| Diagnostic testing: index hospital visit | ||
| ≥3 Cardiac markers | 49/57 (86) | 51/53 (96) |
| Stress CMR | 9/57 (16) | 49/53 (92) |
| Resting echo | 1/57 (2) | 1/53 (2) |
| Stress echo | 31/57 (54) | 2/53 (4) |
| Cardiac catheterization | 9/57 (16) | 8/53 (15) |
| Cardiac catheterization without antecedent stress testing | 8/57 (14) | 1/53 (2) |
| ≥3 Cardiac markers and ≥1 stress test or cardiac catheterization | 42/57 (74) | 50/53 (94) |
| Clinical outcomes: index hospital visit | ||
| Acute coronary syndrome | 6/57 (11) | 2/53 (4) |
| Cardiovascular death | 0/57 (0) | 0/53 |
| Myocardial infarction | 1/57 (2) | 1/53 (2) |
| Revascularization | 5/57 (9) | 2/53 (4) |
| PCI | 5/57 (9) | 1/53 (2) |
| CABG | 0/57 (0) | 1/53 (2) |
| Unstable angina | 2/57 (4) | 0/53 (0) |
| Length of stay* | 29.9 (26.7, 35.7) | 25.7 (20.7, 31.3) |
| Hospital admission (defined by transfer to an inpatient bed) | 54/57 (95) | 11/53 (21) |
| Unadjusted direct medical cost, $* | 2,680 (2,408, 3,448) | 2,062 (1,918, 2,367) |
| Clinical outcomes: after discharge through 30 days | ||
| Acute coronary syndrome | 0/57 (0) | 0/53 (0) |
| Cardiac-related health care after discharge through 30 days † | ||
| Cardiac-related office visit | 4/57 (7) | 7/53 (13) |
| Cardiac-related ED visit | 4/57 (7) | 0/53 (0) |
| Cardiac-related hospitalization | 3/57 (5) | 0/53 (0) |
| Cardiac-related procedures | 4/57 (7) | 0/53 (0) |
| Cardiac catheterization | 3/57 (5) | 0/53 (0) |
| Stress test | 1/57 (2) | 0/53 (0) |
| Resting echo | 1/57 (2) | 0/53 (0) |
CMR, Cardiac magnetic resonance; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft.
Data presented as median (first quartile, third quartile).
Data from all participants are included according to results of telephone follow-up or record review and are presented as the number of participants with at least 1 of the events.
On cardiac MRI testing, observation unit–cardiac MRI participants had a median left ventricular ejection fraction of 61% (Q1=57%, Q3=65%). No evidence of acute myocardial ischemia on any imaging component was observed for 43 of 49 examinations. Inducible ischemia was present in 6 of 49 examinations, all detected as an unmatched defect on myocardial perfusion imaging. Resting wall motion abnormalities were present in 6 of 49 subjects, none of whom had acute coronary syndrome. Among participants with resting wall motion abnormalities, 2 had associated abnormal delayed enhancement, 2 were of uncertain cause, 1 was due to left ventricular noncompaction, and 1 was due to a displaced papillary muscle. Abnormal delayed enhancement was present in 6 of 49 examinations, with 5 related to a previous infarction. No participants had abnormal T2-weighted imaging; however, frequent artifacts limited the clinical utility of this imaging component. Additional details on the diagnostic performance of cardiac MRI are provided in Table E2 (available online at http://www.annemergmed.com).
In the inpatient care group, 6 of 57 participants experienced acute coronary syndrome (11%; 95% CI 4% to 22%) compared with 2 of 53 (4%; 95% CI 0% to 13%) in the observation unit–cardiac MRI group (Table 4). Qualifying acute coronary syndrome events in the inpatient care group included ischemic symptoms plus percutaneous coronary intervention (n=3), unstable angina diagnosis and ischemic symptoms plus percutaneous coronary intervention (n=1), myocardial infarction plus percutaneous coronary intervention (n=1), and unstable angina diagnosis (n=1). Two patients in the observation unit–cardiac MRI group met acute coronary syndrome criteria, one because of ischemic symptoms plus coronary artery bypass graft surgery after a positive cardiac MRI examination result and the other because of a myocardial infarction plus percutaneous coronary intervention. This second patient did not undergo a cardiac MRI examination. No patients in either group experienced acute coronary syndrome after discharge from the hospital.
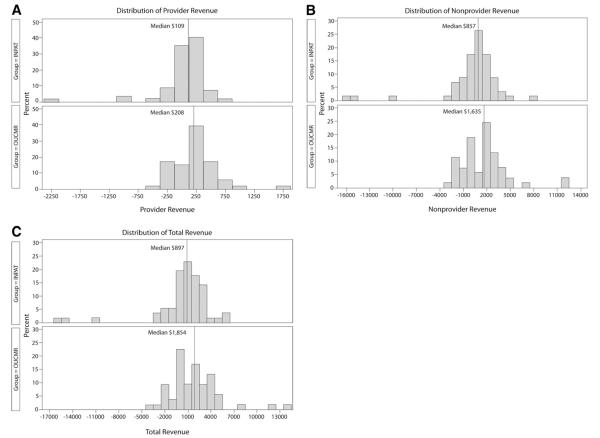
The median direct cost of the index hospital visit was lower in observation unit–cardiac MRI participants ($2,062 versus $2,680; Table 4). The estimated median difference among groups was $588 (95% CI $336 to $811). Comparative histograms of cost by descriptive category are presented in Figure 2. Cost by Thrombolysis in Myocardial Infarction risk score is displayed in Figure 3. With ranked analysis of covariance, full and reduced models had only a small effect and did not change the overall results (Appendix E1 and Table E3, available online at http://www.annemergmed.com). In subgroup analysis, observation unit–cardiac MRI demonstrated a reduction in median cost across most subgroups examined (Table E4, available online at http://www.annemergmed.com). Median total revenue was $897 in the inpatient care group compared with $1,854 in the observation unit–cardiac MRI group, resulting in a median difference of $655 (95% CI −$282 to $1,678) (Figure 4).
Figure 2.
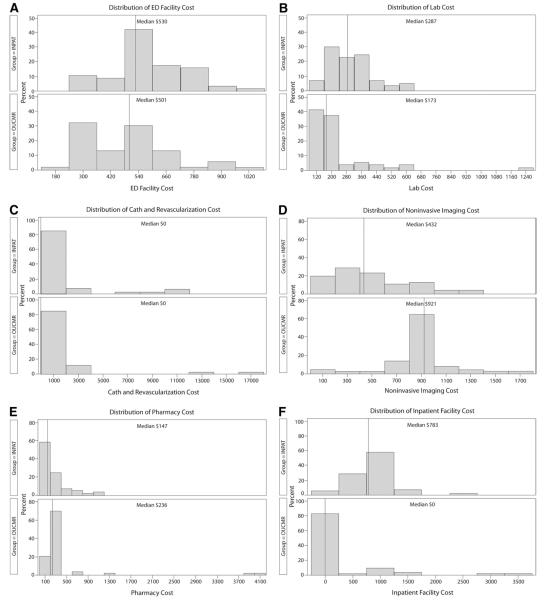
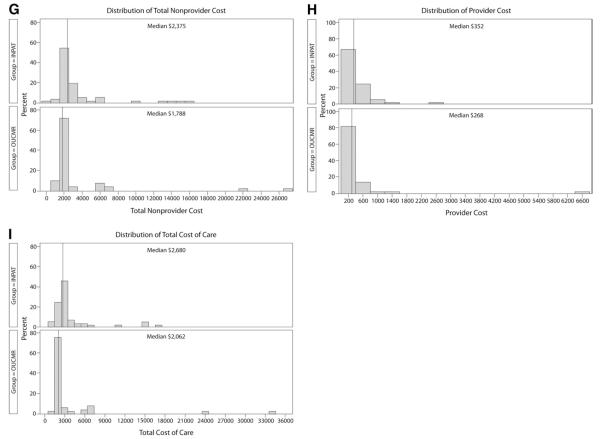
Distributions of cost for study participants in the inpatient care group (INPAT) (n=57) and observation unit–cardiac MRI (OUCMR) group (n=53). All participants have some reported cost for each category unless otherwise noted. A, ED facility cost. B, Laboratory testing cost. C, Catheterization and revascularization cost, no cost n=48 INPAT, n=45 OUCMR. D, Noninvasive imaging cost. E, Pharmacy-associated cost, no cost n=1 INPAT, n=0 OUCMR. F, Inpatient facility cost, no cost n=3 INPAT, n=30 OUCMR. G, Total nonprovider cost representing the sum of cost in A to F. H, Total provider cost. I, Total cost of care, representing the sum of cost in G and H.
Figure 3.
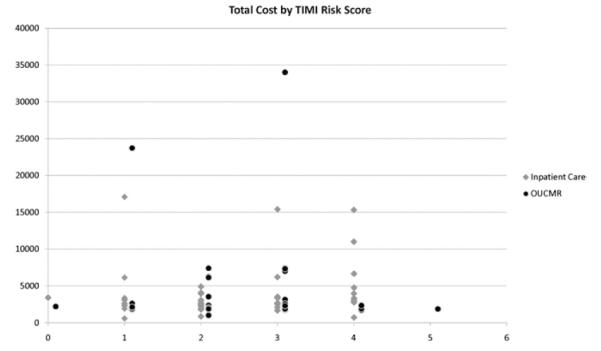
Total cost by TIMI risk score, inpatient care, n=57, and observation unit–cardiac MRI (OUCMR), n=53.
Figure 4.
Distributions of revenue from participants by study group, inpatient care, n=57, and observation unit–cardiac MRI (OUCMR), n=53. A, Provider revenue. B, Nonprovider revenue. C, Total revenue, representing the sum of A and B.
The primary finding of reduced cost in the observation unit–cardiac MRI group was not sensitive to the handling of censored data from the 4 participants who left against medical advice. When the highest-rank cost was assigned to the observation patient and the lowest-rank cost to the 3 patients in the inpatient care group, the estimated median difference was $554 (95% CI $283 to $768), favoring reduced cost in the observation group. The reduction in median cost associated with the observation unit–cardiac MRI group also persisted when participants undergoing revascularization were removed (estimated median difference $542; 95% CI $300 to $741).
LIMITATIONS
This trial was conducted in a single center with substantial experience with cardiac MRI. It is uncertain whether these results can be duplicated at other centers with less cardiac MRI experience. Further, the observed cost difference does not account for the cost of establishing a cardiac MRI program. Second, participants could be enrolled only when cardiac MRI was available within approximately 24 hours; this may have biased length of stay in favor of the cardiac MRI group. Third, we obtained a convenience sample; therefore, selection bias could have affected our results. The magnitude of this potential bias cannot be precisely determined because screening data are incomplete, which precludes a comparison of screened with enrolled participants. Furthermore, some patients were likely screened but not included on the screening log. Fourth, changes in care delivery can have many unanticipated downstream effects not reflected by index hospital visit cost, which will be the subject of future research. Fifth, our cost model used to design this trial was based on higher catheterization rates, longer lengths of stay, and higher cost than those observed. We anticipated a mean cost of $6,000 to 8,000 yet observed a mean cost of only $3,800, which is similar to that reported by others.21 This result ultimately did not impair our ability to detect a difference in our primary endpoint. Sixth, calculating cost with departmental-specific cost-to-charge ratios from a single institution limits external validity. However, cost-to-charge ratios have been proposed as an acceptable method for cross-hospital cost comparisons.22 Seventh, despite randomization and stratification, more patients in the inpatient care group had previous cardiovascular events. It is possible that differences in cost relate to the inpatient care group’s having a higher acuity of illness. Attempts to adjust for this with statistical modeling did not suggest this was the case. Finally, because this trial evaluated the cost of a care strategy, we cannot ascertain whether the effects resulted from cardiac MRI, observation unit care, or both. To further elucidate this, future work could compare observation unit care with and without cardiac MRI.
DISCUSSION
Short-stay units are widely used in low-risk patients with emergent chest pain where protocols guide imaging selection. In contrast, care of non-low-risk patients with emergent chest pain is commonly delivered in the inpatient arena because of the complexities of these patients’ illness. In the inpatient arena, comorbid conditions such as older age, previous infarctions, and revascularizations frequently lead to aggressive diagnostic evaluations incorporating a multitude of imaging techniques, including cardiac catheterization. This investigation examined a population that is not commonly managed in an observation unit, those with non-low-risk chest pain. A commonly cited trial of observation unit care with conventional testing in non-low-risk patients improved efficiency but had a low discharge rate (46%) at the conclusion of the care algorithm.23
This trial investigated whether the advantages of stress cardiac MRI in non-low-risk patients would allow algorithm-driven care to be provided in a short-stay unit in place of the highly individualized care occurring in the inpatient arena. Eligibility criteria were designed to ensure enrollment of a non-low-risk study population; success of this is evidenced by a median age of 55 years, a high prevalence of coronary risk factors, and a 21% prevalence of previous myocardial infarctions. At the study institution, and at most institutions in the United States, these patients are routinely admitted to an inpatient service. In this investigation, participants managed in an observation unit with stress cardiac MRI accumulated lower medical cost during the index hospital visit. Additionally, the observation unit–cardiac MRI strategy was able to manage 79% of patients, without inpatient admission and without any missed cardiovascular events at 30 days.
In the conceptual model of this project, it was anticipated that the intervention would reduce cost mainly by reducing the rate of cardiac catheterization. Comparing care strategies, both groups had similar rates of cardiac catheterization. According to the histograms, it appears that both groups had similar ED cost, catheterization and revascularization cost, pharmacy cost, and provider cost. The cost savings from observation unit–cardiac MRI appear to originate from reduced laboratory cost and reduced inpatient facility cost; these savings offset the increase in noninvasive imaging cost associated with this care pathway.
In the inpatient care group, 6 participants met criteria for acute coronary syndrome compared with 2 in the observation unit–cardiac MRI group. A simple explanation would be that despite randomization, inpatient participants were more ill, which is supported by the higher proportion of patients in the inpatient care group with previous MI and revascularization. However, we also propose an alternative hypothesis. Five of the 6 inpatient care participants with acute coronary syndrome underwent catheterization and revascularization, without antecedent stress testing or biomarker increase. In the observation unit–cardiac MRI group, a similar number of cardiac catheterizations were performed, but only 2 underwent revascularization. We hypothesize that clinicians were less likely to perform revascularization when armed with cardiac MRI results before catheterization compared with when cardiac catheterization was the first imaging test. Appropriateness criteria for revascularization are focused on noninvasive imaging results.24 With increased scrutiny of procedure use, an up-front stress cardiac MRI approach may decrease overall revascularizations and improve appropriateness of those performed.
With aging of the population, the proportion of patients with non-low-probability chest pain will increase. One potential solution is coronary computed tomography angiography. In the emergent chest pain setting, coronary computed tomography angiography has been predominantly tested in patients with low probability where it accurately detects the presence of coronary disease.25,26 However, when testing non-low-probability patients, determining the presence of coronary disease is not sufficient because these patients commonly have coronary disease and many have had previous acute coronary syndrome events. Stress cardiac MRI is able to accurately detect recent infarction, define cardiac structure and function, and detect inducible ischemia without radiation exposure. Because of these characteristics, we anticipate cardiac MRI to be an increasingly important testing modality for ED patients with non-low-risk chest pain.
Implementing a cardiac MRI program is associated with a heavy burden of fixed cost associated with scanner purchase, clinician training or recruitment, and supporting staff, including technologists, nurses, schedulers, and billing personnel.27 Therefore, time to breakeven is highly dependent on the volume of scans. Data specific to cardiac MRI are scarce, but older data addressing all MRI examinations estimate that 1,500 to 2,000 scans per year are required for financial viability.28 With these estimates, the volume of scans that could be generated from an observation unit would represent only a fraction of the examinations required to support the program. Therefore, decisions about whether to implement a cardiac MRI program must take into account a multitude of variables, including the volume of examinations anticipated from other sources.
Stress cardiac MRI can be integrated with observation unit care. In this single-center trial, at a facility with cardiac MRI experience, this combination reduces index hospital cost compared with an inpatient care strategy among patients with non-low-risk emergent chest pain. The observation unit–cardiac MRI strategy was able to manage 79% of participants without hospital admission, and no participants had missed acute coronary syndrome at 30 days. Larger multicenter investigations should further build on these results.
Supplementary Material
Editor’s Capsule Summary.
What is already known on this topic
MRI offers advantages in cardiac imaging over other modalities but is a generally expensive option.
What question this study addressed
Whether rule-out acute coronary syndrome patients who would ordinarily be admitted to a hospital can be managed cost-effectively in an observation unit with a magnetic resonance imaging (MRI)–based protocol.
What this study adds to our knowledge
In this randomized trial of 110 intermediate- to high-risk acute coronary syndrome patients, an MRI-based observation unit strategy was cost-effective compared with inpatient care.
How this is relevant to clinical practice
Facilities with cardiac MRI availability might be able to cost effectively reduce hospital admission of intermediate- to high-risk patients with potential acute coronary syndrome.
Acknowledgments
The authors thank the residents, attending physicians, research staff, and the cardiac MRI technologists and staff for assistance in implementing this trial.
Funding and support: See the Manuscript Submission Agreement in this issue for examples of specific conflicts covered by this statement. Funded by the Translational Science Institute of Wake Forest University School of Medicine. Dr. Miller has received research support from Biosite, Schering-Plough, Siemens, and Heartscape Technologies Inc; has been a consultant for Molecular Insight and the Medicines Co; and has been a speaker for Sanofi-Aventis (indirect sponsor of a CME event). Dr. Lefebvre has received research support from Heartscape Technologies Inc.
Footnotes
Supervising editor: Judd E. Hollander, MD
Author contributions: CDM, WH, JWH, DC, CAH, and WGH were responsible for conceiving and designing the study. CDM and WGH obtained research funding. CDM supervised the conduct of the trial. HB was the study safety monitor and analyzed and interpreted events. Data acquisition was performed by CDM, JWH, CL, and ENH. Data analysis was performed by CDM and DC. Data interpretation was performed by all authors. BH and DBD analyzed, interpreted, and adjudicated clinical endpoints. CDM drafted the article. All authors revised it critically for important intellectual content and granted final approval of the article. CDM takes responsibility for the paper as a whole.
By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article that might create any potential conflict of interest.
Presented at the American Heart Association Scientific Sessions, November 2009, Orlando, FL; and the Society for Cardiac Magnetic Resonance annual meeting, January 2009, Orlando, FL.
Reprints not available from the authors.
REFERENCES
- 1.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Gomberg-Maitland M, Murphy SA, Moliterno DJ, et al. Are we appropriately triaging patients with unstable angina? Am Heart J. 2005;149:613–618. doi: 10.1016/j.ahj.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Tatum JL, Jesse RL, Kontos MC, et al. Comprehensive strategy for the evaluation and triage of the chest pain patient. Ann Emerg Med. 1997;29:116–125. doi: 10.1016/s0196-0644(97)70317-2. [DOI] [PubMed] [Google Scholar]
- 4.Stowers SA, Eisenstein EL, Wackers FJ, et al. An economic analysis of an aggressive diagnostic strategy with single photon emission computed tomography myocardial perfusion imaging and early exercise stress testing in emergency department patients who present with chest pain but nondiagnostic electrocardiograms: results from a randomized trial. Ann Emerg Med. 2000;35:17–25. doi: 10.1016/S0196-0644(00)70100-4. [DOI] [PubMed] [Google Scholar]
- 5.Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99:763–770. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 6.Kwong RY, Schussheim AE, Rekhraj S, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531–537. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
- 7.Ingkanisorn WP, Kwong RY, Bohme NS, et al. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427–1432. doi: 10.1016/j.jacc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 8.Cury RC, Shash K, Nagurney JT, et al. Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118:837–844. doi: 10.1161/CIRCULATIONAHA.107.740597. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Aty H, Zagrosek A, Schulz-Menger J, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 10.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Antman EM, Cohen M, Bernink PJLM, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 12.Pollack CV, Jr, Sites FD, Shofer FS, et al. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006;13:13–18. doi: 10.1197/j.aem.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberger WF, Lachin JM. Randomization in Clinical Trials: Theory and Practice. Wiley; New York, NY: 2002. [Google Scholar]
- 14.Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. 2005;20:187–191. doi: 10.1016/j.jcrc.2005.04.005. discussion 191-193. [DOI] [PubMed] [Google Scholar]
- 15.Budoff MJ, Cohen MC, Garcia MJ, et al. ACCF/AHA clinical competence statement on cardiac imaging with computed tomography and magnetic resonance: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2005;46:383–402. doi: 10.1016/j.jacc.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 16.Kline JA, Mitchell AM, Runyon MS, et al. Electronic medical record review as a surrogate to telephone follow-up to establish outcome for diagnostic research studies in the emergency department. Acad Emerg Med. 2005;12:1127–1133. doi: 10.1197/j.aem.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Puskas JD, Williams WH, Mahoney EM, et al. Off-pump vs conventional coronary artery bypass grafting: early and 1-year graft patency, cost, and quality-of-life outcomes: a randomized trial. JAMA. 2004;291:1841–1849. doi: 10.1001/jama.291.15.1841. [DOI] [PubMed] [Google Scholar]
- 18.Hollander M, Wolfe DA. Nonparametric Statistical Methods. John Wiley & Sons; New York, NY: 1973. [Google Scholar]
- 19.Decker C. [Accessed January 3, 2010];Calculating a nonparametric estimate and confidence interval using SAS software. (PharmaSUG Proceedings, 2000-05.) Available at: http://www.lexjansen.com/pharmasug/2000/Coders/cc01.pdf.
- 20.Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics. 1982;38:715–724. [PubMed] [Google Scholar]
- 21.Chang AM, Shofer FS, Weiner MG, et al. Actual financial comparison of four strategies to evaluate patients with potential acute coronary syndromes. Acad Emerg Med. 2008;15:649–655. doi: 10.1111/j.1553-2712.2008.00159.x. [DOI] [PubMed] [Google Scholar]
- 22.Shwartz M, Young DW, Siegrist R. The ratio of costs to charges: how good a basis for estimating costs? Inquiry. 1995;32:476–481. [PubMed] [Google Scholar]
- 23.Farkouh ME, Smars PA, Reeder GS, et al. A clinical trial of a chest-pain observation unit for patients with unstable angina. N Engl J Med. 1998;339:1882–1888. doi: 10.1056/NEJM199812243392603. [DOI] [PubMed] [Google Scholar]
- 24.Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 appropriateness criteria for coronary revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530–553. doi: 10.1016/j.jacc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Hollander JE, Chang AM, Shofer FS, et al. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med. 2009;53:295–304. doi: 10.1016/j.annemergmed.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction Using Computer Assisted Tomography) Trial. J Am Coll Cardiol. 2009;53:1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff SD, Comeau CR. Setting up a clinical cardiac MR imaging program: practical issues and economics. Magn Reson Imaging Clin N Am. 2003;11:19–26. v. doi: 10.1016/s1064-9689(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 28.Bell RA. Economics of MRI technology. J Magn Reson Imaging. 1996;6:10–25. doi: 10.1002/jmri.1880060105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.