Abstract
The development of injectable and biocompatible vehicles for delivery, retention, growth, and differentiation of stem cells is of paramount importance for regenerative medicine. For cell therapy and the development of clinical combination products, we created a hyaluronan (HA)-based synthetic extracellular matrix (sECM) that provides highly reproducible, manufacturable, approvable, and affordable biomaterials. The composition of the sECM can be customized for use with progenitor and mature cell populations obtained from skin, fat, liver, heart, muscle, bone, cartilage, nerves, and other tissues. This overview describes the design criteria for “living” HA derivatives, and the many uses of this in situ crosslinkable HA-based sECM hydrogel for three-dimensional (3-D) culture of cells in vitro and translational use in vivo. Recent advances allow rapid expansion and recovery of cells in 3-D, and the bioprinting of engineered tissue constructs. The uses of HA-derived sECMs for cell and molecule delivery in vivo will be reviewed, including applications in cancer biology and tumor imaging.
Keywords: synthetic extracellular matrix, hyaluronic acid, cell delivery vehicle, bioprinting, glycosaminoglycan, hydrogel
1. Why clinical-grade biomaterials are important
Hyaluronic acid (HA) is a non-sulfated, linear polysaccharide with the repeating disaccharide, β-1,4-D-glucuronic acid - β-1,3-N-acetyl-D-glucosamine. This ubiquitous and highly hydrated polyanion occurs in sizes ranging from 100 kDa in serum to 8,000 kDa in the vitreous. HA is an essential component of the ECM and its structural and biological properties mediate cellular signaling, wound repair, morphogenesis, and matrix organization [1]. HA and its derivatives have been clinically used as medical products for over three decades [2]. In the past decade, HA has become recognized as an important building block for the creation of new biomaterials for use in cell therapy, three-dimensional (3-D) cell culture, and tissue engineering [3-7].
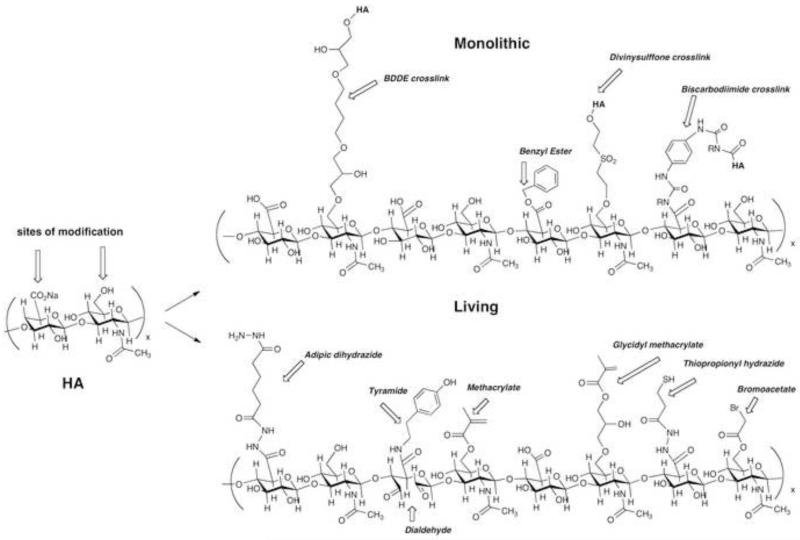
Chemical modification of HA alters its material and biological properties [8, 9], and target three functional groups: the glucuronic acid carboxylic acid, the primary and secondary hydroxyl groups, and the N-acetyl group (following deamidation). As shown in Figure 1, the resulting HA derivatives are categorized as “monolithic” or “living” [10]. Monolithic HA derivatives are “terminally modified” forms of HA that do not form new chemical bonds in the presence of added cells or molecules, while living HA derivatives can form new covalent bonds in the presence of cells, tissues, and small or large molecules. Living HA derivatives are generally needed for clinical and preclinical uses in 3-D cell culture and in vivo cell delivery [11].
Figure 1.
Sample chemical substructures of monolithic (top) and living (bottom) chemical modifications of hyaluronic acid.
The chemical, mechanical, and biological criteria for clinical and preclinical biomaterials are design constraints that must be incorporated into the biomaterial design [4, 11]. One must build in versatility (user control of stiffness and composition), simplicity (ease of use by researchers or physicians), manufacturability, approvability, and cost-effectiveness into the initial design to achieve success as a drug evaluation tool or a clinical biomaterial. One source of clinical biomaterials based on living HA derivatives meet these criteria is Glycosan Biosytems (www.glycosan.com). This overview summarizes the research leading to the development of these HA-based biomaterials and subsequent uses by researchers studying a wide range of primary and progenitor cells.
2. Why living hydrogels are important
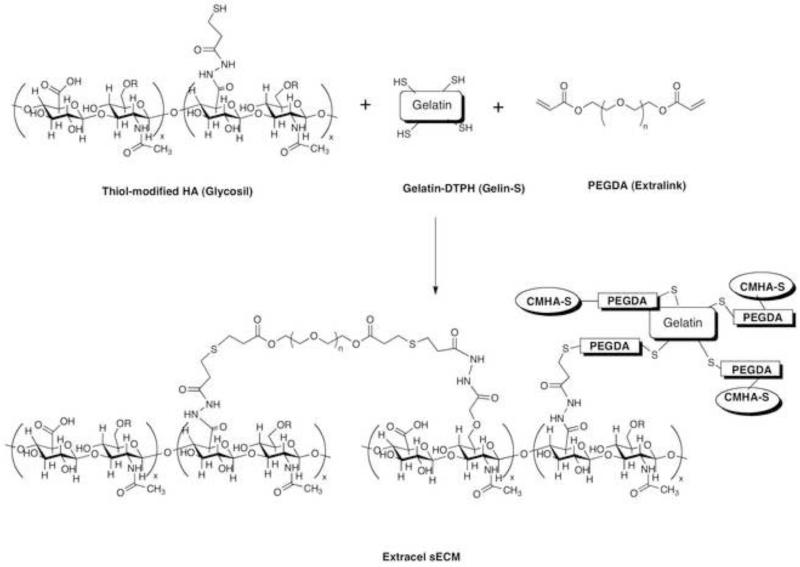
Modular HA-based synthetic extracellular matrices (sECMs) were developed for use in drug evaluation and regenerative medicine [12, 13]. As shown in Figure 2, the sECMs were based on modification of the carboxylate groups of glycosaminoglycans (GAGs) and proteins such as gelatin using hydrazides containing disulfides [14, 15]. The resulting thiol-modified biopolymers slowly crosslinked in air to a hydrogel, this gel could be lyophilized to produce a macroporous sponge [16]. More importantly, in vivo-injectable cell suspensions in the sECM macromonomers can be crosslinked with cytocompatible bifunctional polyethylene glycol (PEG) derived crosslinkers [17]. The mechanical properties and rates of biodegradation could be altered by several varying parameters [18]: (i) molecular weight of starting HA employed; (ii) percentage of thiol modification; (iii) concentrations of thiolated HA and thiolated gelatin; (iv) molecular weight of the crosslinker poly(ethylene glycol) diacrylate (PEGDA); and (v) ratio of thiols to acrylates. Detailed osmotic and neutron scattering studies of this process were performed using hydrogels with PEGDA crosslinking ratios from 0.15 to 1.0 and thiolated HA concentrations from 0.5 to 3.2% (w/w) [19]. The shear modulus was strongly dependent on pre-gel concentration while osmotic mixing pressure was the mainly affected by crosslink density. Moreover, the thermodynamic properties of the composite sECM hydrogels such as HyStem are governed primarily by total polymer concentration; that is, specific interactions between the PEGDA and thiolated HA (CMHA-S) chains are not important.
Figure 2.
Formation of the crosslinked synthetic ECM Extracel from thiol-modified HA, thiol-modified gelatin, and poly(ethylene glycol) diacrylate.
Living hydrogels allow control of gel composition and mechanics, and permit incorporation of cells and a wide variety of small molecules, large molecules, nanoparticles, and microparticles. Moreover, living hydrogels can be processed using fabrication techniques that include electrospinning, bioprinting, molding, or centrifugal casting.
3. Fabrication techniques using living hydrogels
3.1. Centrifugal casting
Endothelial cells encapsulated within an sECM was achieved by allowing crosslinking of the living thiolated HA and gelatin to occur during axial spinning [20]. Since so many of the human body’s tissues are tubular–from capillaries to bones, GI tract, kidney tubules, genitourinary structures–centrifugal casting using living sECMs could play a major role in engineering tissues [21].
3.2 Electrospinning and electrospraying
The applications of electrospinning in tissue engineering were recently reviewed [22]. Included in these applications are the electrospinning of thiolated HA into three-dimensional nanofibrous scaffolds; the fibers were crosslinked with PEDGA prior to removal of the diluent. The scaffold was the coated with fibronectin and seeded with fibroblasts, which attached and spread to a dendritic morphology [23].
However, a common drawback of electrospun scaffolds is that cells cannot infiltrate into the dense nanofibrous structure. To address this, micron-sized fibers electrospun fibers were combined with co-deposition of the thiolated HA-heparin product Heprasil. The resulting mPCL/Col fibers were simultaneously electrosprayed with Heprasil, and the hybrid construct showed optimal penetration of fetal osteoblasts [24].
3.3. Bioprinting
Robotic layer-by-layer fabrication of three-dimensional (3-D) constructs can create functional living tissues by depositing “bio-ink” (cell aggregates or spheroids) and “bio-paper” (scaffold materials)[25] into computer-designed 3-D assemblies [21, 26, 27]. The success of bioprinting so far has been limited by the availability of extrudable, cytocompatible, robust, degradable biomaterials that are compatible with printing devices. Several new materials based on thiolated HA have begun to remedy this deficiency.
First, a four-armed polyethylene glycol 3400 tetracrylate, was used to crosslink thiolated HA and gelatin derivatives into sECM hydrogels that could be extruded from capillaries [28]. High-density suspensions of NIH3T3 cells in a 2% (w/v) crosslinked hydrogel (25 million cells/mL) were printed from microcapillaries into macroscopic filaments that retained their shape during printing. In this way, cellularized tubular constructs were fabricated using a rapid prototyping device, and these vessel-like structures were viable in culture for 4 weeks [28].
In a second strategy, 24-nm gold nanoparticles (AuNP) were employed as multifunctional crosslinkers for thiol-modified HA and gelatin. These AuNP-crosslinked sECM hydrogels exhibited an unusual property that was designated “dynamic crosslinking” [29]. After 24 hr to allow the formation of intra-gel crosslinks, robust hydrogel tubes were extruded and layered in a computer-driven printer. Within hours, the extruded gel filaments formed inter-gel crosslinks, leading to fusion of the macrofilaments. When cellularized AuNP-crosslinked sECM hydrogels were bioprinted, the dynamic crosslinking allowed cell growth and maturation within the printed constructs. After the constructs matured, N-acetyl-cysteine (NAcCys) was added to dissolve the hydrogel, leaving only the cells and the cell-secreted ECM.
4. Cell Delivery with sECMs
Autologous bone-marrow MSCs were delivered to full-thickness defects in the patellar groove of rabbit femoral articular cartilage. Defects were completely repaired at 12 weeks and the sECM was remodeled to trabecular bone and translucent zonated cartilage [30]. The primary role of the sECM appeared to be cell retention, thereby enhancing the natural biological repair processes mediated by endogenous cells. More recently, chondrogenic cells derived from human embryonic stem cells (H9 hESCs) were encapsulated in Extracel and used to repair an osteochondral defect. Spatiotemporally controlled remodeling took place over 12 weeks and produced hyaline-like neocartilage integrated with existing cartilage and regenerated subchondral bone [31].
Injection of bone marrow-derived MSCs in Extracel into injured rat vocal folds led to reduced scarring and favorable ECM production, HA metabolism, myofibroblast differentiation, and TGF-β1 production [32]. Thus, grafting of MSCs in an sECM hydrogel into injured vocal folds offers an attractive therapeutic strategy.
Muscle-derived stems cells (MDSCs) from rat anterior tibialis were cultured in vitro in a 95% collagen-5% HyStem-HP gel combination at densities of 2.2, 3.3, and 4.4 million cells/ml. At the higher concentrations, the scaffold collapsed from traction forces within 48 h, but the lowest concentration gave an intact construct stable for 21 days. Balancing scaffold composition and cell density was particularly critical to create MDSC-seeded scaffolds suitable for implantation [33].
In order to maintain the undifferentiated phenotype, the stem cell microenvironment requires careful control [6]. In one example, encapsulation of embryonic endothelial progenitor cells (murine eEPC) into the sECM HyStem-C created a bioartificial stem cell niche.[34] Implantation of the eEPC-hydrogel into the ears of mice with drug-induced nephropathy or renal ischemia allowed hyaluronidase-mediated eEPC mobilization to injured kidneys and improved renal function. HA hydrogels with eEPCs supported renal regeneration in ischemic and cytotoxic nephropathy, and promoted neovascularization in an ischemic hind limb model [34].
An sECM hydrogel composed of crosslinked thiol-modified heparin, gelatin, and HA (HyStem-HP) significantly promoted the survival of two neural progenitor cell (NPC) lines in vitro under conditions of stress, and in vivo delivery into the cavity of a stroke-infarcted brain [35]. Cell survival was improved, glial scar formation was reduced, and local inflammation was minimized for HyStem-delivered cells in comparison to NPCs delivered in buffer only. Thus, stem cell transplantation into the infarct cavity within a pro-survival hydrogel matrix may provide a translational therapy for stroke recovery [35]. In a separate model, axonal sprouting after stroke was enhanced by delivery of a Lingo1 function blocking protein encapsulated in Extracel-HP in the peri-infarct cavity [36].
When human MSCs encapsulated in Extracel-X were injected subcutaneously into nude mice, those MSCs engineered to produce an anti-tumor antibody showed dramatic reduction of HCT-116 tumor volume. Interestingly, tumors produced after 40 days in which wild-type MSCs were co-cultured with HCT-116 colon cancer cells were over twice as large as tumors obtained from HCT-116 cells alone in Extracel-X [37].
5. Matrix stiffness of sECMs affects cell phenotype
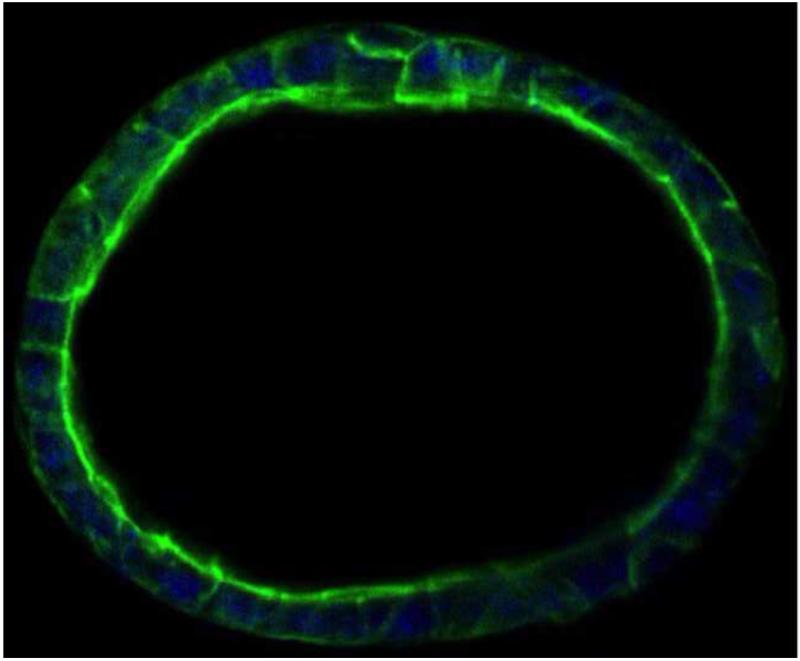
Both composition and gel stiffness can be controlled by varying the ratios of thiolated HA, thiolated gelatin, and crosslinker. The mechanical properties of the sECM largely determined cell phenotype. From shear moduli ranging from 11 Pa to 3500 Pa, the concentration of the thiolated HA and the cross-linking density were the main determinants of gel stiffness. In essence, addition of thiol-modified gelatin reduced gel stiffness by diluting the thiolated HA concentration [18]. One standard sECM, HyStem-C, has an elastic modulus in the 200 Pa range, and has been found suitable for the formation of acini by encapsulated canine kidney MDCK cells (Figure 3). Primary adult human hepatocytes retained a rounded morphology when encapsulated in Extracel (HyStem-C) and cultured for 5 days, as illustrated by the SEM image in Figure 4.
Figure 3.
Culture of the canine kidney MDCK cell line in HyStem-C, supplemented with 100 μg/mL mouse laminin, leads to formation of acinar structures. Key: green, Alexa 488 phalloidin for actin; blue, Draq-5 for cell nuclei. Image courtesy of Glycosan Biosystems, Y. Qui, R. McCall, V. Mironov, X. Wen.
Figure 4.
Scanning electron micrograph of human hepatocytes encapsulated in Extracel and cultured for 5 days. Image obtained by G. Yang and provided by Glycosan Biosystems.
Using a physiologically relevant ECM mimic containing covalently bound fibronectin domains, adult human dermal fibroblasts modified their mechanical response in order to match substrate stiffness. Cells on stiffer substrates had more organized actin cytoskeletons and more extended phenotype.[38] Similarly, migration of human dermal fibroblasts (HDFs) was examined with the same HA-fibronectin hydrogels. Traction stresses correlated to the stiffness of the hydrogel sECM, and high stresses led to nuclear distortion [39].
Human bone-marrow-derived multipotent MSCs were cultured on Extracel at elastic moduli that matched muscle or brain tissue elasticity. Over ninety MSC-secreted cytokines and growth factors were assayed, and many exhibited elasticity-dependent expression. Importantly, IL-8 was up-regulated almost 100-fold on hard surfaces relative to soft surfaces [40].
Young and Engler found that the time-dependent stiffening of thiol-modified HA hydrogels enhanced cardiomyocyte differentiation in vitro [41]. The increase of the elastic modulus was tuned to mimic the increase in modulus as the heart developed during embryogenesis, suggesting that temporally-changing material properties could enhance cell maturation in engineered tissues.
Finally, hESCs grown on Extracel-HP showed reduced vimentin levels relative to hESCs cultured on Matrigel or on murine embryonic fibroblast layers. The expression of vimentin exemplifies a stress-induced response by hESCs to growth on stiff substrata [42]. Proteomic and morphological indicators characteristic of 3D culture were retained in these soft HA-based hydrogels. Combining these observations with the techniques for cell expansion and recovery discussed below suggests that soft HA-rich matrices have the potential for clinical-grade stem cell expansion, differentiation, and implantation in regenerative medicine.
6. Molecule and particle delivery
6.1. Growth factor delivery
One common use of sECM hydrogels is to achieve spatiotemporal control of growth factor (GF) release, accomplishing four main objectives: (i) prolonging biological activity in vivo, (ii) protecting GFs from proteolysis, (iii) localizing GF release, and (iv) reducing GF expense. Often, multiple GFs are required to recapitulate a desired biologic outcome. A heparan sulfate-mimetic sECMs was prepared by co-crosslinking thiolated HA with thiol-modified heparin [43]. Cell growth and rates of neovascularization were increased in this sECM, which released basic fibroblast growth factor (bFGF) in vitro with a half-life of 4 weeks [43]. By varying the thiolated GAG composition and adding thiolated gelatin, different release rates were realized for specific growth factors [44]. Co-release of VEGF with bFGF, angiopoietin-1, or keratinocyte growth factor (KGF) increased microvessel density and maturity using Extracel-HP [45, 46]. Optimal vascularization and vascular maturation using films implanted in mouse ear pinnae in vivo was accomplished by dual release of VEGF and KGF [45, 46].
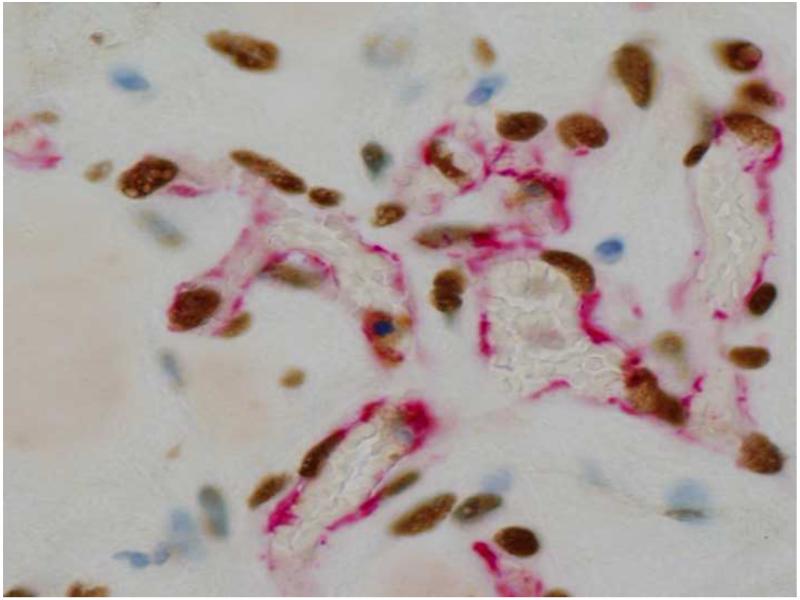
Both GF release and the appropriate mechanical environment of the ECM are required for vasculogenesis. For example, VEGF and substrate mechanics co-regulated tubulogenesis by endothelial progenitor cells (EPCs) encapsulated in Extracel-HP. Higher VEGF concentrations paired with softer gels promoted (i) EPC migration, (ii) increased cellular elongation of EPCs, and (iii) formation of longer tubes with more defined lumens [47]. In a separate study with EPCs, injection of human endothelial colony forming cells (ECFCs), a type of EPC with growth factors and human adipose-derived stem cells into a depot adjacent to the heart in a rat myocardial infarct model appeared to produce a human vasculature connected to the rat circulatory system (Figure 5).
Figure 5.
Subcutaneous injection of Endgenitor human endothelial colony forming cells (ECFCs) with human adipose-derived stem cells and a cocktail of growth factors in Extracel-HP after 1 week. An anastomosed human vasculature (red-stained endothelium by anti-human CD31 and brown nuclei stained with anti-human nuclear matrix protein) is visible in the presence of rat cells (blue) and rat blood (uncolored in red-lined blood vessels). Image courtesy of Glycosan Biosystems and R. I. Grove (Endgenitor).
Endogenous stem cells and precursor cells can be attracted to a defect for de novo tissue regeneration. For example, hepatocyte growth factor (HGF) induces migration of MSCs in vitro but is rapidly degraded in vivo. Extended, localized delivery of HGF was achieved with Heprasil, resulting in controlled release of HGF coupled with migration of human bone marrow MSCs into the hydrogel in vitro [48].
Using a hybrid biomaterial fabricated by electrospinning of poly (ε-caprolactone)-collagen (PCL/Col) microfibers with concomitant electrospraying of Heprasil-entrapped VEGF165 and PDGF-BB were released over a five-week period in vitro. These hybrid meshes were co-cultured with human umbilical vein endothelial cells (HUVECs) and lung fibroblasts, thereby recapitulating a primitive vascular network within the scaffold [49].
6.2. Small molecule delivery
Small hydrophobic molecules, such as steroidal anti-inflammatory drugs, release over the course of days to weeks. For PEG dialdehyde crosslinked adipic hydrazide modified HA (HA-ADH), an inverse correlation between the hydrophobicity of steroid (log P for octanol water partition coefficient) and the rate of release was observed [50]. The steroids were encapsulated in the hydrogels as powders, so that release required both solubilization and diffusion. Analogous results were obtained with thiolated HA hydrogels crosslinked with PEGDA (X. Shu, G. D. Prestwich, unpublished results). In contrast, small water soluble molecules rapidly diffuse out of the hydrogels, unless the small molecules are cationic and retained by charge-charge interactions with the glucuronate residues.
6.3. Large molecule and particle delivery
For standard formulations of PEGDA-crosslinked HA hydrogels such as Extracel and HyStem, large molecules, i.e., molecules >40,000 Da, are retained within the gel and released only as the gel is degraded by hyaluronidase and/or collagenase. This allows co-encapsulation of a variety of ECM proteins such as collagen, laminin, vitronectin, and fibronectin in the hydrogel. It also permits entrapment of antibodies, DNA, siRNA, or macroscopic particles within the gel, although to date few examples of these uses have been described. Several are noted below.
In a mouse model for control of axonal sprouting after stroke, Nogo signaling was by blocked by delivery of Lingo1 function blocking protein (Lingo1-Fc) encapsulated in Extracel-HP injected into the peri-infarct cavity [36]. This resulted in enhanced forelimb motor cortical connections, similar to those in Nogo receptor knock-out mice.
Using nanoporous 10 μm microparticles of crosslinked of HA-aldehyde - HA-ADH, a perlecan domain containing HS chains was coupled to the pendant hydrazides [51]. The perlecan domain-conjugated HA hydrogel particles were used to release bone morphogenetic protein 2 (BMP-2), which stimulated the production of cartilage-specific ECM.
Finally, Bae and coworkers used Extracel to deliver three types of magnetic resonance imaging (MRI) contrast agents to localize them subcutaneously in mice. Superparamagnetic gadolinium-labeled magnetite nanoparticles (GMNPs) were prepared as dual-contrast agents for both T1 and T2-weighted MRI. Extracel-delivered Feridex was dark in T1, while Magnevist and GMNPs were bright in both T1 and T2 relaxivity images [52].
7. Cell expansion and recovery
In order to recover cells after expansion without using enzymatic treatment to disrupt cell-matrix interaction, disulfide groups were introduced into the PEGDA crosslinkers [53]. One, two, or three disulfide blocks were introduced; PEGSSDA contained a single disulfide-containing block. Cells were released from PEGSSDA crosslinked sECMs using a thiol-disulfide exchange reaction; e.g., a 1-h incubation with 25 mM NAcCys dissolved the sECM, permitting cell recovery under non-enzymatic conditions. Fibroblasts, hepatocytes, bone marrow-derived MSCs, and HUVECs were recovered by gentle centrifugation and their viability established by re-culturing the cells on Extracel.
It is also possible to coat an sECM Extracel onto porous crosslinked dextran beads [54]. In this case, the hydrogel was crosslinked by disulfide bond formation. Cells were allowed to proliferation in 3-D in a rotating wall vessel (RWV) bioreactor that recapitulated the low fluid shear stress environments in the body. One cell type, human intestinal epithelial cells (Int 407), formed multilayered cell aggregates on the sECM beads. The cell clusters were released by dissolving the gel with NAcCys, and the cell aggregates were further expanded in a scaffold-free manner in the RWV bioreactor to produce spheroidal microtissues. Such tissue spheroids can be used to investigate host-pathogen interactions, evaluate new therapeutic agents, and creating clusters for bioprinting or cell therapy [54].
Human hepatoblasts (hHBs) and human hepatic stem cells (hHpSCs) were maintained on thiol-modified HA hydrogels mixed containing ECM proteins such as type I collagen and laminin. Both hHpSCs and hHBs survived and expanded for more than 4 weeks in a soft, disulfide-bonded Glycosil (thiolated HA) hydrogel. The hHpSCs in hydrogels were differentiated into hHBs that expressed α-fetoprotein, albumin, and urea [55]. In a related study, human fetal liver cells were embedded in soft, disulfide-crosslinked Glycosil, and the hydrogel was placed within the capillary system of a 3-D perfusion bioreactor. This system was optimized for liver cell survival and differentiation [56]. In earlier preliminary studies, we had found that primary rat hepatocytes cultured in Extracel retained cytochrome P-450 activity, a key metabolic function for testing hepatic function and hepatotoxicity [12].
8. Drug evaluation and tumor models
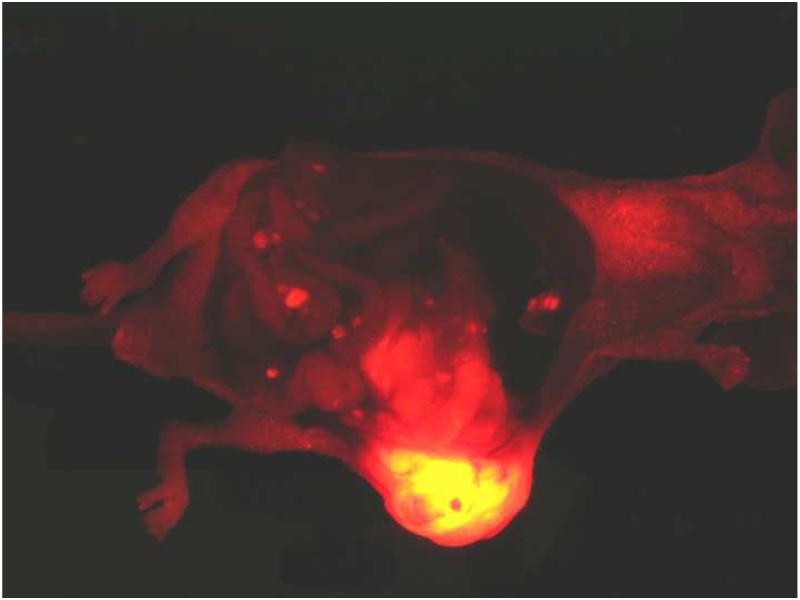
The strategy of “tumor engineering” involves injection of cancer cells in Extracel into orthotopic locations of immune compromised mice and is a versatile method to harness 3-D cell culture for studying tumor biology and for evaluating new anti-cancer drugs [57]. Engineered tumors showed improved “take” for hard-to-culture and poorly tumorigenic cell lines. The 3-D injection in Extracel allows experimental control of important xenograft parameters: (i) more consistent tumor size, (ii) better tissue integration and vascularization, (iv) reduced necrosis, (iv) control of tumor location, and (v) generally improved animal health compared with tail vein or intraperitoneal injection of cells injection in medium [57]. Tumor growth and metastasis were also enhanced in a pancreatic adenocarcinoma model [58], in which RFP-modified MiaPaCa-2 cells injected into the pancreas created both a primary tumor and distant metastases (Figure 6). As noted above, mice implanted with tumors in which wild-type human MSCs were co-injected with HCT-116 colon cancer cells in Extracel-X produced larger tumors in 40 days compared to mice receiving an Extracel-HCT-116 suspension without MSCs [37].
Figure 6.
Injection of MiaPaCa-2 cells labeled with red fluorescent protein as an Extracel suspension into the pancreas of a nude mouse. After 4 weeks, the tumor mass is visible as a large orange mass (lower center); individual metastases are visible as red foci in the intestinal folds and elsewhere in the abdomen. Image courtesy of Jill Shea.
Novel cell types and cell aggregates have been produced using Extracel. In one study, tumor-like stem cells derived from human keloid (keloid precursor cells, KPCs) were suspended in Extracel-HP containing IL-6 or IL-17 and implanted subcutaneously in immune compromised mice. This inflammatory niche contributed to a benign tumor-like stem cell phenotype of the KPCs characterized by uncontrolled self-renewal and increased proliferation. Modification of this pathological stem cell niche with anti-cytokine antibodies had an anti-tumor effect [59].
Using cells isolated from human placentas, placenta-derived adherent cells (hPDACs) prevented bone loss, stimulated bone formation, and suppressed growth of multiple myeloma in preclinical models [60]. HyStem-C encapsulated hPDACs survived subcutaneously for 8 weeks in immunocompromised mice, while hPDACs injected into tumor lesions or into bone were cleared within 3 weeks. Only intrabone injections were effective as cytotherapy for myeloma.
Finally, engineered tumors have been employed to explore stabilized lipids that modulate the lysolipid signaling [61]. First, a novel dual function bromophosphonate analog of LPA, BrP-LPA, was identified as a dual activity LPA antagonist/ATX inhibitor (LPAa/ATXi). Intraperitoneal injection of BrP-LPA inhibited growth and angiogenesis in MB-231 breast tumors grown in Extracel [62]. Second, addition of GFs to Extracel-HP allowed tumorigenic expansion of A-549 non-small cell lung carcinoma (NSCLC) cells in vivo [63]. Reproducibly-sized subcutaneous lung tumors were formed, and growth and vascularization were inhibited by the BrP-LPA. Third, injection of a suspension of HCT-116 colon cancer cells in Extracel directly into the liver of a nude mouse, created vascularized colon tumor grafted onto the liver, mimicking a colon cancer metastasis site.[61] BrP-LPA also significantly reduced tumor growth and angiogenesis in this model. Taken together, these improved, more realistic xenografts show considerable utility for evaluating the potential of novel anti-metastatic, anti-proliferative, and anti-angiogenic compounds.
9. Conclusions
Simple, manufacturable ECM-mimetic hydrogels based on HA have broad utility in wound repair [64], drug evaluation [12], stem cell niche engineering [6], and regenerative medicine [4]. The concentrations, compositions, and mechanics of these HA-based sECMs can be experimentally optimized for primary and stem cells for both ex vivo and in vivo uses. Moreover, sECMs can be used for delivery of growth factors, small molecules, antibodies, microparticles and nanoparticles. Meeting the initial design criteria for clinical biomaterials is now facilitating the translation of HyStem and Extracel into preclinical, veterinary, and human clinical applications.
Acknowledgements
This research was supported in part by NIH Grant 2R01 DC 04336, NSF FIBR Grant (EF-0526854), and the State of Utah Centers of Excellence Program. We thank the scientists at Glycosan BioSystems and our many colleagues for helpful discussions and for preprints.
References
- [1].Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- [2].Kuo JW. Practical aspects of hyaluronan based medical products. CRC/Taylor & Francis; Boca Raton: 2006. [Google Scholar]
- [3].Allison DD, Grande-Allen KJ. Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12:2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- [4].Prestwich GD. Engineering a clinically-useful matrix for cell therapy. Organogenesis. 2008;4:42–47. doi: 10.4161/org.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Condie RC, Prestwich GD. Engineering Clinically Useful Hyaluronan Matrices. In: Vernon B, editor. Injectable Biomaterials: Science and Application. Woodhead Publishing; London: 2010. [Google Scholar]
- [6].Prestwich GD, Ghaly T, Brudnicki P, Ratliff B, Goligorsky MS. Bioartificial Stem Cell Niches: Engineering a Regenerative Microenvironment. In: Goligorsky MS, editor. Regenerative Nephrology. Elsevier; 2010. [Google Scholar]
- [7].Burdick J, Prestwich G. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011 doi: 10.1002/adma.201003963. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Prestwich GD. Biomaterials from chemically-modified hyaluronan, Glycoforum. 2001 http://glycoforum.gr.jp/science/hyaluronan/HA18/HA18E.html. [Google Scholar]
- [9].Kuo JW, Prestwich GD. Hyaluronic acid. In: Ducheyne P, Healy K, Hutmacher D, Kirkpatrick J, editors. Materials of biological origin- Materials analysis and implant uses, Comprehensive biomaterials. Elsevier; 2010. [Google Scholar]
- [10].Prestwich GD, Kuo JW. Chemically-modified HA for therapy and regenerative medicine. Curr Pharm Biotechnol. 2008;9:242–245. doi: 10.2174/138920108785161523. [DOI] [PubMed] [Google Scholar]
- [11].Prestwich GD. Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: a pragmatic approach. J Cell Biochem. 2007;101:1370–1383. doi: 10.1002/jcb.21386. [DOI] [PubMed] [Google Scholar]
- [12].Prestwich GD. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc Chem Res. 2008;41:139–148. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- [13].Serban MA, Prestwich GD. Making modular extracellular matrices: Solutions for the puzzle. Methods. 2008;45:93–98. doi: 10.1016/j.ymeth.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide crosslinked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- [15].Shu XZ, Ahmad S, Liu Y, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J. Biomed. Mater. Res. 2006;79A:902–912. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- [16].Shu XZ, Liu Y, Palumbo F, Prestwich GD. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials. 2003;24:3825–3834. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- [17].Vanderhooft JL, Mann BK, Prestwich GD. Synthesis and characterization of novel thiol-reactive poly(ethylene glycol) cross-linkers for extacellular-matrix-mimetic biomaterials. Biomacromolecules. 2007;8:2883–2889. doi: 10.1021/bm0703564. [DOI] [PubMed] [Google Scholar]
- [18].Vanderhooft JL, Alcoutlabi M, Magda JJ, Prestwich GD. Rheological properties of cross-linked hyaluronan-gelatin hydrogels for tissue engineering. Macromol Biosci. 2009;9:20–28. doi: 10.1002/mabi.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Horkay F, Magda J, Alcoutlabi M, Atzet S, Zarembinski T. Structural, mechanical and osmotic properties of injectable hyaluronan-based composite hydrogels. Polymer. 2010;51:4424–4430. doi: 10.1016/j.polymer.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mironov V, Kasyanov V, Shu XZ, Eisenberg C, Eisenberg L, Gonda S, Trusk T, Markwald RR, Prestwich GD. Fabrication of tubular tissue constructs by centrifugal casting of cells suspended in an in situ crosslinkable hyaluronan-gelatin hydrogel. Biomaterials. 2005;26:7628–7635. doi: 10.1016/j.biomaterials.2005.05.061. [DOI] [PubMed] [Google Scholar]
- [21].Mironov V, Kasyanov V, Markwald RR, Prestwich GD. Bioreactor-free tissue engineering: directed tissue assembly by centrifugal casting. Expert Opin Biol Ther. 2008;8:143–152. doi: 10.1517/14712598.8.2.143. [DOI] [PubMed] [Google Scholar]
- [22].Agarwal S, Wendorff J, Greiner A. Progress in the field of electrospinning for tissue engineering applications. Adv. Mater. 2009;21:3343–3351. doi: 10.1002/adma.200803092. [DOI] [PubMed] [Google Scholar]
- [23].Ji Y, Ghosh K, Shu XZ, Li BQ, Sokolov JC, Prestwich GD, Clark RAF, Rafailovich MH. Electrospun three-dimensional hyaluronic acid nanofibrous scaffolds. Biomaterials. 2006;27:3782–3792. doi: 10.1016/j.biomaterials.2006.02.037. [DOI] [PubMed] [Google Scholar]
- [24].Ekaputra AK, Prestwich GD, Cool SM, Hutmacher DW. Combining electrospun scaffolds with electrosprayed hydrogels leads to three-dimensional cellularization of hybrid constructs. Biomacromolecules. 2008;9:2097–2103. doi: 10.1021/bm800565u. [DOI] [PubMed] [Google Scholar]
- [25].Mironov V, Prestwich GD, Forgacs G. Bioprinting living structures. J. Mater. Chem. 2007;17:2054–2060. [Google Scholar]
- [26].Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJA. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Engineering. 2007;13:1905–1925. doi: 10.1089/ten.2006.0175. [DOI] [PubMed] [Google Scholar]
- [27].Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–2174. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Skardal A, Zhang J, Prestwich G. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31:6173–6181. doi: 10.1016/j.biomaterials.2010.04.045. [DOI] [PubMed] [Google Scholar]
- [29].Skardal A, Zhang J, McCoard L, Oottamasathien S, Prestwich G. Dynamically crosslinked gold nanoparticle – hyaluronan hydrogels. Advanced Materials. 2010;22:4736–4740. doi: 10.1002/adma.201001436. [DOI] [PubMed] [Google Scholar]
- [30].Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3405–3416. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- [31].Toh W, Lee E, Guo X, Chan J, Yeow C, Choo A, Cao T. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31:6968–6980. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- [32].Johnson B, Fox R, Chen X, Thibeault S. Tissue regeneration of the vocal fold using bone marrow mesenchymal stem cells and synthetic extracellular matrix injections in rats. Laryngoscope. 2010;120:537–545. doi: 10.1002/lary.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim J, Wilson H-M, Lucioni A, Kobashi K, Allen M. Neurourol. Urodynam; 2011. Optimizing muscle stem cell constructs for pelvic floor; p. 239. doi 10.1002/nau. [Google Scholar]
- [34].Ratliff B, Ghaly T, Brudnicki P, Yasuda K, Rajdev M, Bank M, Mares J, Hatzopoulous A, Goligorsky MS. Endothelial progenitors encapsulated in bioartifical niches are insulated from systemic cytotoxicity and are angiogenesis competent. Am. J. Physiol. Renal Physiol. 2010;299:F178–F186. doi: 10.1152/ajprenal.00102.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil. Neural Repair. 2010;24:636–644. doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li S, Overman J, Katsman D, Kozlov S, Donnelly C, Twiss J, Giger R, Coppola G, Gewschind D, Carmichael S. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nature Neurosci. 2010;13:1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Compte M, Cuesta AM, Sanchez-Martin D, Alonso-Camino V, Vicario JL, Sanz L, Alvarez-Vallina L. Tumor immunotherapy using gene-modified human mesenchymal stem cells loaded into synthetic extracellular matrix scaffolds. Stem Cells. 2009;27:753–760. doi: 10.1634/stemcells.2008-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, Ren XD, Rafailovich M, Clark RA. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671–679. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pan Z, Ghosh K, Liu Y, Clark RA, Rafailovich M. Traction stresses and translational distortion of the nucleus during fibroblast migration on a physiologically relevant ECM mimic. Biophys. J. 2009;96:4286–4298. doi: 10.1016/j.bpj.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Seib FP, Prewitz M, Werner C, Bornhauser M. Matrix elasticity regulates the secretory profile of human bone marrow-derived multipotent mesenchymal stromal cells (MSCs) Biochem Biophys Res Commun. 2009;389:663–667. doi: 10.1016/j.bbrc.2009.09.051. [DOI] [PubMed] [Google Scholar]
- [41].Young J, Engler A. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32:1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Van Hoof D, Braam SR, Dormeyer W, Ward-van Oostwaard D, Heck AJ, Krijgsveld J, Mummery CL. Feeder-free monolayer cultures of human embryonic stem cells express an epithelial plasma membrane protein profile. Stem Cells. 2008;26:2777–2781. doi: 10.1634/stemcells.2008-0365. [DOI] [PubMed] [Google Scholar]
- [43].Cai S, Liu Y, Shu XZ, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- [44].Peattie RA, Pike DB, Yu B, Cai S, Shu XZ, Prestwich GD, Firpo MA, Fisher RJ. Effect of gelatin on heparin regulation of cytokine release from hyaluronan-based hydrogels. Drug Delivery. 2008;15:389–397. doi: 10.1080/10717540802035442. [DOI] [PubMed] [Google Scholar]
- [45].Elia R, Fuegy PW, VanDelden A, Firpo MA, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis by in situ crosslinked, dual growth factor-loaded, glycosaminoglycan hydrogels. Biomaterials. 2010;31:4630–4638. doi: 10.1016/j.biomaterials.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hosack L, Firpo MA, Scott JA, Prestwich GD, Peattie RA. Microvascular maturity elicited in tissue treated with cytokine-loaded hyaluronan-based hydrogels. Biomaterials. 2008;29:2336–2347. doi: 10.1016/j.biomaterials.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hanjaya-Putra D, Yee J, Ceci D, Truitt R, Yee D, Gerecht S. Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells. J. Cell. Molec. Med. 2009;14:2436–2447. doi: 10.1111/j.1582-4934.2009.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhao J, Zhang N, Prestwich GD, Wen X. Recruitment of endogenous stem cells for tissue repair. Macromol Biosci. 2008;8:836–842. doi: 10.1002/mabi.200700334. [DOI] [PubMed] [Google Scholar]
- [49].Ekaputra A, Prestwich G, Cool S, Hutmacher D. Electrospun hybrid mesh of poly (e-caprolactone)/collagen fibers and hyalruonic acid hydrogel as a growth facdtor delviery system and three-dimensional vascularized scaffold. J. Controlled Release. 2010 submitted. [Google Scholar]
- [50].Luo Y, Kirker KR, Prestwich GD. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J. Controlled Rel. 2000;69:169–184. doi: 10.1016/s0168-3659(00)00300-x. [DOI] [PubMed] [Google Scholar]
- [51].Jha AK, Yang W, Kirn-Safran C, Farach-Carson M, Jia X. Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release. Biomaterials. 2009;30:6964–6975. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bae K, Kim Y, Lee Y, Hwang J, Park H, Park T. Bioinspired synthesis and charcterization of gadolinium-labeled magnetite nanoparticles for dual contrast T1- and T2-weighted magnetic resonance imaging. Bioconj. Chem. 2010;21:505–512. doi: 10.1021/bc900424u. [DOI] [PubMed] [Google Scholar]
- [53].Zhang J, Skardal A, Prestwich GD. Engineered extracellular matrices with cleavable crosslinkers for cell expansion and easy cell recovery. Biomaterials. 2008;29:4521–4531. doi: 10.1016/j.biomaterials.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Skardal A, Sarker S, Crabbé A, Nickerson CA, Prestwich GD. The generation of 3D tissue models based on hyaluronan hydrogel-coated microcarriers within a rotating wall vessel bioreactor. Biomaterials. 2010;31:8426–8435. doi: 10.1016/j.biomaterials.2010.07.047. [DOI] [PubMed] [Google Scholar]
- [55].Turner WS, Seagle C, Galanko JA, Favorov O, Prestwich GD, Macdonald JM, Reid LM. Nuclear magnetic resonance metabolomic footprinting of human hepatic stem cells and hepatoblasts cultured in hyaluronan-matrix hydrogels. Stem Cells. 2008;26:1547–1555. doi: 10.1634/stemcells.2007-0863. [DOI] [PubMed] [Google Scholar]
- [56].Schmelzer E, Triolo F, Turner ME, Thompson RL, Zeilinger K, Reid LM, Gridelli B, Gerlach JC. Three-dimensional perfusion bioreactor culture supports differentiation of human fetal liver cells. Tissue Eng Part A. 2010;16:2007–2016. doi: 10.1089/ten.TEA.2009.0569. [DOI] [PubMed] [Google Scholar]
- [57].Liu Y, Shu XZ, Prestwich GD. Tumor engineering: orthotopic cancer models in mice using cell-loaded, injectable, cross-linked hyaluronan-derived hydrogels. Tissue Eng. 2007;13:1091–1101. doi: 10.1089/ten.2006.0297. [DOI] [PubMed] [Google Scholar]
- [58].Scaife CL, Shea JE, Dai Q, Firpo MA, Prestwich GD, Mulvihill SJ. Synthetic extracellular matrix enhances tumor growth and metastasis in an orthotopic mouse model of pancreatic adenocarcinoma. J Gastrointest Surg. 2008;12:1074–1080. doi: 10.1007/s11605-007-0425-3. [DOI] [PubMed] [Google Scholar]
- [59].Zhang Q, Yamaza T, Kelly AP, Shi S, Wang S, Brown J, Wang L, French SW, Le AD. Tumor-like stem cells derived from human keloid are governed by the inflammatory niche driven by IL-17/IL-6 axis. PLoS One. 2009;4:e7798. doi: 10.1371/journal.pone.0007798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Li X, Ling W, Pennisi A, Wang Y, Khan S, Heidaran M, Pal A, Zhang X, He S, Zeitlin A, Abbot S, Faleck H, Hariri R, Shaughnesssy J, van Rhee F, Nair B, Barlogie B, Epstein J, Yaccoby S. Human placental-derived adherent cells prevent bone loss, stimulate bone formation, and suppress growth of multiple myeloma in bone. Stem Cells. 2010 doi: 10.1002/stem.572. doi: 10.1002/stem.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Xu X, Yang G, Zhang H, Prestwich GD. Evaluating dual activity LPA receptor pan-antagonist/autotaxin inhibitors as anti-cancer agents in vivo using engineered human tumors. Prostaglandins Other Lipid Mediat. 2009;89:140–146. doi: 10.1016/j.prostaglandins.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhang H, Xu X, Gajewiak J, Tsukahara R, Fujiwara Y, Liu J, Fells JI, Perygin D, Parrill AL, Tigyi G, Prestwich GD. Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 2009;69:5441–5449. doi: 10.1158/0008-5472.CAN-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Xu X, Prestwich GD. Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in a engineered three-dimensional lung cancer xenograft model. Cancer. 2010;116:1739–1750. doi: 10.1002/cncr.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Prestwich GD. Clinical biomaterials for scar-free healing and localized delivery of cells and growth factors. Adv. Wound Care. 2010;1:394–399. [Google Scholar]