Abstract
Objective:
Our aim was to determine the incidence of peripheral neuropathy in a population-based inflammatory bowel disease (IBD) cohort from Olmsted County, Minnesota.
Methods:
We retrospectively ascertained neuropathy incidence in a population-based cohort of adult persons newly diagnosed with IBD between 1940 and 2004 in Olmsted County, Minnesota, using the medical records linkage system of the Rochester Epidemiology Project. The Kaplan-Meier method was used to estimate the cumulative incidence of neuropathy.
Results:
A total of 772 Olmsted County residents aged 18 to 91 years were diagnosed with IBD. After 12,476 person-years, 9 patients developed neuropathy, providing an overall incidence rate of 72 (95% confidence interval [CI] 33–137) cases per 100,000 IBD person-years. The cumulative incidence rates after 10, 20, and 30 years were 0.7% (95% CI 0.0%–1.3%), 0.7% (95% CI 0.0%–1.5%), and 2.4% (95% CI 0.6%–4.6%), respectively. Neuropathy was diagnosed after 1 to 44 years from IBD onset. Only 2 patients had active bowel disease at the time of neuropathy onset. The clinical spectrum consisted of 1) monophasic immune radiculoplexus neuropathy (comorbid diabetes in 2 of 4 patients) and 2) chronic distal sensorimotor polyneuropathy (comorbid diabetes in 2 of 5 patients).
Conclusions:
Our population-based study suggests that neuropathy is uncommon in the patient population of IBD. Radiculoplexus neuropathy and sensorimotor polyneuropathy were both observed, commonly during periods of bowel disease inactivity. Clinicians should consider other etiologies of neuropathy in patients with IBD.
Crohn disease (CD) and ulcerative colitis (UC), collectively referred to as inflammatory bowel disease (IBD), are clinically heterogeneous disorders with potential for immune-mediated extraintestinal manifestations.1
Peripheral neuropathy (PN) is one of the most frequently reported neurologic complications of IBD.2–4 In addition to nutritional (e.g., B12 deficiency) and iatrogenic (e.g., metronidazole neurotoxicity) causes, patients with IBD may experience severe immune-mediated neuropathies as extraintestinal manifestations throughout the clinical course.5–8 It is important to quantify the morbidity burden of clinically significant PN in IBD, particularly those of immune etiology, as they may be more incapacitating than the bowel involvement (e.g., paralysis) or may indicate a higher risk of severe combined clinical course (e.g., relapsing course) potentially representing an important treatment target for future studies. The reported frequency of PN in IBD varies greatly among published studies, with estimates ranging from 0% to 39% depending on the study population characteristics and neuropathy criteria,2–4 and longitudinal population-based incidence studies are lacking.
In this study, we calculated the annual and cumulative incidence rates of clinical PN among patients with IBD from Olmsted County, Minnesota, who were diagnosed between 1940 and 2004. We also studied the clinical spectrum of PN and its association with bowel disease activity.
METHODS
Standard protocol approvals, registrations, and patient consents.
The Olmsted Medical Center and Mayo Clinic Institutional Review Boards approved the study. Written informed consent was obtained before chart review.
Design.
This was a retrospective, observational population-based cohort study of incident PN throughout the course of incident IBD among community adults in Olmsted County, Minnesota, followed from January 1, 1940, to December 31, 2004, by using diagnostic data generated by all the community health care providers and gathered and indexed by the Rochester Epidemiology Project. We cross-matched the list of confirmed community-incident IBD with lists of PN diagnoses generated from clinical, electrophysiologic, and pathologic databases at Mayo Clinic. We describe below the details of the database source capabilities and case ascertainment strategy.
Database source.
The population of Olmsted County, Minnesota, is well suited for a population-based epidemiologic study of PN in IBD. The Rochester Epidemiology Project maintains a comprehensive medical records linkage system; thus, all diagnoses (including medical, surgical, and postmortem) made by all health care providers in the area are indexed and retrievable. Also, the medical care of the residents of Olmsted County is virtually self-contained within the community. Subspecialty tertiary care services, including gastroenterology and neurology, are provided almost entirely by Mayo Clinic. EMG services within the area are provided only by Mayo Clinic. A detailed description of the data sources and their capabilities is given elsewhere.9 Ninety-eight percent of the general population in Olmsted County is seen at Mayo Clinic over a period of 5 years,9,10 and the Rochester Epidemiology Project enumerates the entire population of Olmsted County more effectively than the US Census.10 The actual county general population in the 2010 Census was 142,248, and 83.4% of the population was non-Hispanic white. According to the 2010 US Census, the percentage of non-Hispanic whites in the national population is 63%.11
IBD case ascertainment.
Identified potential adult cases were verified to have features consistent with IBD on the basis of available clinical, endoscopic, radiologic, surgical, and histologic findings. All charts were reviewed by a gastroenterologist to confirm the diagnoses. The diagnostic criteria used have been previously reported.12–14 Only incident cases were included. Patients diagnosed with IBD before moving to the area were excluded. The incident cases comprised the inception cohort for our study. Severity at presentation was assessed by the initial distribution of bowel involvement. Localized initial involvement was classified as proctitis, left-sided colitis, colitis, ileocolitis, ileocecal, oral/esophageal, gastroduodenal, or small-bowel involvement. Extensive initial involvement was defined as any combination of previously mentioned sites. Follow-up time was defined as time between initial diagnosis of IBD and last follow-up visit.
Incident neuropathy ascertainment in the IBD cohort.
We identified potential PN cases from centralized databases of clinical diagnoses and billing codes indexed from Mayo Clinic medical records by using a comprehensive and extensive list of key words and billing codes. The following is a representative sample of queried words or codes: neuropathy, peripheral neuropathy, diabetic neuropathy, uremic neuropathy, alcoholic neuropathy, small-fiber neuropathy, demyelinating neuropathy, autonomic neuropathy, multifocal motor neuropathy, neuropathy not otherwise specified, burning feet, neuropathic, neuropathic pain, nerve pain, neuralgia, leg neuralgia, neuritis, leg neuritis, polyneuritis, paralysis, palsy, plexus, plexopathy, plexitis, diabetic amyotrophy, Parsonage-Turner syndrome, Guillain-Barré syndrome, Miller Fisher syndrome, Landry' disease, Landry-Guillain-Barré syndrome, mononeuropathy, mononeuropathy multiplex, mononeuritis multiplex, myeloradiculitis, radiculomyelitis, polyradiculitis, polyradiculoneuropathy, polyradiculopathy, chronic inflammatory demyelinating polyneuropathy, deafferentation syndrome, polyganglionopathy, ganglioneuropathy, neuronopathy, motor neuronopathy, sensory neuronopathy, and neuronitis. We cross-matched the list of confirmed IBD cases with all the generated PN lists and with the Mayo Clinic Electromyography and Nerve Laboratory Databases. We reviewed the Mayo Clinic and Olmsted County medical records of patients with IBD with a potential PN diagnosis. The minimal criteria for definite PN were 1) neuropathic symptoms suggestive of PN; 2) neuropathic deficits suggestive of PN; and 3) abnormal nerve conduction and EMG, autonomic function test, or quantitative sensory testing. If these tests were not performed but the patient had neuropathic symptoms and deficits, then the diagnosis of PN was considered probable. Severity of neuropathic deficits, both motor and sensory, was quantified in a standardized fashion by Neuropathy Impairment Score (NIS).15 Cranial mononeuropathies (e.g., Bell palsy), isolated limb mononeuropathies (e.g., carpal tunnel syndrome), and compressive radiculopathies were excluded. Standard diagnostic workup for neuropathy was performed in all patients and included nutritional (hemoglobin, mean corpuscular volume, serum vitamin B12, folate, and various vitamin levels), metabolic (fasting glucose, hemoglobin A1C, creatinine, and thyroid function), autoimmune/inflammatory (erythrocyte sedimentation rate, antinuclear antibodies, rheumatoid factor, and serum protein electrophoresis), and infectious (testing for hepatitis B and C, syphilis, and HIV, when appropriate) studies. Drug treatment history, including metronidazole and biologic agents, and history of tobacco and alcohol use were also noted. Treatment and subsequent evaluations were documented. Coexistence of potential alternative culprits of neuropathy, exposure to drug-related neurotoxicity, or presence of risk factors for neuropathy was not an exclusion criterion.
Statistical analysis.
The annual incidence rate was calculated by using the cases of PN as the numerator and the follow-up in the IBD inception cohort as the denominator and was expressed as cases per person-years of study. The cumulative incidence of PN (1 minus survival-free of PN) was estimated for up to 30 years after the date of IBD diagnosis by using Kaplan-Meier methods. Death or lost at follow-up before neuropathy event was treated as censored data.
RESULTS
A total of 772 adult Olmsted County residents (402 men; 370 women) were newly diagnosed with IBD between 1940 and 2004 at a median age of 36 years (range, 18–91 years). The proportions of CD (n = 342; 44.3%) and UC (n = 430; 55.7%) cases were comparable. The cohort was followed up for a total of 12,476 person-years (median follow-up, 13.8 years; range 0.1–57.6 years), with all cases accounted for at last follow-up including 148 (19.2%) deaths. Severity at presentation as assessed by initial distribution of bowel involvement was available in 601 cases (78%), of whom 105 (18%) were considered to have an initial extensive involvement.
After 12,476 person-years, only 9 patients (5 women; 4 men) with IBD (6 with UC; 3 with CD) developed 12 events of PN (2 events in 3 patients), providing an overall crude annual incidence rate of PN in the IBD population of 72 cases per 100,000 person-years (95% confidence interval [CI], 35–134 cases per 100,000 person-years). The cumulative incidence rates of PN after IBD at 10, 20, and 30 years of follow-up were 0.7% (95% CI, 0.0%–1.3%), 0.7% (95% CI, 0.0%–1.5%), and 2.4% (95% CI, 0.6%–4.6%), respectively (figure).
Figure. Cumulative incidence of peripheral neuropathy in patients with inflammatory bowel disease from Olmsted County, Minnesota, 1940–2004.
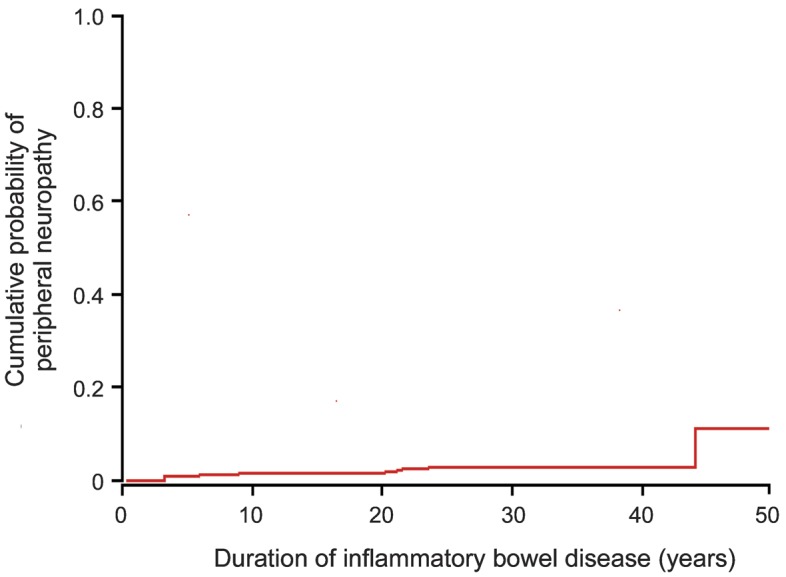
The 9 patients with neuropathy were initially diagnosed with IBD at a median age of 50 years (range, 21–83 years) and subsequently with PN after a median of 17.5 years (range, 0.8–44 years). Of the 12 episodes of neuropathies, 11 were definite, and 1 was probable (sensory-predominant distal polyneuropathy). Neuropathies comprised 2 different phenotypes: 1) monophasic immune radiculoplexus neuropathy seen in 4 patients, 2 of them with comorbid diabetes and 2 idiopathic; and 2) chronic sensorimotor distal polyneuropathy seen in 5 patients, 2 of them with comorbid diabetes and 3 idiopathic. Except for diabetes, standard neuropathy workup was unrevealing for alternative neuropathy causes, including absence of B12 deficiency. No patient had exposure to metronidazole or biologic agents at the onset of peripheral neuropathy. Of 8 patients with available information, 6 had inactive bowel disease at the onset of neuropathy (table).
Table.
Characteristics of 9 community patients with inflammatory bowel disease who subsequently developed peripheral neuropathy
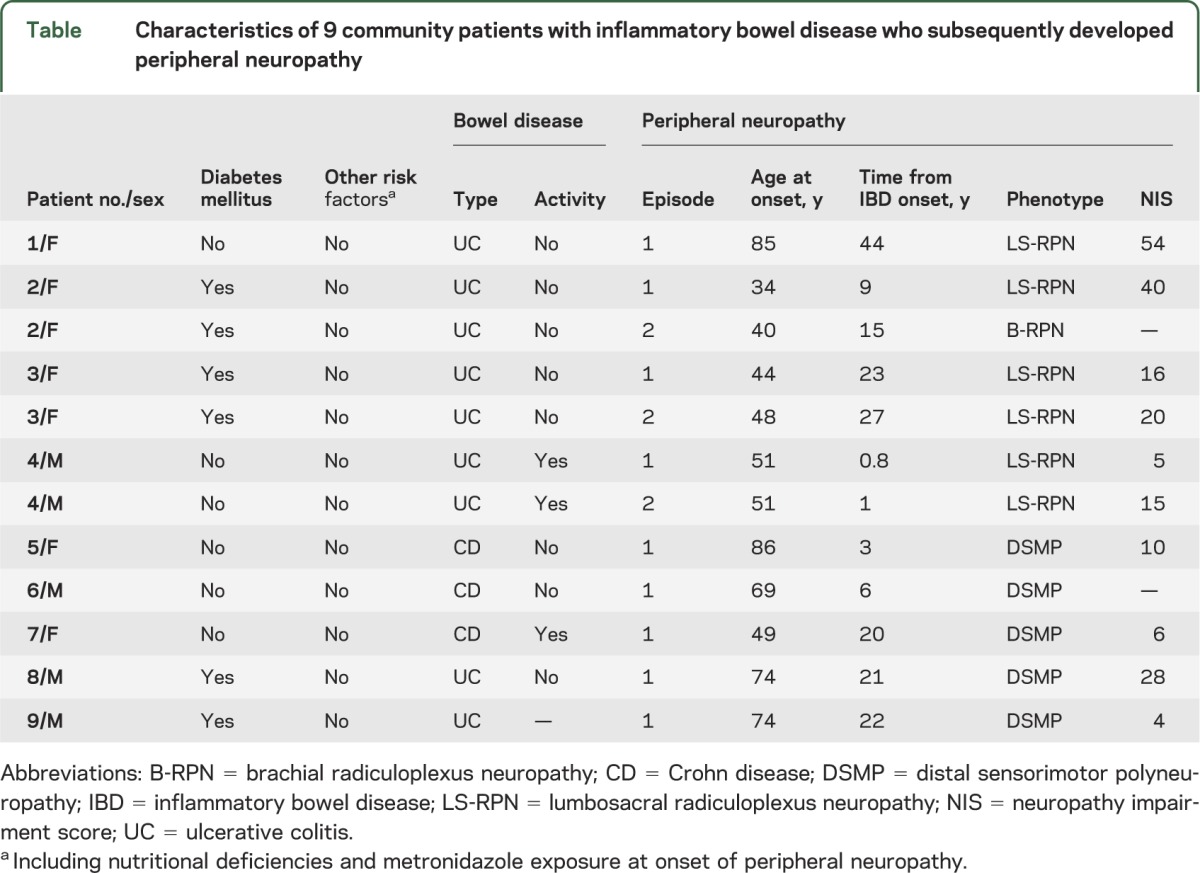
The immune radiculoplexus neuropathies (diabetic and nondiabetic) were characterized by limb pain at onset, followed by a monophasic course of limb weakness and sensory impairment of moderate severity (NIS, 5–54), which would translate into moderate impairment of activities of daily living. Three of 4 patients had recurrence of radiculoplexus neuropathies after variable intervals (table). Pain and weakness of the lower extremities in patients with radiculoplexus neuropathies with available neurologic follow-up information improved as follows: 1) in patient 1, from moderately severe to mild at 6-month follow-up without immunomodulatory treatment; 2) patient 2 (first episode), from moderate to mild at 4-month follow-up after a 1-week course of tapering doses of oral methylprednisolone; 3) patient 3 (second episode), from moderate to mild at 1-year follow-up after 4 weekly doses of 1 g IV methylprednisolone; and 4) patient 4 (second episode), from moderate to mild at 1-year follow-up without immunomodulatory treatment. The polyneuropathies (diabetic and nondiabetic) were chronic, indolent, and of mild severity (NIS, 4–28). No antecedent triggers such as anti–tumor necrosis factor-α therapy, recent surgery, or recent viral illness preceded onset of the neuropathies.
Postpartum Parsonage-Turner syndrome was recorded in 1 case, preceding the diagnosis of IBD by 25 years. Given that this was not an incident case of neuropathy in IBD, we did not include this case in the analysis.
DISCUSSION
The main findings of our study are that in the community, incident PN in IBD patients 1) was rare; 2) occurred late in the course of the disease, mainly during periods of bowel disease inactivity; and 3) was characterized by 2 equally common phenotypes: mild chronic large-fiber sensory-predominant polyneuropathy and moderately severe immune radiculoplexus neuropathy. These findings suggest that PN in IBD is not as common as thought from referral-based cohort evaluations, highlighting the need for careful exclusion of other causes of neuropathy when both conditions are encountered in clinical practice. Although infrequent, some association between PN and IBD may exist on the basis of 1) several case reports6 of putative immune neuropathies paralleling the IBD course; 2) frequent reports6 of comorbid inflammatory neuropathies and IBD; 3) subclinical or minimally symptomatic unexplained polyneuropathies detected frequently in prospective referral-based studies7,16; and 4) a recent case-control study reporting increased frequency of PN among IBD cases followed at a gastrointestinal specialty clinic from a tertiary referral center compared with their unaffected relatives and medical center staff.4 The severity of bowel disease may be a contributory factor for neuropathy. Nutritional or systemic inflammatory issues may be predicted more frequently among more severely affected individuals and may explain high neuropathy frequencies in prior reports. Only one-fifth of our patients had defined extensive bowel involvement at IBD onset, and this suggests a milder IBD phenotype of our cohort. The Olmsted County IBD population has previously been characterized as milder compared with referral IBD populations.17 Contributing to the health of our IBD population is the routine close monitoring of nutritional status. Milder disease severity and close nutritional monitoring could potentially explain the infrequent development of neuropathy in our community IBD population.
A recent study18 on comorbid conditions in 8,972 patients with IBD, using physician-based billing claims from universal health insurance administrative data in the Canadian province of Manitoba, reported a population-based prevalence of neuropathy in IBD between 0.1% and 0.18%. The concordant low population-based frequencies, expressed as incidence in Olmsted County and as prevalence in Manitoba, argue that neuropathy is rare among community patients with IBD. We recognize that our population is different in race and social environment compared with one large Brazilian prospective study7 in which neuropathy was believed to be common (13.4%), but the differences in the selection of cases may in fact be the larger factor determining the differences with our findings. Nevertheless, their identified patients also had generally mild neuropathy.
The observed overrepresentation of immune plexopathies among the small number of neuropathy-affected IBD cases found in our study does raise the possibility of a common pathogenic mechanism (i.e., an inflammatory/immune cause).19–22 The annual incidence rate of immune brachial plexus neuropathy in the general population of Olmsted County is 1.64 cases per 100,000 person-years.23 Our single case of brachial plexus neuropathy yields an overall crude annual incidence rate of 7.3 per 100,000 with a wide CI. To achieve enough statistical power to prove a major increase in the incidence of brachial plexus neuropathy among patients with IBD compared with the general population, we would require a population sample in the hundreds of thousands. The incidence of lumbosacral plexus neuropathy is not known; therefore, we are unable to state whether there is higher incidence in IBD, and again the statistical issues likely remain for this association as well. It is reasonable, however, according to our study, to consider coexistent IBD in patients with idiopathic or diabetic lumbosacral plexus neuropathies.
Whether the relatively good nutritional status and overall milder bowel disease severity of our IBD cohort may have contributed to the low frequency of neuropathy remains to be determined.
Glossary
- CD
Crohn disease
- CI
confidence interval
- IBD
inflammatory bowel disease
- NIS
Neuropathy Impairment Score
- PN
peripheral neuropathy
- UC
ulcerative colitis
AUTHOR CONTRIBUTIONS
Study concept and design, collecting the data, drafting and revising the manuscript, and interpretation of the data: J.J. Figueroa, W.S. Harmsen, E.V. Loftus Jr., P.J.B. Dyck, and C.J. Klein. Obtaining funding: E.V. Loftus Jr. Statistical analysis: W.S. Harmsen.
STUDY FUNDING
Supported by the Mayo Foundation for Medical Education and Research, Rochester Epidemiology Project (grant R01 AG034676 from the National Institute on Aging), and NIH grant K08 NS065007-01A1, NS36797.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347:417–429 [DOI] [PubMed] [Google Scholar]
- 2.Elsehety A, Bertorini TE. Neurologic and neuropsychiatric complications of Crohn's disease. South Med J 1997;90:606–610 [DOI] [PubMed] [Google Scholar]
- 3.Lossos A, River Y, Eliakim A, Steiner I. Neurologic aspects of inflammatory bowel disease. Neurology 1995;45:416–421 [DOI] [PubMed] [Google Scholar]
- 4.Shen TC, Lebwohl B, Verma H, et al. Peripheral neuropathic symptoms in celiac disease and inflammatory bowel disease. J Clin Neuromuscul Dis 2012;13:137–145 [DOI] [PubMed] [Google Scholar]
- 5.Crespin V, Bogliun G, Marzorati L, et al. Inflammatory bowel disease and peripheral neuropathy. J Neurol 1994;241suppl 1:63 [Google Scholar]
- 6.Gondim FA, Brannagan TH, III, Sander HW, Chin RL, Latov N. Peripheral neuropathy in patients with inflammatory bowel disease. Brain 2005;128:867–879 [DOI] [PubMed] [Google Scholar]
- 7.Oliveira GR, Teles BC, Brasil EF, et al. Peripheral neuropathy and neurological disorders in an unselected Brazilian population-based cohort of IBD patients. Inflamm Bowel Dis 2008;14:389–395 [DOI] [PubMed] [Google Scholar]
- 8.Sassi SB, Kallel L, Ben Romdhane S, Boubaker J, Filali A, Hentati F. Peripheral neuropathy in inflammatory bowel disease patients: a prospective cohort study. Scand J Gastroenterol 2009;44:1268–1269 [DOI] [PubMed] [Google Scholar]
- 9.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266–274 [DOI] [PubMed] [Google Scholar]
- 10.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, III, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 2011;173:1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United States Census Bureau State & county QuickFacts: Olmstead County, Minnesota. Available at: http://quickfacts.census.gov/qfd/states/27/27109.html.
- 12.Loftus CG, Loftus EV, Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis 2007;13:254–261 [DOI] [PubMed] [Google Scholar]
- 13.Loftus EV, Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn's disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology 1998;114:1161–1168 [DOI] [PubMed] [Google Scholar]
- 14.Loftus EV, Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Ulcerative colitis in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gut 2000;46:336–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyck PJ, Sherman WR, Hallcher LM, et al. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol 1980;8:590–596 [DOI] [PubMed] [Google Scholar]
- 16.Stahlberg D, Barany F, Einarsson K, Ursing B, Elmqvist D, Persson A. Neurophysiologic studies of patients with Crohn's disease on long-term treatment with metronidazole. Scand J Gastroenterol 1991;26:219–224 [DOI] [PubMed] [Google Scholar]
- 17.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology 2001;121:255–260 [DOI] [PubMed] [Google Scholar]
- 18.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology 2005;129:827–836 [DOI] [PubMed] [Google Scholar]
- 19.Dyck PJ, Engelstad J, Norell J. Microvasculitis in non-diabetic lumbosacral radiculoplexus neuropathy (LSRPN): similarity to the diabetic variety (DLSRPN). J Neuropathol Exp Neurol 2000;59:525–538 [DOI] [PubMed] [Google Scholar]
- 20.Dyck PJ, Norell JE. Microvasculitis and ischemia in diabetic lumbosacral radiculoplexus neuropathy. Neurology 1999;53:2113–2121 [DOI] [PubMed] [Google Scholar]
- 21.Dyck PJ, Norell JE. Non-diabetic lumbosacral radiculoplexus neuropathy: natural history, outcome and comparison with the diabetic variety. Brain 2001;124:1197–1207 [DOI] [PubMed] [Google Scholar]
- 22.Suarez GA, Fealey RD, Camilleri M, Low PA. Idiopathic autonomic neuropathy: clinical, neurophysiologic, and follow-up studies on 27 patients. Neurology 1994;44:1675–1682 [DOI] [PubMed] [Google Scholar]
- 23.Beghi E, Kurland LT, Mulder DW, Nicolosi A. Brachial plexus neuropathy in the population of Rochester, Minnesota, 1970-1981. Ann Neurol 1985;18:320–323 [DOI] [PubMed] [Google Scholar]


