Abstract
Adenovirus infections frequently complicate allogeneic stem cell transplants but nervous system involvement, usually presenting as encephalitis, is atypical. Progression from encephalitis to myeloradiculitis has not been described previously.1 We present a unique case of fatal adenoviral encephalomyeloradiculitis with imaging and pathologic correlates.
Adenovirus infections frequently complicate allogeneic stem cell transplants, but nervous system involvement, usually presenting as encephalitis, is atypical. Progression from encephalitis to myeloradiculitis has not been described previously.1 We present a unique case of fatal adenoviral encephalomyeloradiculitis with imaging and pathologic correlates.
Case report.
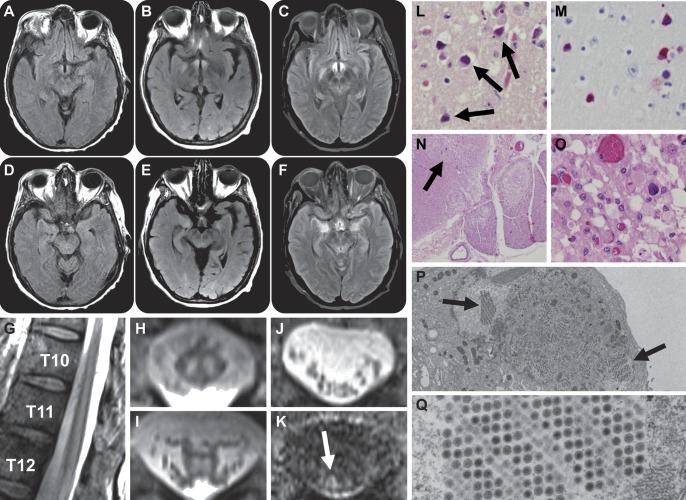
A 58-year-old right-handed woman with T-cell prolymphocytic leukemia, status post double umbilical cord blood stem cell transplantation (DUCBT) with fludarabine, melphalan, and anti-thymocyte globulin (ATG) conditioning 6 months prior, was admitted to our hospital with altered mental status and fevers to 40°C. She had been admitted on 2 prior occasions for idiopathic mental status changes and persistent fevers which resolved over 3–4 days. Unlike the previous visits, CD4 count on admission was 1 cell/µL. Plasma adenovirus DNA, which had been intermittently detected at low levels since her DUCBT but was negative 2 weeks prior, was now positive with 935 copies/mL. Adenovirus CSF PCR was also positive with 817,296 copies/mL. CSF contained no leukocytes. Sequential brain MRIs revealed progressive abnormal T2 hyperintensity in the hypothalami and medial temporal lobes without mass effect or enhancement (figure, A–F).
Figure. Imaging and histology.
Progressive abnormal T2 hyperintensity is seen in the hypothalami (A–C) and medial temporal lobes (D–F) at the ninth (B, E) and 23rd hospital days (C, F), compared to MRI at presentation (A, D). Sagittal T2-weighted MRI of the lower thoracic spine shows abnormal central cord hyperintensity extending rostrally from the conus medullaris (G). Axial T2-weighted MRI at the T10 (H) and T12 (I) vertebral levels shows abnormal T2 hyperintensity within the spinal cord gray matter. The spinal cord was not expanded and there was no abnormal cord enhancement following administration of gadolinium contrast (not shown). Axial T2 (J) and T1 postgadolinium (K) MRI at the L3 vertebral level shows abnormal nerve root enhancement (K, arrow). Postmortem hematoxylin & eosin section of the hypothalamic region demonstrates vacuolization, neuronal loss, and reactive gliosis as well as intraneuronal and glial adenoviral inclusions (L, arrows, 400×), also seen on adenovirus-specific immunohistochemical staining (M, 400×). The neocortex, thalamus, caudate nucleus, and dorsal motor nucleus of the vagus were similarly affected (not shown). Hematoxylin & eosin section of the lumbar spinal cord shows parenchymal destruction primarily involving the posterior horns (N, arrow, 20×; O, 400×). Adenovirus inclusions were seen, along with macrophages and axonal swellings (O). Electron micrographs of cell culture obtained from brain tissue show intranuclear inclusions containing nonenveloped hexagonal viral particles of adenovirus (P, 2,700×; Q, 21,000×).
She received IV cidofovir and CMX001, an investigational antiviral (clinicaltrials.gov, NCT01143181).2,3 Despite antiviral therapy, the patient developed bilateral spastic paraparesis progressing to areflexia over several days. A nerve conduction study suggested diffuse myeloradiculopathy. MRI of the spine revealed abnormal T2 hyperintensity within the central spinal cord extending rostrally from the conus medullaris to the T2 vertebral level (figure, G–I) with abnormal enhancement of cauda equina nerve roots (figure, J and K). Despite broad-spectrum antimicrobial agents and supportive care, the patient died 2 weeks later.
General autopsy and immunohistochemistry demonstrated lymph node, brain, and spinal cord parenchyma evidence of viral cytopathologic change (i.e., amphophilic, “smudgy” or dense nuclear chromatin, and mild nuclear enlargement, without true Cowdry B inclusion formation or cytoplasmic inclusions; figure, L–O). Furthermore, pathologic examination revealed parenchymal destruction with vacuolation, neuronal loss, and reactive gliosis involving predominantly the posterior horns of the entire spinal cord, illustrated in a section of lumbosacral spinal cord (figure, N and O). Several surviving neurons, including the dorsal root ganglia, showed adenovirus inclusions on immunohistochemistry. Serial sections showed destruction of central myelinated fibers with clear demarcation from peripheral myelin in the lumbosacral spinal cord (figure, N). Sections of spinal nerve roots at multiple levels showed focal lymphocytic inflammation and evidence of myelin breakdown (radiculitis). Adenovirus serotype 6 was isolated in cell culture from brain tissue obtained at the time of autopsy. Of note, serial CSF cytomegalovirus (CMV) viral loads before and after the onset of radiculopathy were negative, and cauda equina nerve roots and dorsal root ganglion did not stain positively for CMV.
Discussion.
Adenovirus affects 14%–20% of adult stem cell transplant (SCT) patients.4,5 Neurologic manifestations are uncommon and include meningoencephalitis characterized by lethargy, meningismus, and headache, with mortality of up to 80%.6
Our patient presented 6 months post-SCT with meningoencephalitis that rapidly progressed to myeloradiculitis and ultimately death despite treatment. The first and only other case report of fatal adenoviral meningoencephalitis was in a 17-year-old SCT recipient who at autopsy had bilateral symmetric degeneration of the inferomedial temporal cortex, amygdala, hippocampus, hypothalamus, and brainstem, secondary to relapsing-remitting disease.7 Our patient had abnormal T2 hyperintensity in similar regions, with microscopic evidence of intraneuronal and intraglial viral inclusions suggesting disseminated adenovirus infection with a predilection for these brain regions.
Although enteroviruses (coxsackie, echovirus, poliovirus), rabies virus, CMV, West Nile virus, and Japanese encephalitis virus are known to cause myeloradiculopathy,e1 to our knowledge this is the first case of adenovirus myeloradiculitis. Given the presence of viral inclusions in anterior and posterior horn cells and dorsal root ganglia in this patient, we postulate direct viral infiltration and destruction of spinal cord neurons and dorsal root ganglia neurons as the mechanism of flaccid paralysis. This patient was highly immunocompromised following recent DUCBT, ATG conditioning, alemtuzumab exposure prior to her DUCBT, and severe lymphopenia—all factors associated with poor recovery of adenovirus-specific T-cell immunity and an increased risk of severe, disseminated adenovirus disease.e2
Surveillance and treatment of adenovirus remains controversial. Although the role of routine surveillance is unclear, several studies have illustrated that serial peripheral blood PCR assays in transplant recipients may detect adenovirus up to 3 weeks prior to symptom onset, facilitating early and preemptive therapy4,6; the patient presented here had negative peripheral blood and CSF adenovirus PCR assays shortly before development of her fulminant encephalomyeloradiculitis syndrome.
Supplementary Material
Footnotes
Supplemental data at www.neurology.org
For references e1 and e2, please visit the Neurology® Web site at www.neurology.org.
This article was prepared while Oluwole O. Awosika was employed at the Brigham and Women's Hospital. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Author contributions: Dr. Awosika: drafting/revising the manuscript, study concept and design, acquisition of data, analysis and interpretation of data. Dr. Lyons: drafting/revising the manuscript, study concept and design, acquisition of data, analysis and interpretation of data. Dr. Ciarlni: revising the manuscript, acquisition of data analysis and interpretation of data. Dr. Phillips: drafting/revising the manuscript. Dr. Alfson: drafting/revising the manuscript. Dr. Johnson: revising the manuscript, acquisition of data. Dr. Koo: drafting/revising the manuscript, interpretation of data. Dr. Marty: revising the manuscript, interpretation of data. Dr. Drew: acquisition of data analysis and interpretation of data. Dr. Zaki: acquisition of data analysis and interpretation of data. Dr. Folkerth: study concept or design, interpretation of data. Dr. Klein: critical revision of the manuscript for important intellectual content, study concept, acquisition and interpretation of data.
Study funding: No targeted funding reported.
Disclosure: The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Schmidt-Hieber M, Schwender J, Heinz WJ, et al. Viral encephalitis after allogeneic stem cell transplantation: a rare complication with distinct characteristics of different causative agents. Haematologica 2011;96:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florescu DF, Pergam SA, Neely MN, et al. Safety and efficacy of CMX001 as salvage therapy for severe adenovirus infections in immunocompromised patients. Biol Blood Marrow Transplant 2012;18:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quenelle DC, Lampert B, Collins DJ, et al. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. J Infect Dis 2010;202:1492–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch JP, Fishbein M, Echavarria M. Adenovirus. Semin Respir Crit Care Med 2011;32:494–511 [DOI] [PubMed] [Google Scholar]
- 5.Carrigan DR. Adenovirus infections in immunocompromised patients. Am J Med 1997;102:71–74 [DOI] [PubMed] [Google Scholar]
- 6.Barton LL, Friedman NR, Volpe JJ. The Neurological Manifestations of Pediatric Infectious Diseases and Immunodeficiency Syndromes. Totowa: Humana Press; 2010 [Google Scholar]
- 7.Davis D, Henslee PJ, Markesbery WR. Fatal adenovirus meningoencephalitis in a bone marrow transplant patient. Ann Neurol 1988;23:385–389 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.