Abstract
Objective:
To analyze the relationship between body weight variability and dementia more than 3 decades later.
Methods:
The measurement of body weight variability was based on 3 successive weight recordings taken from over 10,000 apparently healthy tenured working men participating in the Israel Ischemic Heart Disease study, in which cardiovascular risk factors and clinical status were assessed in 1963, 1965, and 1968, when subjects were 40–70 years of age. Groups of men were stratified according to quartiles of SD of weight change among 3 measurements (1963/1965/1968): ≤1.15 kg, 1.16–1.73 kg, 1.74–2.65 kg, and ≥2.66 kg. The prevalence of dementia was assessed more than 36 years later in approximately one-sixth of them who survived until 1999/2000 (minimum age 76 years) and underwent cognitive evaluation (n = 1,620).
Results:
Survivors' dementia prevalence rates were 13.4%, 18.4%, 20.1%, and 19.2% in the first to fourth quartiles of weight change SD, respectively (p for trend = 0.034). Compared to the first quartile of weight change SD and adjusted for diabetes mellitus, body height, and socioeconomic status, a multivariate analysis demonstrated that the odds ratio for dementia was 1.42 (95% confidence interval [CI] 0.95–2.13), 1.59 (95% CI 1.05–2.37), and 1.74 (95% CI 1.14–2.64) in quartiles 2–4 of weight change SD respectively. This relationship was independent of the direction of weight changes.
Conclusion:
Midlife variations in weight may antecede late-life dementia.
Increasing prevalence of obesity and overweight,1 and studies showing that body weight2 and changes in body weight3 are associated with cognitive decline and dementia, stress the importance of understanding their role in cognition. This relationship may be complex since several weight-related factors have been demonstrated to be involved with increased risk for dementia, namely overweight,4 underweight,5 and changes in weight over time.5,6
Body weight variability has been shown7–9 to be associated with significantly increased morbidity and mortality (but see reference 10). Weight fluctuations, but not stable obesity, were associated in some studies with increased risk for all-cause mortality.8 The aim of the present study was to analyze the relationship between body weight variability in over 9,000 men who participated in the Israel Ischemic Heart Disease Project (IIHD), with dementia in more than 1,600 survivors of the original cohort more than 3 decades later. The measurement of body weight variability was based on 3 successive weight recordings in 1963, 1965, and 1968, when subjects were 40–70 years of age. The prevalence of dementia was assessed more than 36 years later in survivors of the original cohort (n = 1,620).
METHODS
The Israel Ischemic Heart Disease Project.
In 1963, the sample of the IIHD was chosen by stratified sampling of civil servants and municipal employees aged 40 years and above at the time of inclusion. Of 11,876 men approached, 10,232 (86.2%) agreed to participate. The subjects were seen upon enrollment (1963) and at 2 follow-up visits (1965 and 1968). Analysis excluded 173 men born outside 6 predefined geographical areas. Further details of the study have been described elsewhere.11
Socioeconomic status index at baseline.
The Socioeconomic Status Index (SES) was based on formal education and the type of employment. Formal education included 9 levels ranging from "no formal education" to "completed university education." Employment was the current one at the time of examination (1963) and included 5 levels ranging from "laborer" to "professional." The SES scale ranged from 1 ("very low SES"; 3 lowest education levels and lowest employment level) to 5 ("very high SES"; 3 highest education and 2 highest employment levels).11
Leisure time physical activity at baseline.
In 1965, subjects were asked the following question: "What degree of leisure time physical activity to you practice?" The answer that describes best the degree of physical activity was to be chosen by the subjects: 1) almost no physical activity, 2) inconsistent physical activity, 3) daily physical activity, 4) daily effortful physical activity.
Intentional weight-losing diet.
Subjects were asked in 1963 if they were on a diet. For those who answered "yes," 6 options were given as main reason for diet (each reason could be answered "yes" or "no"): heart disease, hypertension, diabetes, peptic ulcer, reduction of dietary fat, or "other" (e.g., vegetarian).
Stratification of subjects to groups according to body mass index and SD between weighing.
Subjects were stratified by the 1963 BMI measure to 4 groups: body mass index (BMI) <20 kg/m2 (group I), 20 kg/m2 < BMI < 24.99 kg/m2 (group II), 25 kg/m2 ≤ BMI < 29.99 kg/m2 (group III), and BMI ≥30 kg/m2 (group IV). Groups of men were also stratified according to quartiles of SD of weight change among 3 measurements (1963, 1965, 1968): ≤1.15 kg, 1.16–1.73 kg, 1.74–2.65 kg, and ≥2.66 kg. The analysis regarding associations of weight variation with dementia is based on 4,863 measurements in 1,620 survivors with weight measurements in all 3 examinations.
Diagnosis of dementia.
Dementia assessment was performed in 1999/2000 in survivors of the original cohort, 36–37 years after weight assessments. Cases of dementia were identified using a 2-step procedure as described in detail elsewhere.12 The first step was a telephonic screening interview of subjects who consented to participate. They were administered the Hebrew version of the Modified Telephone Interview for Cognitive Status (TICS-m).13 The second step was a face-to-face interview with all the subjects with a TICS-m score of 27 or lower, aimed to ascertain the diagnosis of dementia among subjects who were identified by the TICS-m as possibly cognitively impaired. The subjects were assessed by a neurologist or psychiatrist, blind to the TICS-m score. The clinical assessment included the Dementia Questionnaire,14 Mini-Mental State Examination,15 Global Deterioration Scale,16 and Hachinski Ischemic Scale.17 Dementia was diagnosed using DSM-IV criteria.18 Subjects were classified as cognitively impaired no dementia (CIND) if both subject and informant reported a memory problem but they had normal activities of daily living and no dementia. The primary analysis compares subjects unequivocally with dementia to subjects unequivocally without dementia, excluding the CIND subjects, who are heterogeneous, with some subjects early in the course of cognitive decline.
According to the Israel Mortality Registry, 7,136 men had died by the beginning of the study in 1999; another 306 men died before being approached for a phone interview and 13 subjects were lost in the matching process. The remaining 2,604 subjects qualified for a telephone interview, of whom 2,038 had phone contact. The telephone screening identified 799 subjects potentially with dementia for a home interview. Of these, 149 could not be examined. Of the remaining 1,239 subjects who were not identified as potentially having dementia by the TICS-m, 51 were examined at home for a sensitivity study of the phone interview instruments; 50 were cognitively intact and 1 cognitively impaired but without dementia. The remaining 1,188 subjects were classified as no dementia. Thus, the follow-up study characterized the presence or absence of dementia in 1,889 subjects: 307 had dementia, 175 had CIND, and 1,407 elderly subjects had no cognitive impairment. Individuals who had survived until 1999/2000 were younger and taller, with lower BMI, glucose, and total cholesterol levels, lower systolic and diastolic blood pressure (SBP/DBP) values, and included fewer baseline diabetic and cigarette smokers. Their SES was slightly lower. The groups had similar average weight and high-density lipoprotein cholesterol levels.19
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Sheba Medical Center Helsinki committee.
Statistical analysis.
An order-directed score test for trend20 was used to test the departure of sample results consistent with a dose-response association between BMI categories assessed in 1963 and late-life dementia prevalence from a null hypothesis of an independence of dementia rates from BMI. The same was applied to categories of increasing BMI variation (SD 1963/1965/1968). Logistic regression was used to estimate the covariate (age, SES, body height, weight at baseline, diabetes)–adjusted odds for dementia prevalence, at the 3 highest quartiles of SD (weight), relative to the lowest quartile.
The relationship between survivors' dementia prevalence and weight variation was examined using the Royston-Altman regression using polynomials of continuous covariates.21 A transformation of the SD of weight, computed from the 3 measurements in 1963/1965/1968, was employed.
An issue in the setting of the study is the absence of information on dementia incidence between 1968 (last weight assessment) and 1999/2000. This may present bias to an analysis of the association between the weight variability in 1963/1965/1968 and dementia in 1999/2000. To account partly for the potential bias, we used an adaptation of a marginal structural model (MSM)22 applying inverse probability weights (IPW). We estimated the probability of every individual to actually reach the assessment phase in 1999/2000. In subsequent analysis the SD of the 1963/1965/1968 weights for each person was multiplied by the inverse of the individual probability. This was introduced into a logistic regression to examine the association between survivors' dementia and weight variation 1963/1965/1968. We compare the results of analysis using this method with analysis that did not take the above potential bias into account.
RESULTS
Table 1 depicts the numbers and baseline characteristics of men who were originally weighed in 1963/1965/1968 and of those who survived until 1999/2000 by groups of BMI. A total of 1,620 men survived until 1999/2000, had their weights assessed 3 times, participated in the dementia assessment, and were diagnosed as cognitively normal or having dementia. At baseline, lower BMI was associated with higher rates of smoking among the entire sample and among those who survived until 1999/2000 (table 1). Higher baseline BMI was associated with higher rates of diabetes in the original cohort and lower rates of diabetes in those who survived until 1999/2000 (table 1). Survival beyond age 75 years was 59.6%, 66.3%, 64.6%, and 53.1% in groups I–IV, respectively.
Table 1.
Baseline (1963) characteristics of IIHD participants (entire groups and survivors in 1999) by category of BMI in 1963 (for those who had BMI measurements in 1963, 1965, and 1968)
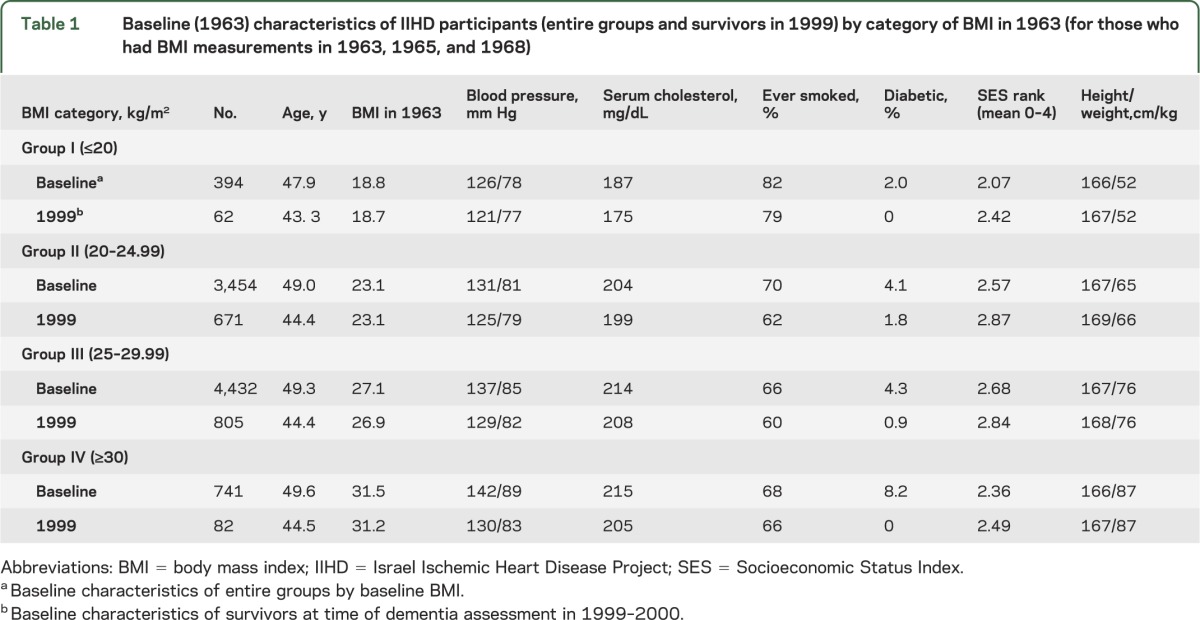
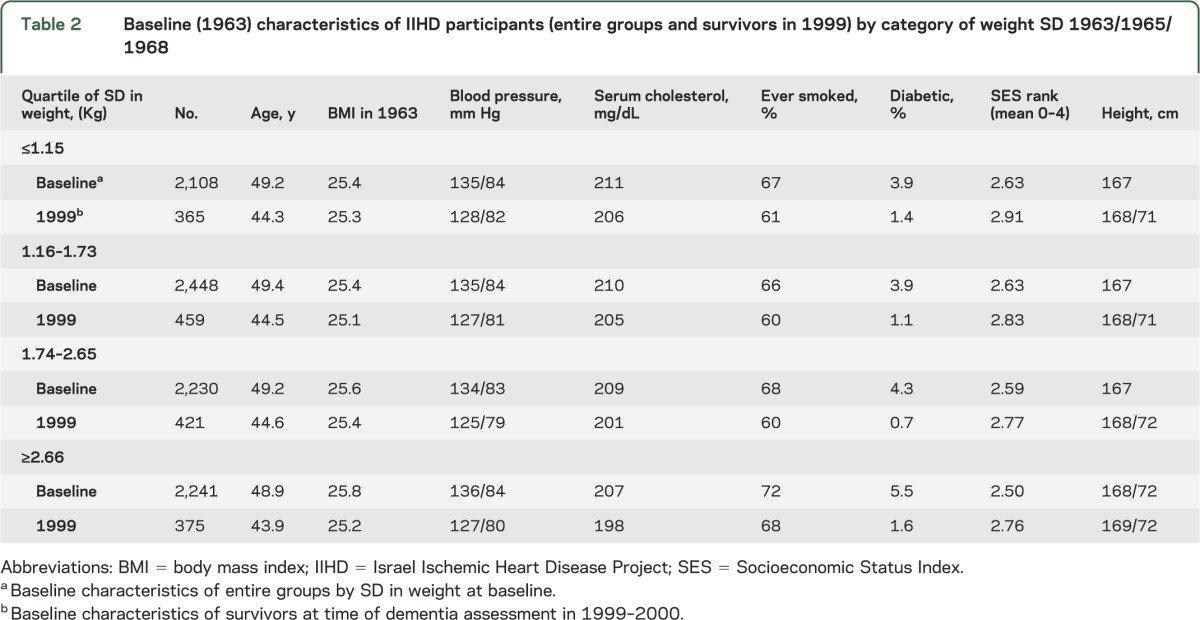
Table 2 depicts the numbers and baseline characteristics of men who were originally weighed in 1963/1965/1968 and of those who survived until 1999/2000 by weight change SD. Survival beyond age 75 years was 64.8%, 66.4%, 65.2%, and 59.4% in quartiles 1–4, respectively. Men in the highest quartile of SD of changes in weight (≥2.66 kg/m2) included relatively more smokers and had lower SES at baseline (table 2). Groups did not differ in total serum cholesterol values or in SBP and DBP values. There was no association between baseline BMI in 1963 and SD of changes in weight (table 2).
Table 2.
Baseline (1963) characteristics of IIHD participants (entire groups and survivors in 1999) by category of weight SD 1963/1965/1968
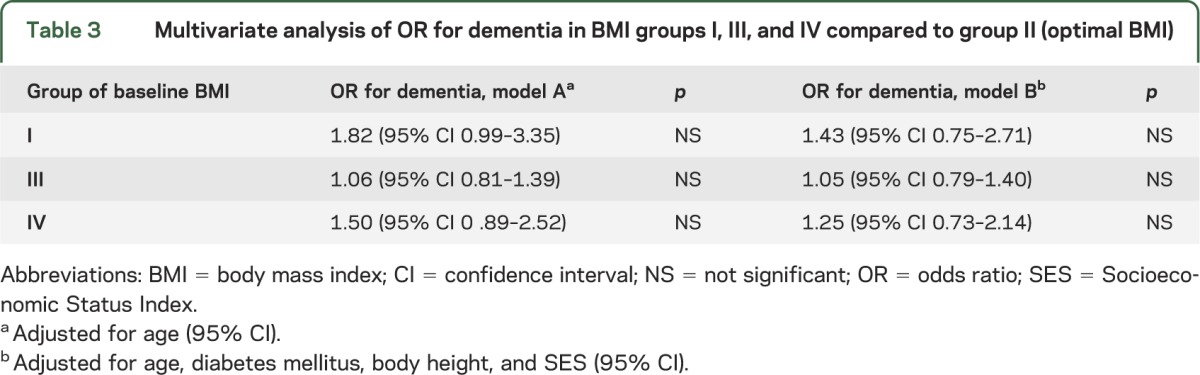
Association of baseline BMI with dementia at old age.
Dementia prevalence was 23.6%, 17.7%, 17.6%, and 23.0% in subjects with midlife BMI groups I, II, III, IV, respectively. Compared to optimal BMI (group II), subjects in the lowest and highest BMI groups were at the highest risk for dementia; however, these differences did not reach statistical significance (table 3).
Table 3.
Multivariate analysis of OR for dementia in BMI groups I, III, and IV compared to group II (optimal BMI)
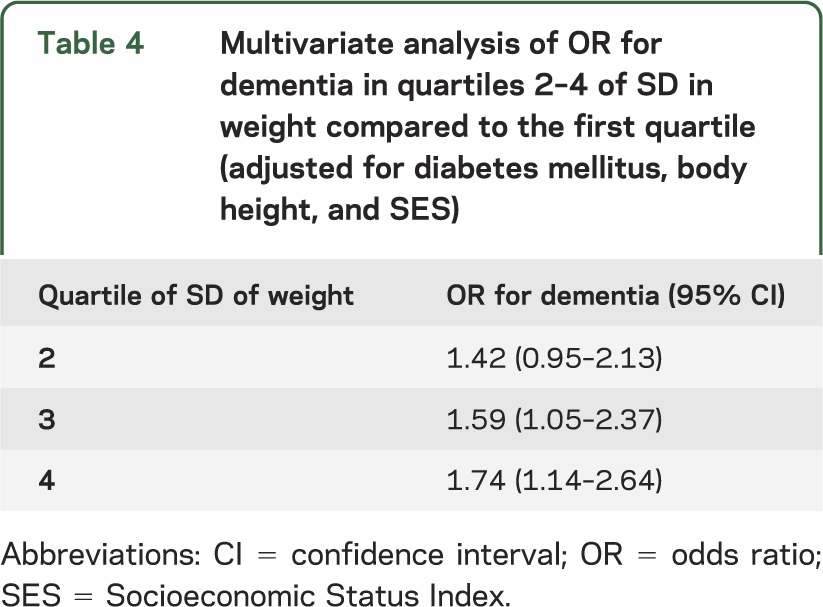
Association of midlife body weight variability with dementia at old age.
Dementia was more prevalent in men who were above the median of weight change SD values than in men who were below the median (median of SD of 3 weight measurements = 1.15 kg, p = 0.0075). Dementia prevalence rates were 13.4%, 18.4%, 20.1%, and 19.2% in quartiles 1–4 of weight change SD, respectively (z = 2.12, p = 0.034 in trend test). Table 4 demonstrates the results of a multivariate analysis adjusted for diabetes mellitus, body height, and SES of ORs for dementia in quartiles 2–4 of weight change SD compared to the first quartile. The risk for dementia increased with increasing weight change SD. A trend test for these rates yielded z = 2.17, p = 0.032 (table 4). Additional adjustment for initial (1963) weight, blood pressure, serum cholesterol, and smoking habits had negligible effect on the results. We further adjusted the analysis for degree of leisure time physical activity, report of intentional weight losing diet in 1963, total caloric consumption in 1963, and percent calories from fat and from saturated fat. The results remained essentially unchanged. The area under the receiver operating characteristic (ROC) curve for this model was 0.73 and the Hosmer-Lemeshow model fit test yielded χ2 (8) = 6.77 (associated p = 0.56), consistent with an adequate model fit. No such associations were found between quartiles of weight change SD and CIND (n = 167) prevalence (6.9%, 6.4%, 7.1%, and 6.8% in the first to fourth quartiles, respectively).
Table 4.
Multivariate analysis of OR for dementia in quartiles 2–4 of SD in weight compared to the first quartile (adjusted for diabetes mellitus, body height, and SES)
Transformation of weight change SD.
We used fractional polynomials to investigate whether the association of variability in weight with dementia may not be linear. The best fit was obtained applying a power of 0, i.e., using log (weight change SD). However, including age as covariate invoked a power of 0.5, namely the square root of weight change SD. When SES was adjusted for, a power of 3 yielded the best fit. Using a second degree polynomial adjusting for age yielded powers −2 (inverse of weight change SD squared) and −1 (inverse), whereas adjusting for SES or both age and SES both the log and third power transformations were obtained as the best transformations. Examining these data applying the Box-Tidwell transformation yielded (for age-adjusted association) a power of 0.25 with an associated odds ratio (OR) of 4.26 for the transformed independent variable. Testing of the deviation from a linear model was not statistically significant (p = 0.27). If the quartiles of weight change SD are used, the best fitting transformation is obtained with power = 1.327, again not departing significantly from linearity.
To address the issue of competing risk with mortality, we performed inverse probability weighting. Compared to the first quartile of weight change SD, the adjusted hazard risk ratios (adjusted for smoking, diabetes, cholesterol, and SES) for mortality by time of dementia assessment were 0.95 (95% confidence interval [CI] 0.89–1.02), 1.01 (95% CI 0.94–1.09), and 1.08 (95% CI 1.01–1.17), respectively, for quartiles 2–4. Nevertheless, we calculated IPW to apply a model accounting for bias due to failure of most participants to reach the dementia assessment phase. The predictors of reaching that phase as estimated by logistic regression were younger age (adjusted OR = 0.44 per 5 years increment), low SBP (OR = 0.68 per 20 mm Hg increase), high SES (OR = 2.69 for lowest vs highest category), absence of baseline diabetes mellitus (OR = 0.35 for diabetics), not smoking at any time (OR = 0.65 for smokers), and low serum cholesterol (0.88 per 40 mg/dL increase). Probabilities of reaching the above phase were calculated on the basis of the above risk profile. Individual weight change SD (1963–1968) were subsequently multiplied by the inverse of the corresponding probabilities to create the weighted variable. Using quartiles of the weighted variables, the adjusted odds for dementia associated with quartiles 2–4 relative to the lowest quartile of SD in weight were 2.09 (95% CI 1.53–2.86), 3.01 (95% CI 2.11–4.30), and 4.61 (95% CI 2.57–8.26), respectively. Estimates were obtained using logistic regression adjusted for height. Adding 1963 weight as confounder left the above estimates literally unchanged. Adjusting the latter logistic regression also for age and SES, variables were initially used in the computation of the probabilities that led to IPW values, the above 3 relative odds associated with quartiles 2–4 diminished to 1.56 (95% CI 1.12–2.17), 1.53 (95% CI 1.01–2.32), and 1.63 (95% CI 0.81–3.28). The area under the ROC for this model was 0.79, suggesting good prediction of individual survival to the dementia assessment.
Direction of weight change.
A total of 445 subjects exhibited a continuous rise in weight between assessments, 804 had a continuous decrease in weight, 412 had identical weight measurements in at least 2 of 3 measurements, and 718 showed inconsistent trends in weight change (i.e., increase in 1965 and decrease in 1968 or vice versa). The corresponding survivors’ dementia rates were 19.6%, 19.1%, 15.1%, and 18.0%.). No statistical trend was thus observed across directions of weight changes.
DISCUSSION
The present study demonstrated that instability in body weight over 5 years between ages 40 and 70 years, independently of the direction of changes in weight, was associated with increased risk for dementia. This association was maintained after adjustment for factors associated with risk for dementia such as SES, original weight, diabetes mellitus, and body height. Variables such as physical activity,23 intentionality of weight loss,24 and dietary factors23 known to be associated with weight change, morbidity, and mortality did not appear to confound the association. Results remained essentially unchanged when we analyzed the effect of survival bias (using the IPW model).
The relationship of baseline (1963) BMI with risk for dementia was consistent with a U-shaped curve such that compared to men with desirable BMI, lean body mass and obesity were at higher risk for dementia more than 3 decades later. Overweight was not associated with increased risk for dementia. The latter findings, however, did not reach statistical significance.
The association of variation in midlife body weight and risk for dementia varies across studies. In the Honolulu-Asia Aging Study, subjects with and without dementia at old age did not differ with respect to baseline weight or weight change from mid to late life.6 Others reported that women, but not men, who subsequently developed dementia began to lose weight compared to subjects without dementia as long as 11–20 years prior to onset of dementia.25 Similarly, a slower weight gain between midlife and old age was observed in women who subsequently developed dementia.26 Discrepancies between studies may arise from the different populations studied (sex, age at baseline) and methodologies applied (number and frequency of weight measurements, method of dementia assessment, definitions used for variability in weight).
The importance of the current findings relates to the observation that body weight variability in a relatively short time period (5 years) during midlife or late adulthood is associated with dementia.
Pathways associating variability in body weight and dementia may be mediated by cardiovascular risk factors,27,28 insulin resistance,29 or personality factors30–32 (high neuroticism and low conscientiousness), all of which are associated with both weight cycling and dementia.33,34
Previous studies differed in their findings regarding the relationship between body weight per se and dementia. Some have shown that both low and high BMI at midlife were associated with increased risk for dementia at old age,35,36 while others have found an association of overweight or obesity (but not low BMI) with risk for dementia.37,38 Inconsistencies may be explained by the different measures used for body fat, or by lack of association leading to coincidental findings.
The strengths of our study are the relatively large sample size and longitudinal design and an abundance of data regarding midlife demographic, social, biomedical, physical activity, and nutritional data, including intention to lose weight, all of which may mediate the relationships of body weight during midlife with cognitive compromise at late life. Weight was measured more than 3 decades prior to dementia assessment, thus minimizing the possibility that changes in weight were affected by the dementing process itself.
The main limitation of this study is the lack of information on the incidence of dementia in the subjects from the original IIHD study reported dead before the follow-up study was conducted, or in those who were not accessible to us at the phase of dementia assessment. However, when we applied the MSM model to compensate for the probability of selective survival, results remained essentially unaltered.
The effect of weight and changes in weight may differ according to sex,25 limiting our ability to generalize the results.
Brain imaging was not performed as part of dementia assessment, thus preventing information about vascular/structural changes that may underlie the association between variability in body weight during midlife. It should be noted that at the relatively old age of the sample at dementia assessment, the degree of mixed neuropathology is high,39 perhaps adding complexity to the potential mechanisms underlying the association between stability in weight and cognition.
GLOSSARY
- BMI
body mass index
- CI
confidence interval
- CIND
cognitively impaired no dementia
- DBP
diastolic blood pressure
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- IIHD
Israel Ischemic Heart Disease Project
- IPW
inverse probability weight
- MSM
marginal structural model
- OR
odds ratio
- ROC
receiver operating characteristic
- SBP
systolic blood pressure
- SES
Socioeconomic Status Index
- TICS-m
Modified Telephone Interview for Cognitive Status
AUTHOR CONTRIBUTIONS
Dr. Ravona-Springer: data ascertainment, interpretation of results, manuscript writing. Dr. Schnaider-Beeri: study design, data ascertainment, interpretation of results, manuscript writing. Dr. Goldbourt: hypothesis, study design, data ascertainment, statistical analysis, interpretation of results, manuscript writing.
STUDY FUNDING
Supported by NIA R01 AG034087 and the Graubard 431 Fund (M.S.-B.), the American Federation for Aging Research (AFAR), Young Investigator Award 2011, and NIRG-11-205083 Alzheimer's Association, 2012 (R.R.-S.).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA 2002;288:1723–1727 [DOI] [PubMed] [Google Scholar]
- 2.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62:1556–1560 [DOI] [PubMed] [Google Scholar]
- 3.Gustafson D. A life course of adiposity and dementia. Eur J Pharmacol 2008;585:163–175 [DOI] [PubMed] [Google Scholar]
- 4.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med 2003;163:1524–1528 [DOI] [PubMed] [Google Scholar]
- 5.Chen YC, Chen TF, Yip PK, Hu CY, Chu YM, Chen JH. Body mass index (BMI) at an early age and the risk of dementia. Arch Gerontol Geriatr 2010;50(suppl 1):S48–S52 [DOI] [PubMed] [Google Scholar]
- 6.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol 2005;62:55–60 [DOI] [PubMed] [Google Scholar]
- 7.Arnold AM, Newman AB, Cushman M, Ding J, Kritchevsky S. Body weight dynamics and their association with physical function and mortality in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 2010;65:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rzehak P, Meisinger C, Woelke G, Brasche S, Strube G, Heinrich J. Weight change, weight cycling and mortality in the ERFORT Male Cohort Study. Eur J Epidemiol 2007;22:665–673 [DOI] [PubMed] [Google Scholar]
- 9.Shimazu T, Kuriyama S, Ohmori-Matsuda K, Kikuchi N, Nakaya N, Tsuji I. Increase in body mass index category since age 20 years and all-cause mortality: a prospective cohort study (the Ohsaki Study). Int J Obes 2009;33:490–496 [DOI] [PubMed] [Google Scholar]
- 10.Field AE, Malspeis S, Willett WC. Weight cycling and mortality among middle-aged or older women. Arch Intern Med 2009;169:881–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton CB, Medalie JH, Flocke SA, Zyzanski SJ, Yaari S, Goldbourt U. Self-reported physical activity predicts long-term coronary heart disease and all-cause mortalities twenty-one-year follow-up of the Israeli Ischemic Heart Disease Study. Arch Fam Med Care 1995;4:323–329 [DOI] [PubMed] [Google Scholar]
- 12.Beeri MS, Werner P, Davidson M, Schmidler J, Silverman J. Validation of the modified telephone interview for cognitive status (TICS-m) in Hebrew. Int J Geriatr Psychiatry 2003;18:381–386 [DOI] [PubMed] [Google Scholar]
- 13.Gallo JJ, Breitner JC. Alzheimer's disease in the NAS-NRC Registry of aging twin veterans, IV: performance characteristics of a two-stage telephone screening procedure for Alzheimer's dementia. Psychol Med 1995;25:1211–1219 [DOI] [PubMed] [Google Scholar]
- 14.Silverman JM, Keefe RS, Mohs RC, Davis KL. A study of the reliability of the family history method in genetic studies of Alzheimer disease. Alzheimer Dis Assoc Disord 1989;3:218–223 [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 16.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982;139:1136–1139 [DOI] [PubMed] [Google Scholar]
- 17.Moroney JT, Bagiella E, Hachinski VC, et al. Misclassification of dementia subtype using the Hachinski Ischemic Score: results of a meta-analysis of patients with pathologically verified dementias. Ann NY Acad Sci 1997;826:490–492 [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2010 [Google Scholar]
- 19.Schnaider Beeri M, Goldbourt U, Silverman JM, et al. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology 2004;63:1902–1907 [DOI] [PubMed] [Google Scholar]
- 20.Liu Q. An order-directed score test for trend in ordered 2 X K tables. Biometrics 1998;54:1147–1154 [PubMed] [Google Scholar]
- 21.Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Appl Statist 1994;43:429–467 [Google Scholar]
- 22.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–570 [DOI] [PubMed] [Google Scholar]
- 23.Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 2010;304:1795–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorensen TI, Rissanen A, Korkeila M, Kaprio J. Intention to lose weight, weight changes, and 18-y mortality in overweight individuals without co-morbidities. PLoS Med 2005;2:e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology 2007;69:739–746 [DOI] [PubMed] [Google Scholar]
- 26.Gustafson DR, Backman K, Joas E, et al. 37 years of body mass index and dementia: observations from the prospective population study of women in Gothenburg, Sweden. J Alzheimers Dis 2012;28:163–171 [DOI] [PubMed] [Google Scholar]
- 27.Peters ET, Seidell JC, Menotti A, et al. Changes in body weight in relation to mortality in 6441 European middle-aged men: the Seven Countries Study. Int J Obes Relat Metab Disord 1995;19:862–868 [PubMed] [Google Scholar]
- 28.Montani JP, Viecelli AK, Prevot A, Dulloo AG. Weight cycling during growth and beyond as a risk factor for later cardiovascular diseases: the 'repeated overshoot' theory. Int J Obes 2006;30(suppl 4):S58–S66 [DOI] [PubMed] [Google Scholar]
- 29.Yatsuya H, Tamakoshi K, Yoshida T, et al. Association between weight fluctuation and fasting insulin concentration in Japanese men. Int J Obes Relat Metab Disord 2003;27:478–483 [DOI] [PubMed] [Google Scholar]
- 30.Evers C, Marijn Stok F, de Ridder DT. Feeding your feelings: emotion regulation strategies and emotional eating. Pers Soc Psychol Bull 2010;36:792–804 [DOI] [PubMed] [Google Scholar]
- 31.Rush CC, Becker SJ, Curry JF. Personality factors and styles among college students who binge eat and drink. Psychol Addict Behav 2009;23:140–145 [DOI] [PubMed] [Google Scholar]
- 32.Sutin AR, Ferrucci L, Zonderman AB, Terracciano A. Personality and obesity across the adult life span. J Pers Soc Psychol 2011;101:579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craft S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis Assoc Disord 2006;20:298–301 [DOI] [PubMed] [Google Scholar]
- 34.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men: The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol 2000;20:2255–2260 [DOI] [PubMed] [Google Scholar]
- 35.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med 2005;165:321–326 [DOI] [PubMed] [Google Scholar]
- 36.Chiang CJ, Yip PK, Wu SC, et al. Midlife risk factors for subtypes of dementia: a nested case-control study in Taiwan. Am J Geriatr Psychiatry 2007;15:762–771 [DOI] [PubMed] [Google Scholar]
- 37.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res 2007;4:103–109 [DOI] [PubMed] [Google Scholar]
- 38.Gelber RP, Petrovitch H, Masaki KH, et al. Lifestyle and the risk of dementia in Japanese-American men. J Am Geriatr Soc 2012;60:118–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med 2009;6:e1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]


