Abstract
Right ventricle myocardial infarctions (RVMIs) accompany inferior wall ischemia in up to one-half of cases. The clinical sequelae of RVMIs vary from no hemodynamic compromise to severe hypotension and cardiogenic shock. Diagnosis is based on physical examination, electrocardiography, echocardiography and coronary angiography. Because the standard 12-lead electrocardiogram is insufficient for the assessment of RV involvement, right-sided precordial leads should always be included. Adequate fluid administration in combination with positive inotropic agents and early coronary reperfusion are crucial components of treatment, while diuretics and nitrates should be avoided. Intra-aortic balloon counterpulsation and right ventricle assist devices may be used with success in RVMIs associated with medically refractory heart failure. Right ventricular involvement appears to be an independent prognostic factor that dramatically increases in-hospital mortality, due, in part, to a significantly higher risk of hemodynamically compromising arrhythmias. Thus, using right-sided precordial leads and early RVMI identification to trigger an appropriately aggressive treatment protocol may improve patients’ prognosis.
Keywords: Arrhythmias, Revascularization, Right ventricular myocardial infarction, Treatment
Cardiovascular disease (CVD) continues to be the main cause of mortality and morbidity in developed countries. Annually, more than one-half of the deaths in the Czech Republic are due to CVD (1), and approximately 2200 Americans die of CVD each day. CVD claims more lives each year than cancer, chronic lower respiratory disease and accidents combined (2). Coronary artery disease accounts for approximately one-half of CVD deaths.
Patients with acute coronary syndrome may present with either acute myocardial infarction (MI) with or without ST segment elevation, or unstable angina. These conditions share common patho-physiological mechanisms related to coronary plaque instability (erosion or rupture), thrombosis and vasospasm, resulting in either subendocardial or transmural ischemia.
In contrast to the lengthy historical interest in acute MIs of the left ventricle (LVMIs), the clinical consequences of right ventricular (RV) involvement were first described only in 1974 (3). Specific RV perfusion from both the right and left coronary arteries results in relatively small RVMIs, with the majority of the myocardium remaining viable even in the absence of reperfusion. Recovery from RV myocardial impairment (mainly linked to stunning or hibernation) may be rather slow and is associated with a high rate of in-hospital mortality (4).
INCIDENCE
RVMI is usually associated with LVMI and, in practice, does not exist in isolation (5). The occurrence of RV impairment depends primarily on the location of the MI, which ranges from rare cases in the anterior heart wall (6) to more common locations (depending on the type of diagnostic method used) such as in the inferior wall in 24% to 50% of cases (5–7). The relatively small percentage of RVMIs may be explained by several factors: lower oxygen requirements of the RV due to its smaller muscle mass and workload; increased blood flow during diastole and systole; more extensive collateralization of the RV, primarily from the left coronary system; and diffusion of oxygen from intrachamber blood through the thin wall of the RV and into the Thebesian veins (7–9).
The clinical sequelae of RVMI vary widely, and range from no hemodynamic compromise to severe hypotension and cardiogenic shock depending on the extent of RV ischemia (6). According to the literature, approximately 25% to 50% of RV infarctions are hemodynamically significant (7,10).
DIAGNOSTICS
Physical examination
It is important to consider a diagnosis of RVMI, particularly in the presence of an inferior wall MI. The typical triad observed on physical examination is hypotension occurring with jugular vein distention and clear lungs. Preserved left ventricular (LV) function confirms the diagnosis (3). A tricuspid regurgitation murmur, Kussmaul’s sign (an increase in inspiratory central venous pressure, visible as jugular vein distention) and pulsus paradoxus are signs of significant hemodynamic effects due to RV ischemia (11). In some cases, these symptoms are not present at admission and do not occur until diuretics or nitrates are administered.
Two-dimensional echocardiography
This technique can show RV dilation with depressed systolic function (Figure 1) and RV free wall dyskinesia with paradoxical septal motion (12–16). A Doppler examination of specific pulmonary regurgitation patterns can add hemodynamic insight and confirm the diagnosis of RV involvement, especially in cases of technically inadequate two-dimensional images (17).
Figure 1).
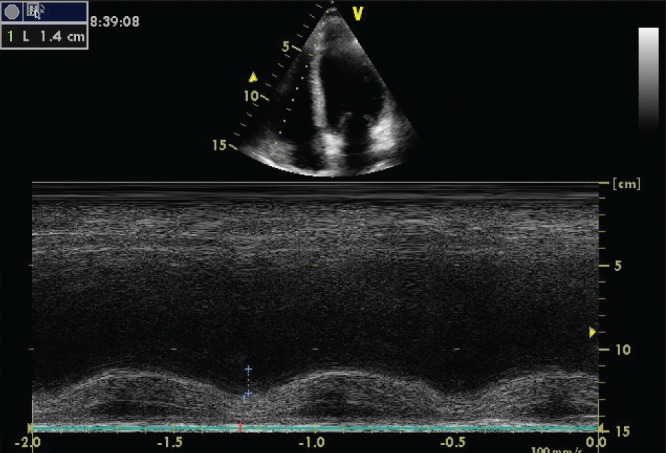
Right ventricle systolic dysfunction, estimated using tricuspid annular systolic plane excursion-method echocardiography
Radionuclide ventriculography and 99mTc pyrophosphate myocardial scintigraphy
These noninvasive techniques may be used to detect RV dysfunction. A dilated right ventricle with hypokinesia, akinesia or dyskinesia of its free wall associated with a depressed RV ejection fraction (EF) and a normal or only mildly depressed LV EF are indicative of an RVMI (7). A 99mTc pyrophosphate myocardial scintigraphy examination requires proper timing, and the scans are usually not diagnostic until 72 h after the onset of symptoms. Excessive uptake of the radionuclide in non-cardiac structures (chest wall, bone, cartilage) can lead to issues regarding the interpretation of the acquired images (18).
Hemodynamic examination
Hemodynamic examination using right-sided cardiac catheterization may reveal a disproportionate elevation of right-sided filling pressures compared with left-sided filling pressures. The generally accepted criteria for hemodynamically-significant RVMIs originate from an autopsy/hemodynamic study by Lopez-Sendon et al (19) and include right atrial pressure (RAP) >10 mmHg, a RAP to pulmonary capillary wedge pressure (PCWP) ratio >0.8, or RAP within 5 mmHg of the PCWP. However, with concomitant and significant LV dysfunction, the close relationship between the RAP and the PCWP is not preserved, although the RAP will continue to be elevated (18,19).
Coronary angiography
Angiography often reveals occlusion of the right coronary artery (RCA) proximal to the acute marginal branch (Figure 2), while more proximal occlusions usually suggest more extensive necrosis of the posterior and, potentially, the anterior RV myocardial wall (5,6). In patients with left coronary artery dominance, a left circumflex coronary artery (LCX) occlusion may also be found. Although uncommon, RV involvement may be present in patients with an occlusion in the left anterior descending artery (5). Cabin et al (20) studied 97 hearts with anterior MIs and found that 13% were RVMIs.
Figure 2).
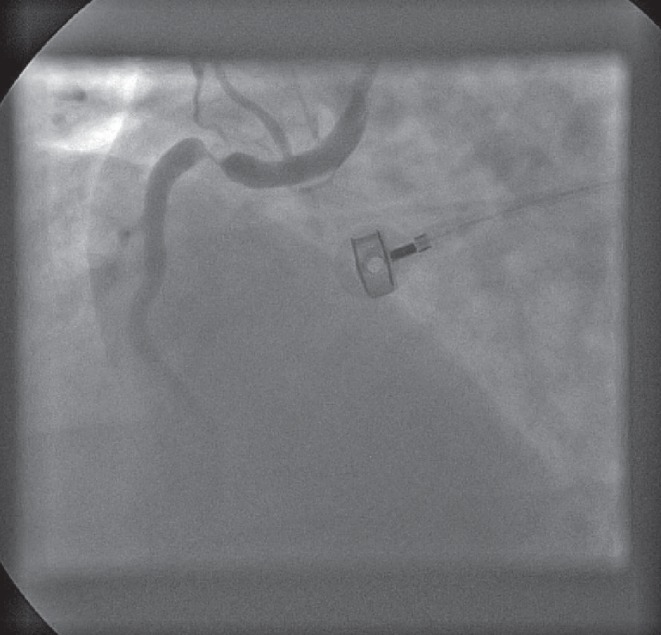
Angiogram showing critical stenosis of the proximal right coronary artery
Electrocardiography
Because RVMIs are usually associated with an inferior wall MI, evaluation using standard 12-lead electrocardiography (ECG) often reveals corresponding ST segment elevations in leads II, III and aVF. Disproportionate ST segment elevation with greater ST elevation in lead III than in lead II is pathognomonic for an RVMI, and RV involvement should be fully and carefully considered (21). Because standard 12-lead ECG images mainly assess the LV, right-sided precordial leads should always be used. These can show ST segment elevation across the entire right precordium from V1R through V6R; a sole ST segment elevation in lead V4R >1.0 mm (Figure 3) is a reliable marker of an RV infarction, with 100% sensitivity, 87% specificity and 92% predictive accuracy (22,23). Furthermore, higher ST segment elevations in V4R have been found to be independent predictive factors for more significant RV dysfunction and higher mortality rates (24,25).
Figure 3).
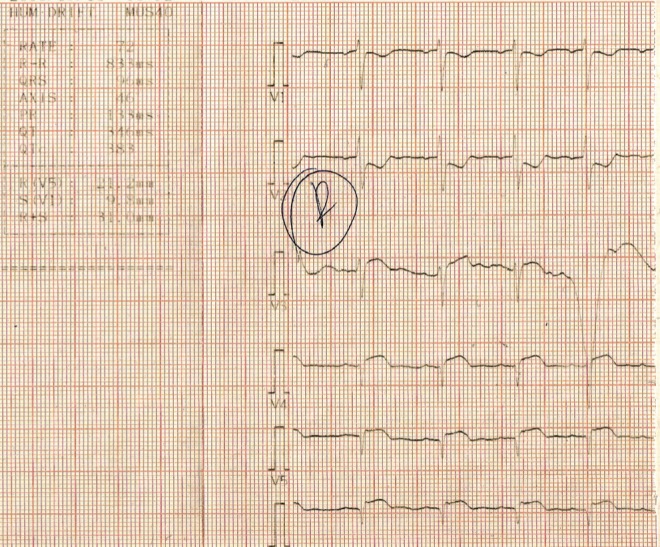
Electrocardiogram showing right-sided precordial leads and V3-V6 ST segment elevation in a patient with right ventricular myocardial infarction.
ARRHYTHMIAS
RVMIs are more often complicated by all types of arrhythmias compared with ‘simple’ inferior or anterior wall LVMIs (24,26–28). Barrillon et al (29) were the first to recognize the significantly higher risk of severe conduction disorders in patients with RV involvement. Complete atrioventricular (AV) or sinoatrial blocks occurred in one-half of cases in which ST segment elevation or a QS pattern in V3R and/or V4R were present. On the other hand, these complications were found in only 14% of cases in which these signs were absent. In a prospective study of 200 consecutive patients with acute inferior wall LVMI, Zehender et al (24) demonstrated a higher incidence of sustained ventricular tachycardia (16% versus 8%; P=0.08) and ventricular fibrillation (21% versus 9%; P=0.05) in patients with ECG signs of RV involvement. Significantly higher incidences of complete AV block (17% versus 4%; P=0.06) and severe bradycardias (9% versus 3%; P=0.09) with pacing requirements (18% versus 3%; P=0.01) have been reported in cases of RVMI (24). Mehta et al (26) studied complications and prognoses in a large number of patients hospitalized for anterior (n=971) and inferior LVMI with (n=491) or without (n=638) RV involvement. Anterior wall LVMI was associated with the highest overall and in-hospital mortality rates, while the number of arrhythmias (ventricular fibrillation, sustained ventricular tachycardia and high-degree AV blockade) was the highest in patients with inferior LVMI with RV involvement.
THERAPY
RV ischemia may lead to systolic and diastolic dysfunction, resulting in a serious deficit in LV preload with a subsequent drop in cardiac output and consequent systemic hypotension. Adequate filling (preload) of the impaired RV is thus crucial to maintain sufficient RV output volume and LV function (30). Initial therapy, therefore, requires the administration of sufficient volume to increase RV filling; at the same time, it is critically important to avoid drugs that cause venodilation and a decrease in RV filling (eg, nitrates, diuretics). Treatment is generally recommended to begin with a volume challenge of 300 mL to 600 mL normal saline over 10 min to 15 min through a central line or through a large-bore peripheral intravenous site (31). However, some studies have indicated that volume loading may not increase cardiac output (7,32,33). This may be due to variable initial volume status among patients. Some patients may be relatively volume-depleted and could benefit from a volume infusion, while others who present with a normal intravascular volume show no changes in cardiac index or blood pressure following a fluid load because the RV preload is already at a maximum for maintaining RV stroke output (18). Invasive hemodynamic monitoring is, therefore, recommended, because further infusion may be harmful if additional increases in RV volume prevent sufficient LV filling via inter-ventricular interactions and intrapericardial pressure equalization. Based on hemodynamic monitoring studies, exceeding a RAP or PCWP of 20 mmHg is generally not recommended (32).
If initial volume loading fails to improve arterial pressure and cardiac output despite significant increases in RAP and PCWP, then positive inotropic agent therapy can be effective in stabilizing patients. Dell’Italia et al (32) studied the effect of dobutamine in patients with RVMI after volume loading and concluded that dobutamine produced a statistically significant increase in cardiac index, stroke volume index and RVEF.
Restoration of sufficient coronary blood flow represents the only treatment that addresses the underlying problem, and early reperfusion (Figure 4) improves RV performance as well as the clinical course and survival (4). Bowers et al (16) studied clinical outcomes and RV function using two-dimensional echocardiography in 53 patients with acute RVMI before and after reperfusion therapy. The authors reported dramatic recovery of RV performance and excellent clinical outcomes after early and complete reperfusion of the RCA using primary percutaneous coronary intervention. In contrast, unsuccessful reperfusion was associated with impaired recovery of RV function, persistent hemodynamic compromise and high mortality rates. Early reperfusion was also crucial in preventing ventricular arrhythmias, which were observed much more frequently in patients with unsuccessful coronary reperfusion (16).
Figure 4).
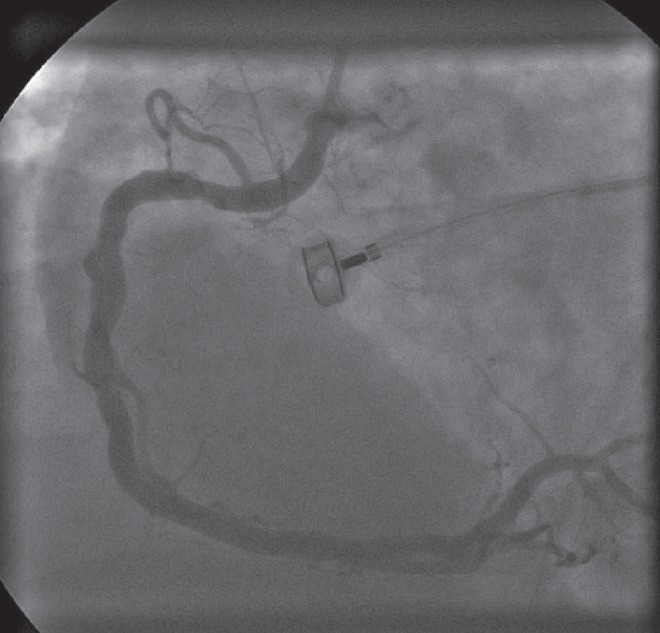
Angiogram showing the right coronary artery post-revascularization
In patients with refractory hypotension and low cardiac output, intra-aortic balloon counterpulsation (IABC) may be beneficial. Although IABC does not directly influence RV performance, it can increase coronary perfusion pressure and thereby improve RV function, particularly if the RCA has been recanalized. Furthermore, the performance of a dysfunctional RV is largely dependent on LV septal contraction, which can be improved using IABC (4).
The limitations of medical management of RV failure mentioned above have led to the development of a number of RV assist devices designed to bypass the impaired RV and/or pulmonary circulation to allow the right ventricle to recover. The TandemHeart Percutaneous Ventricular Assist Device (CardiacAssist Inc, USA) is an extracorporeal centrifugal pump that generates continuous flow with a minimal low amplitude pulsatile componentF. It can provide flow up to 5.0 L/min and pumps the blood from the right atrium to the main pulmonary artery, bypassing a poorly functioning RV. Kapur et al (34) studied nine patients with medically refractory RV failure and found that, although four patients died early, the Percutaneous Ventricular Assist Device was associated with significantly improved hemodynamics. Venoarterial extra-corporeal membrane oxygenation systems can provide both cardiac and respiratory support. These can be fully established through the cannulation of a femoral vein and artery using the Seldinger technique; therefore, surgery is not required. In contrast to RV assist devices, this technique allows total bypassing of the pulmonary bed and, therefore, does not cause further elevation of the pulmonary pressures and relieves the RV overload. Particularly in patients presenting with an obstructive hemodynamic pattern (pulmonary artery hypertension, pulmonary embolism), extracorporeal membrane oxygenation method may be considered to be the only reasonable approach (35,36).
PROGNOSIS
RV involvement significantly increased mortality in patients with inferior wall LVMIs, although it does not achieve the same rates observed in anterior wall LVMIs (26). Zehender et al (24) studied 200 consecutive patients admitted with acute inferior wall LVMI. RV involvement presenting with an ST elevation in lead V4R was found to be a highly negative predictive factor of both in-hospital mortality (31% versus 6%) and all major in-hospital complications (64% versus 28%). These included sustained ventricular tachycardia, ventricular fibrillation, myocardial rupture, second- and third-degree AV block requiring cardiac pacing, reinfarction and cardiogenic shock. Jacobs et al (37) evaluated 933 patients with acute MI presenting with cardiogenic shock due to either predominant RV (n=49) or LV failure (n=884). Despite the younger age, shorter time to shock diagnosis, higher prevalence of single-vessel coronary disease, lower prevalence of previous MI and similar revascularization outcomes in patients with RV failure, there was no significant difference in mortality between the groups (53.1% versus 60.8%; P=0.296), representing an unexpectedly high mortality rate among the RV shock patients.
On the other hand, recently published research by Foussas et al (38) found no significant difference in long-term mortality between patients with and without a RVMI. Therefore, the poor in-hospital prognosis for patients with RVMIs appears to be mainly due to the increased risk of life-threatening arrhythmias (26). Proper monitoring and appropriate antiarrhythmic treatment until hospital discharge plays a key role in the overall prognosis and survival of patients.
CONCLUSION
RVMI often accompanies an inferior wall LVMI presenting with typical clinical, ECG and echocardiographic findings. In the presence of hypotension or cardiogenic shock without signs of LV failure and 1 mm ST segment elevation in the V4R lead, a diagnosis of RVMI is highly probable. Specific treatment includes fluid loading and vasopressors, which should be administered immediately. In addition, patients with RV involvement require continuous careful monitoring because they are at a significantly higher risk for ventricular fibrillation, sustained ventricular tachycardia and high-degree AV blockade, any of which would worsen the overall prognosis.
Thus, right-sided precordial leads should be used in all patients presenting with an acute inferior LVMI. This simple approach may facilitate early identification and risk stratification of patients with an accompanying RVMI and trigger a protocol of appropriately aggressive treatments required to manage the increased risk of life-threatening arrhythmic complications.
Acknowledgments
Supported by a Grant from the Ministry of Health of the Czech Republic, NT13767-4.
REFERENCES
- 1.Czech Health Statistics Yearbook 2010. Institute of Health Information and Statistics of the Czech Republic; 2011. pp. 32–35. [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn JN, Guiha NH, Broder MI, Limas CJ. Right ventricular infarction. Clinical and hemodynamic features. Am J Cardiol. 1974;33:209–14. doi: 10.1016/0002-9149(74)90276-8. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JA. Pathophysiology and management of right heart ischemia. J Am Coll Cardiol. 2002;40:841–53. doi: 10.1016/s0735-1097(02)02048-x. [DOI] [PubMed] [Google Scholar]
- 5.Andersen HR, Falk E, Nielsen D. Right ventricular infarction: Frequency, size and topography in coronary heart disease: A prospective study comprising 107 consecutive autopsies from a coronary care unit. J Am Coll Cardiol. 1987;10:1223–32. doi: 10.1016/s0735-1097(87)80122-5. [DOI] [PubMed] [Google Scholar]
- 6.Isner JM, Roberts WC. Right ventricular infarction complicating left ventricular infarction secondary to coronary heart disease: Frequency, location, associated findings and significance from analysis of 236 necropsy patients with acute or healed myocardial infarction. Am J Cardiol. 1978;42:885–94. doi: 10.1016/0002-9149(78)90672-0. [DOI] [PubMed] [Google Scholar]
- 7.Shah PK, Maddahi J, Berman DS. Scintigraphically detected predominant right ventricular dysfunction in acute myocardial infarction: Clinical and hemodynamic correlates and implications for therapy and prognosis. J Am Coll Cardiol. 1985;6:1264–72. doi: 10.1016/s0735-1097(85)80212-6. [DOI] [PubMed] [Google Scholar]
- 8.Haupt HM, Hutchins GM, Moore GW. Right ventricular infarction: Role of the moderator band artery in determining infarct size. Circulation. 1983;67:1268–72. doi: 10.1161/01.cir.67.6.1268. [DOI] [PubMed] [Google Scholar]
- 9.Berger PB, Ryan TJ. Inferior myocardial infarction. High-risk subgroups. Circulation. 1990;81:401–11. doi: 10.1161/01.cir.81.2.401. [DOI] [PubMed] [Google Scholar]
- 10.Horan LG, Flowers NC. Right ventricular infarction: Specific requirements of management. Am Fam Physician. 1999;60:1727–34. [PubMed] [Google Scholar]
- 11.Dell’Italia LJ, Starling MR, O’Rourke RA. Physical examination for exclusion of hemodynamically important right ventricular infarction. Ann Intern Med. 1983;99:608–11. doi: 10.7326/0003-4819-99-5-608. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein JA, Barzilai B, Rosamond TL, Eisenberg PR, Jaffe AS. Determinants of hemodynamic compromise with severe right ventricular infarction. Circulation. 1990;82:359–68. doi: 10.1161/01.cir.82.2.359. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein JA. Right heart ischemia: Pathophysiology, natural history, and clinical management. Prog Cardiovasc Dis. 1998;40:325–41. doi: 10.1016/s0033-0620(98)80051-0. [DOI] [PubMed] [Google Scholar]
- 14.Bellamy GR, Rasmussen HH, Nasser FN, Wiseman JC, Cooper RA. Value of two-dimensional echocardiography, electrocardiography, and clinical signs in detecting right ventricular infarction. Am Heart J. 1986;112:304–9. doi: 10.1016/0002-8703(86)90266-8. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda T, Okada RD, Leinbach RC, et al. Serial evaluation of right ventricular dysfunction associated with acute inferior myocardial infarction. Am Heart J. 1990;119:816–22. doi: 10.1016/s0002-8703(05)80317-5. [DOI] [PubMed] [Google Scholar]
- 16.Bowers TR, O’Neill WW, Grines C, Pica MC, Safian RD, Goldstein JA. Effect of reperfusion on biventricular function and survival after right ventricular infarction. N Engl J Med. 1998;338:933–40. doi: 10.1056/NEJM199804023381401. [DOI] [PubMed] [Google Scholar]
- 17.Cohen A, Logeart D, Chauvel C. Right ventricular infarction. N Engl J Med. 1998;339:479–80. doi: 10.1056/NEJM199808133390714. [DOI] [PubMed] [Google Scholar]
- 18.O’Rourke RA, Dell’Italia LJ. Diagnosis and management of right ventricular myocardial infarction. Curr Probl Cardiol. 2004;29:6–47. doi: 10.1016/j.cpcardiol.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Sendon J, Coma-Canella I, Gamallo C. Sensitivity and specificity of hemodynamic criteria in the diagnosis of acute right ventricular infarction. Circulation. 1981;64:515–25. doi: 10.1161/01.cir.64.3.515. [DOI] [PubMed] [Google Scholar]
- 20.Cabin HS, Clubb KS, Wackers FJ, Zaret BL. Right ventricular myocardial infarction with anterior wall left ventricular infarction: An autopsy study. Am Heart J. 1987;113:16–23. doi: 10.1016/0002-8703(87)90004-4. [DOI] [PubMed] [Google Scholar]
- 21.Moye S, Carney MF, Holstege C, Mattu A, Brady WJ. The electrocardiogram in right ventricular myocardial infarction. Am J Emerg Med. 2005;23:793–9. doi: 10.1016/j.ajem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Somers MP, Brady WJ, Bateman DC, Mattu A, Perron AD. Additional electrocardiographic leads in the ED chest pain patient: Right ventricular and posterior leads. Am J Emerg Med. 2003;21:563–73. doi: 10.1016/j.ajem.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Robalino BD, Whitlow PL, Underwood DA, Salcedo EE. Electrocardiographic manifestations of right ventricular infarction. Am Heart J. 1989;118:138–44. doi: 10.1016/0002-8703(89)90084-7. [DOI] [PubMed] [Google Scholar]
- 24.Zehender M, Kasper W, Kauder E, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med. 1993;328:981–8. doi: 10.1056/NEJM199304083281401. [DOI] [PubMed] [Google Scholar]
- 25.Yoshino H, Udagawa H, Shimizu H, et al. ST-segment elevation in right precordial leads implies depressed right ventricular function after acute inferior myocardial infarction. Am Heart J. 1998;135:689–95. doi: 10.1016/s0002-8703(98)70287-x. [DOI] [PubMed] [Google Scholar]
- 26.Mehta SR, Eikelboom JW, Natarajan MK, et al. Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J Am Coll Cardiol. 2001;37:37–43. doi: 10.1016/s0735-1097(00)01089-5. [DOI] [PubMed] [Google Scholar]
- 27.Berger PB, Ruocco NA, Jr, Ryan TJ, et al. Frequency and significance of right ventricular dysfunction during inferior wall left ventricular myocardial infarction treated with thrombolytic therapy (results from the Thrombolysis in Myocardial Infarction [TIMI] II trial) Am J Cardiol. 1993;71:1148–52. doi: 10.1016/0002-9149(93)90637-r. [DOI] [PubMed] [Google Scholar]
- 28.Bueno H, López-Palop R, Bermejo J, López-Sendón JL, Delcán JL. In-hospital outcome of elderly patients with acute inferior myocardial infarction and right ventricular involvement. Circulation. 1997;96:436–41. doi: 10.1161/01.cir.96.2.436. [DOI] [PubMed] [Google Scholar]
- 29.Barrillon A, Chaignon M, Guize L, Gerbaux A. Premonitory sign of heart block in acute posterior myocardial infarction. Br Heart J. 1975;37:2–8. doi: 10.1136/hrt.37.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein JA, Vlahakes GJ, Verrier ED, et al. Volume loading improves low cardiac output in experimental right ventricular infarction. J Am Coll Cardiol. 1983;2:270–8. doi: 10.1016/s0735-1097(83)80163-6. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein JA, Vlahakes GJ, Verrier ED, et al. The role of right ventricular systolic dysfunction and elevated intrapericardial pressure in the genesis of low output in experimental right ventricular infarction. Circulation. 1982;65:513–22. doi: 10.1161/01.cir.65.3.513. [DOI] [PubMed] [Google Scholar]
- 32.Dell’Italia LJ, Starling MR, Blumhardt R, Lasher JC, O’Rourke RA. Comparative effects of volume loading, dobutamine, and nitroprusside in patients with predominant right ventricular infarction. Circulation. 1985;72:1327–35. doi: 10.1161/01.cir.72.6.1327. [DOI] [PubMed] [Google Scholar]
- 33.Siniorakis EE, Nikolaou NI, Sarantopoulos CD, Sotirelos KT, Iliopoulos NE, Bonoris PE. Volume loading in predominant right ventricular infarction: Bedside haemodynamics using rapid response thermistors. Eur Heart J. 1994;15:1340–7. doi: 10.1093/oxfordjournals.eurheartj.a060391. [DOI] [PubMed] [Google Scholar]
- 34.Kapur NK, Paruchuri V, Korabathina R, et al. Effects of a percutaneous mechanical circulatory support device for medically refractory right ventricular failure. J Heart Lung Transplant. 2011;30:1360–7. doi: 10.1016/j.healun.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Belohlavek J, Rohn V, Jansa P, et al. Veno-arterial ECMO in severe acute right ventricular failure with pulmonary obstructive hemodynamic pattern. J Invasive Cardiol. 2010;22:365–9. [PubMed] [Google Scholar]
- 36.Berman M, Tsui S, Vuylsteke A, Klein A, Jenkins DP. Life-threatening right ventricular failure in Pplmonary hypertension: RVAD or ECMO? J Heart Lung Transplant. 2008;27:1188–9. doi: 10.1016/j.healun.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs AK, Leopold JA, Bates E, et al. Cardiogenic shock caused by right ventricular infarction: A report from the SHOCK registry. J Am Coll Cardiol. 2003;41:1273–9. doi: 10.1016/s0735-1097(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 38.Foussas SG, Zairis MN, Tsiaousis GZ, et al. The impact of right ventricular involvement on the postdischarge long-term mortality in patients with acute inferior ST-segment elevation myocardial infarction. Angiology. 2010;61:179–83. doi: 10.1177/0003319709335032. [DOI] [PubMed] [Google Scholar]


