Abstract
BACKGROUND:
Although hematological parameters have been associated with prognosis in patients with various cardiovascular diseases, their relationship with coronary collateral (CC) circulation in patients with stable coronary artery disease (CAD) is unknown.
OBJECTIVE:
To investigate the relationship between hematological parameters and CC vessel development in patients with stable CAD.
METHODS:
A total of 96 patients who underwent coronary angiography were retrospectively enrolled. All study participants had at least one occluded major coronary artery. Development of CCs was classified using the method of Rentrop. Rentrop grades of 0 and 1 indicate poor CCs, whereas grades 2 and 3 indicate good CCs. Hematological parameters, including mean platelet volume (MPV) and neutrophil/lymphocyte (N/L) ratio, were measured. Multivariate logistic regression analysis was performed to identify independent variables.
RESULTS:
The MPV and N/L ratio were significantly higher in the poor CC group compared with the good CC group. Negative correlations were found in the analyses comparing Rentrop score with MPV and N/L ratio (r=−0.274; P=0.012 and r=−0.339; P=0.001, respectively). In multivariate analysis, the N/L ratio was independently related to CC circulation (OR 0.762 [95% CI 0.587 to 0.988]; P=0.04).
CONCLUSION:
The results suggest that N/L ratio and MPV are associated with poor CCs, and a high N/L ratio is a significant predictor of poor CC development in patients with stable CAD.
Keywords: Coronary collateral circulation, Hematological parameters, Mean platelet volume, Neutrophil/lymphocyte ratio
Development of coronary collateral (CC) circulation is a compensatory response to overcome injury in cases of severe ischemia secondary to tight stenoses or occluded vessels. Well-developed CCs preserve ventricular function and viability, providing an alternative blood supply to an ischemic region in coronary artery disease (CAD). Prominent interindividual variability exists even among patients with a similar degree of CAD (1); however, it remains unclear which factors are responsible for these differences (2). Several studies have shown that the development of CCs is impaired in the presence of many of the risk factors predisposing to atherosclerosis such as age, hypercholesterolemia, diabetes mellitus (DM) and smoking (3–7). In addition, many serum biomarkers, such as high-sensitivity C-reactive protein (CRP), lipoprotein-associated phospholipase A2, paraoxonase activity and asymmetric dimethylarginine, have been reported to be associated with the development of CCs (8–11). Although many studies have reported the role of hematological parameters, such as mean platelet volume (MPV) and the neutrophil/lymphocyte (N/L) ratio in various cardiovascular diseases, their relationship with CC circulation is unknown (12–14). The N/L ratio has emerged as a prognostic marker to predict cardiovascular events and mortality in patients with CAD. MPV is a determinant of platelet activation. It has been reported that elevated MPV values are associated with atherosclerosis and endothelial dysfunction. Accordingly, in the current study, we investigated the role of hematological parameters in CC development in patients with stable CAD.
METHODS
Study population
The present analysis was a retrospective cross-sectional study. Patients who underwent coronary angiography at the Department of Cardiology, Abant Izzet Baysal University (Bolu, Turkey) between January 2010 and April 2012 were evaluated. Only patients with a totally occluded major coronary artery were included.
Demographic, clinical and laboratory data were obtained from the patients’ medical records. DM was defined as a history of DM, the use of antidiabetic drugs or fasting plasma glucose levels of ≥7 mmol/L. Hypertension (HT) was defined as a history of HT or use of antihypertensive drugs, or a blood pressure ≥140/90 mmHg. Smoking status was defined as current smoking. The study protocol was approved by the local ethics committee.
Patients with hepatic or renal dysfunction (serum creatinine level >132.6 μmol/L), revascularization (coronary artery bypass graft operation or percutaneous coronary intervention) history, severe valvular disease, myocardial or pericardial disease, and technically inadequate coronary angiography were excluded from the study. Patients who were diagnosed with hematological disease, cancer, systemic inflammatory or autoimmune disease, thrombocytopenia and the use of anticoagulant agents were also excluded from the present study.
Angiographic evaluation and CC grading
Coronary angiography was performed through the femoral artery for all patients using the Judkin technique. Each angiogram was interpreted by two experienced cardiologists who were blinded to the clinical details and results of the other investigations of each patient. CC vessels were graded according to the Rentrop grading system of 0 to 3: 0 = no filling of any collateral vessel; 1 = filling of the side branches of the artery to be perfused by collateral vessels without visualization of the epicardial segment; 2 = partial filling of the distal epicardial segment by collateral vessels; and 3 = complete filling of the distal epicardial segment by collateral vessels (15). The vessel that had the highest Rentrop grade with collaterals was used for analysis when more than one occluded vessel was present. In cases for which more than one collateral vessel to the same occluded vessel was present, the highest Rentrop grade was used. The study population was divided into two groups according to the Rentrop collateral score: patients with grade 0 to 1 collateral development were classified as the poor collateral group; and patients with Rentrop grade 2 to 3 collateral development were classified as the good collateral group.
Biochemical and hematological parameters
Venous blood samples were collected from all patients after a 12 h fast, and serum glucose, creatinine, total cholesterol, triglyceride, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels were measured by standard methods. Tripotassium EDTA-based anticoagulated blood samples were drawn and stored at 4°C and assessed using the Beckman Coulter LH 750 and Hmx systems (Beckman Coulter, USA) using original reagents. Hemoglobin, platelet count, white blood cell (WBC) count, differential counts (neutrophil, lymphocyte, eosinophil and monocyte) and percentages were analyzed using a blood counter. All measurements were performed 30 min after blood collection using an automatic blood counter. The N/L ratio was calculated. The MPV and other hematological parameters, such as mean corpuscular volume and red cell distribution width, were also measured.
Statistical analysis
All analyses were performed using SPSS version 15 (IBM Corporation, USA) for Windows (Microsoft Corporation, USA). Continuous variables are presented as mean ± SD and categorical variables are presented as percentages. The Kolmogorov-Smirnov test was used to evaluate whether the distribution of variables was normal. For continuous variables, the independent samples t test was used and, for categorical variables, the χ2 test was used. The Spearman’s correlation coefficient was used for correlation analysis. Logistic regression analysis was used for multivariate analysis of independent variables; P<0.05 was considered to be statistically significant.
RESULTS
The study population consisted of 96 patients. Four patients (4%) had Rentrop grade 0, 35 patients (37%) had grade 1, 26 patients (27%) had grade 2 and 31 patients (32%) had grade 3 collateral circulation. The clinical characteristics and biochemical parameters of the patients in the good and the poor CC groups are summarized in Table 1. There were no statistically significant differences between the two groups with respect to age, sex, presence of HT, DM, smoking, and glucose, creatinine, total cholesterol, triglyceride, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol levels. The location of the occluded vessel was similar in both groups.
TABLE 1.
Baseline demographic and clinical characteristics of the study population
|
Coronary collaterals
|
|||
|---|---|---|---|
| Poor (n=39) | Good (n=57) | P* | |
| Age, years | 66.4±9.4 | 66.1±10.9 | 0.90 |
| Sex, male/female, n/n | 29/10 | 49/8 | 0.15 |
| Diabetes mellitus, n (%) | 13 (33) | 10 (18) | 0.08 |
| Hypertension, n (%) | 23 (59) | 25 (44) | 0.15 |
| Smoking, n (%) | 14 (39) | 24 (42) | 0.75 |
| Creatinine, μmol/L | 89.2±35.4 | 78.7±19.4 | 0.80 |
| Total cholesterol, mmol/L | 4.7±1.7 | 4.9±1.2 | 0.45 |
| LDL-C, mmol/L | 2.8±1.0 | 3.2±1.0 | 0.25 |
| HDL-C, mmol/L | 0.8±0.2 | 1.0±0.2 | 0.09 |
| Triglycerides, mmol/L | 2.0±1.0 | 1.8±1.2 | 0.44 |
| TCO location | |||
| LAD | 6 (16) | 17 (29) | 0.31 |
| LCx | 9 (23) | 10 (18) | 0.26 |
| RCA | 24 (61) | 29 (51) | 0.13 |
Data presented as mean ± SD unless otherwise indicated.
* Student’s t test or χ2 test were used. HDL-C High-density lipoprotein cholesterol; LAD Left anterior descending coronary artery; LCx Left circumflex coronary artery; LDL-C Low-density lipoprotein cholesterol; RCA Right coronary artery; TCO Total coronary occlusion
Hematological parameter data are presented in Table 2. Platelet count, red cell distrubition width, mean corpuscular volume and lymphocyte count were similar in both groups. However, MPV was significantly higher in the poor CC group (Figure 1). Hemoglobin level was significantly lower in the poor CC group. WBC count, neutrophil count and N/L ratio was significantly higher in the poor CC group compared with the good CC group (Figure 2). In the correlation analysis comparing Rentrop score with MPV and N/L ratio, a negative correlation was found (r=−0.274; P=0.012 and r=−0.339; P=0.001, respectively). A multivariate logistic regression analysis showed that N/L ratio was the only independent predictor of collateral circulation (OR 0.762 [95% CI 0.587 to 0.988]; P=0.04) (Table 3).
TABLE 2.
Comparison of hematological parameters in the study population
|
Coronary collaterals
|
|||
|---|---|---|---|
| Poor (n=39) | Good (n=57) | P* | |
| Hemoglobin, g/L | 131±15 | 140±16 | 0.02 |
| MCV, fL | 84.9±7.3 | 87.8±7.0 | 0.08 |
| RDW, % | 17.1±0.9 | 17.0±0.8 | 0.41 |
| Platelet count, ×109/L | 236.6±60.0 | 228.1±63.0 | 0.53 |
| MPV, fl | 8.7±2.0 | 7.9±0.9 | 0.01 |
| WBC count, ×109/L | 8.7±2.9 | 7.4±1.8 | 0.01 |
| WBC subytpes | |||
| Neutrophil, ×109/L | 6.5±2.5 | 4.6±1.7 | 0.001 |
| Lymphocyte, ×109/L | 1.8±0.6 | 1.9±0.6 | 0.70 |
| Eosinophils, ×109/L | 0.11±0.10 | 0.2±0.2 | 0.01 |
| N/L ratio | 3.8±2.0 | 2.6±1.6 | 0.002 |
Data presented as mean ± SD unless otherwise indicated.
Student’s t test. MCV Mean corpuscular volume; MPV Mean platelet volume; N/L Neutrophil/lymphocyte; RDW Red cell distribution width; WBC White blood cell
Figure 1).
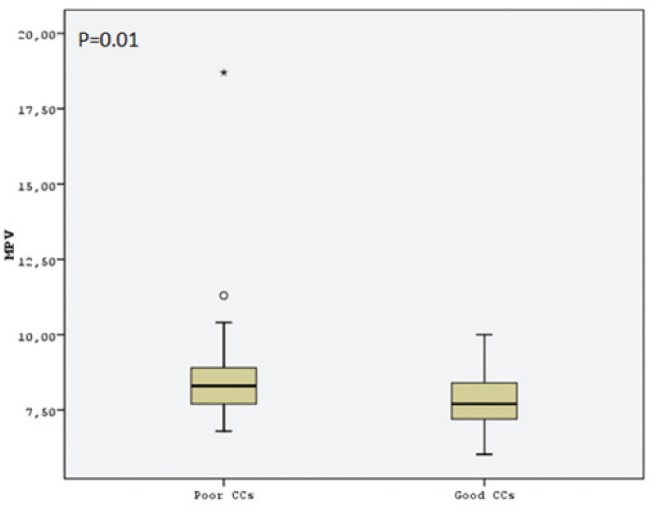
Comparison of mean platelet volume (MPV) levels of the study groups. CCs Coronary collaterals
Figure 2).
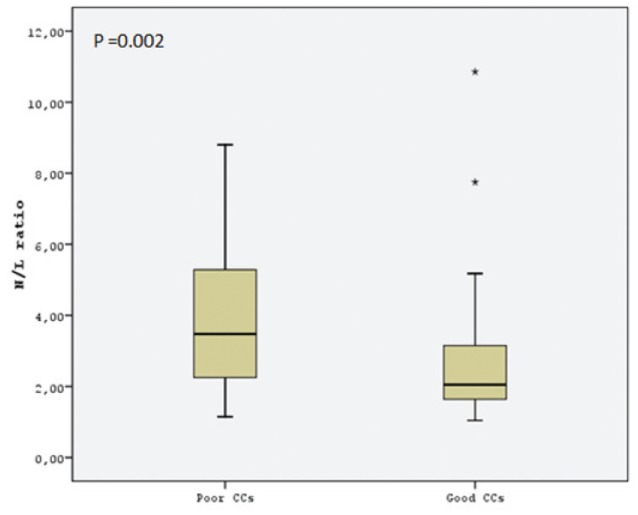
Comparison of the neutrophil/lymphocyte (N/L) ratio of the study groups. CCs Coronary collaterals
TABLE 3.
Relationship between coronary collateral circulation and clinical and hematological parameters
| OR (95% CI) | P | |
|---|---|---|
| Age | 1.003 (0.952–1.057) | 0.91 |
| Sex | 0.716 (0.182–2.811) | 0.63 |
| Diabetes mellitus | 0.595 (0.195–1.814) | 0.36 |
| Hypertension | 0.476 (0.173–1.309) | 0.15 |
| Mean platelet volume | 0.652 (0.397–1.072) | 0.09 |
| Neutrophil/lymphocyte ratio | 0.762 (0.587–0.988) | 0.04 |
DISCUSSION
The main finding of the present study was that N/L ratio and MPV were significantly higher in patients with poor CCs compared with those with good CCs. N/L ratio and MPV also correlated with the Rentrop score. In addition, we found that N/L ratio was an independent predictor of the development of CCs in multivariate analyses.
Well-developed CCs are important compensatory mechanisms in the protection of myocardium in the ischemic region during coronary occlusion. The factors involved in the development of CCs have been investigated in numerous studies. Several demographic, clinical and biochemical factors have been reported to be associated with the degree of CC development (3,5,8).
Inflammation has a significant role in the initiation and progression of atherosclerosis (16,17). WBCs and its subtypes play a major role in modulating the inflammatory response in the atherosclerotic process (18). Previous studies have clearly demonstrated that elevated inflammatory activity was associated with poor CCs (8,19). The N/L ratio is an indicator of inflammatory status that can easily be derived from the WBC count. The N/L ratio has been studied to predict cardiovascular events and mortality (13,20). Recently, Kalay et al (21) reported that higher N/L ratio is independently associated with angiographic progression of atherosclerosis (21). However, the relationship between the N/L ratio and the devolepment of CCs has not yet been studied. In the present study, we showed that the N/L ratio was higher in patients with poor CCs and that this ratio was correlated with the Rentrop score. We also demonstrated that the N/L ratio was an independent predictor of CCs in multivariate analyses. The relationship between the N/L ratio and poor CCs may be explained by increased inflammatory activity and endothelial dysfunction. It is known that the endothelium plays a major role in the development of CCs. Increased N/L ratio also inhibits angiogenesis through its negative effect on endothelial function and, as such, is a risk factor for atherosclerosis. This may represent additional supportive evidence that high CRP levels, which cause endothelial dysfunction, are associated with poor CCs.
Larger platelets have greater prothombotic potential than smaller platelets (22). It has been reported that increased MPV is associated with atherosclerotic risk factors including DM, HT and obesity (23–25). Only one report to date has investigated the relationship between MPV and the development of CCs (26). The authors indicated that MPV levels were significantly higher in patients with poor CCs. Similarly, we found elevated MPV in patients with poor CCs compared with patients with good CCs. Our study also showed that MPV values correlated with Rentrop scores. The relationship between increased MPV and poor CCs may be explained by the prothrombotic factors that are expressed by activated platelets, which contribute to endothelial dysfunction and coronary atherosclerosis.
Our results suggest that N/L ratio and MPV are significantly higher in the patients with poor CCs. Additionally, our findings suggest that platelet size inversely influences the development of CCs, and that increased N/L ratio is related to the presence of inadequte CCs. Another clinical implication of our study, especially N/L ratio and MPV levels, are useful and readily available hematological parameters for the assessment of CCs.
The present study had some limitations. First, the study population was relatively small. Second, inflammatory markers such as CRP and interleukin-6 were not analyzed and compared with N/L ratio in the study population. Finally, we did not quantify and compare CCs with invasive parameters other than the Rentrop scoring system.
Our results revealed that N/L ratio and MPV were associated with poor CCs, and that high N/L ratio was a significant predictor of poor CC development in patients with stable CAD.
REFERENCES
- 1.Pohl T, Seiler C, Billinger M, et al. Frequency distribution of collateral flow and factors influencing collateral channel development: Functional collateral channel measurement in 450 patients with coronary artery disease. J Am Coll Cardiol. 2001;38:1872–8. doi: 10.1016/s0735-1097(01)01675-8. [DOI] [PubMed] [Google Scholar]
- 2.Lambiase PD, Marber MS. Factors determining heterogeneity in coronary collateral development: A clinical perspective. Exp Clin Cardiol. 2002;7:120–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–20. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Van Belle E, Rivard A, Chen D, et al. Hypercholesterolemia attenuates angiogenesis but does not preclude augmentation by angiogenic cytokines. Circulation. 1997;96:2667–74. doi: 10.1161/01.cir.96.8.2667. [DOI] [PubMed] [Google Scholar]
- 5.Weihrauch D, Lohr NL, Mraovic B, et al. Chronic hyperglycemia attenuates coronary collateral development and impairs proliferative properties of myocardial interstitial fluid by production of angiostatin. Circulation. 2004;109:2343–8. doi: 10.1161/01.CIR.0000129225.67353.1F. [DOI] [PubMed] [Google Scholar]
- 6.Tatli E, Altun A, Buyuklu M, Barotçu A. Coronary collateral vessel development after acute myocardial infarction. Exp Clin Cardiol. 2007;12:97–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Koerselman J, de Jaegere PP, Verhaar MC, Grobbee DE, Graaf van der Y. Coronary collateral circulation: The effects of smoking and alcohol. Atherosclerosis. 2007;191:191–8. doi: 10.1016/j.atherosclerosis.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Kerner A, Gruberg L, Goldberg A, et al. Relation of C-reactive protein to coronary collaterals in patients with stable angina pectoris and coronary artery disease. Am J Cardiol. 2007;99:509–12. doi: 10.1016/j.amjcard.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 9.Orem C, Kahraman N, Orem A, Uçar U, Yucesan FB, Mentese A. Increased plasma lipoprotein-associated phospholipase A2 (Lp-PLA2) levels are related to good collateral development in patients with isolated left coronary artery disease. Int J Cardiol. 2011;148:117–9. doi: 10.1016/j.ijcard.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 10.Yildiz A, Sezen Y, Gur M, Yilmaz R, Demirbag R, Erel O. Association of paraoxonase activity and coronary collateral flow. Coron Artery Dis. 2008;19:441–7. doi: 10.1097/MCA.0b013e328309b9f0. [DOI] [PubMed] [Google Scholar]
- 11.Kocaman SA, Sahinarslan A, Biberoglu G, et al. Asymmetric dimethylarginine and coronary collateral vessel development. Coron Artery Dis. 2008;19:469–74. doi: 10.1097/MCA.0b013e328311d32b. [DOI] [PubMed] [Google Scholar]
- 12.Mukamal KJ, Wellenius GA, Mittleman MA. Hematologic parameters, atherosclerotic progression, and prognosis in patients with previous coronary artery bypass grafting (from the Post CABG Trial) Am J Cardiol. 2009;103:328–32. doi: 10.1016/j.amjcard.2008.09.080. [DOI] [PubMed] [Google Scholar]
- 13.Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. 2008;395:27–31. doi: 10.1016/j.cca.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 14.De Luca G, Santagostino M, Secco GG, et al. Mean platelet volume and the extent of coronary artery disease: Results from a large prospective study. Atherosclerosis. 2009;206:292–7. doi: 10.1016/j.atherosclerosis.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–92. doi: 10.1016/s0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 16.Libby P. What have we learned about the biology of atherosclerosis? The role of inflammation. Am J Cardiol. 2001;88(Suppl):3J–6J. doi: 10.1016/s0002-9149(01)01879-3. [DOI] [PubMed] [Google Scholar]
- 17.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 18.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–43. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 19.Kadı H, Ceyhan K, Karayakalı M, et al. The relationship between coronary collateral circulation and blood high-sensitivity C-reactive protein levels. Turk Kardiyol Dern Ars. 2011;39:23–8. [PubMed] [Google Scholar]
- 20.Horne BD, Anderson JL, John JM, et al. Intermountain Heart Collaborative Study Group Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–43. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 21.Kalay N, Dogdu O, Koc F, et al. Hematologic parameters and angiographic progression of coronary atherosclerosis. Angiology. 2012;63:213–7. doi: 10.1177/0003319711412763. [DOI] [PubMed] [Google Scholar]
- 22.Kamath S, Blann AD, Lip GY. Platelet activation: Assessment and quantification. Eur Heart J. 2001;22:1561–71. doi: 10.1053/euhj.2000.2515. [DOI] [PubMed] [Google Scholar]
- 23.Papanas N, Symeonidis G, Maltezos E, et al. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15:475–8. doi: 10.1080/0953710042000267707. [DOI] [PubMed] [Google Scholar]
- 24.Nadar S, Blann AD, Lip GY. Platelet morphology and plasma indices of platelet activation in essential hypertension: Effects of amlodipine based antihypertensive therapy. Ann Med. 2004;36:552–7. doi: 10.1080/07853890410017386. [DOI] [PubMed] [Google Scholar]
- 25.Coban E, Ozdogan M, Yazicioglu G, Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59:981–2. doi: 10.1111/j.1742-1241.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 26.Ege MR, Acıkgoz S, Zorlu A, et al. Mean platelet volume: An important predictor of coronary collateral development. Platelets. 2012 May 30; doi: 10.3109/09537104.2012.675107. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]


