Abstract
BACKGROUND/OBJECTIVE:
Left ventricular (LV) circumferential or longitudinal shortening may be impaired in patients with type 2 diabetes mellitus (DM). In the present study, patients with type 2 DM without myocardial ischemia and combined impairment of circumferential and longitudinal (C+L) shortening were studied to assess the prevalence and factors associated with this condition.
METHODS:
Data from 386 patients with type 2 DM enrolled in the SHORTening of midWall and longitudinAl left Ventricular fibers in diabEtes study were analyzed. One hundred twenty healthy subjects were used to define C+L dysfunction. Stress-corrected midwall shortening and mitral annular peak systolic velocity were considered as indexes of C+L shortening and classified as low if <89% and <8.5 cm/s, respectively (10th percentiles of controls).
RESULTS:
Combined C+L dysfunction was detected in 66 patients (17%). The variables associated with this condition were lower glomerular filtration rate (OR 0.98 [95% CI 0.96 to 0.99], greater LV mass (OR 1.05 [95% CI 1.02 to 1.08]), high pulmonary artery wedge pressure (OR 1.23 [95% CI 1.04 to 1.44]) and mitral annular calcifications (OR 3.35 [95% CI 1.71 to 6.55]). Considering the entire population, the relationship between stress-corrected midwall shortening and peak systolic velocity was poor (r=0.20), and the model was linear. The relationship was considerably closer and nonlinear in patients with combined C+L dysfunction (r=0.61; P<0.001), having the best fit by cubic function.
CONCLUSIONS:
Combined C+L dysfunction was present in one-sixth of patients with type 2 DM without myocardial ischemia. This condition was associated with reduced renal function, worse hemodynamic status and structural LV abnormalities, and may be considered a preclinical risk factor for heart failure.
Keywords: Diabetes, Left ventricular systolic dysfunction, Longitudinal function, Midwall shortening, Mitral calcification
A significant proportion of patients with type 2 diabetes mellitus (DM) without overt cardiac disease and, in particular, myocardial ischemia, experience left ventricular (LV) systolic dysfunction (LVSD) that is not detected by the traditional measure of global LV systolic function at the chamber level by ejection fraction (1–6). In fact, this index largely overestimates LV systolic function in these patients, particularly in those with concentric geometry (1,2,6). These patients may exhibit depressed midwall shortening (1,2,4), suggesting the presence of systolic dysfunction of circumferential LV myocardial fibres and/or reduced shortening of longitudinal fibres – an index of longitudinal LVSD (3,5).
The aims of the present study were to analyze, in patients with DM without clinical evidence of coronary artery disease (CAD), the prevalence and clinical characteristics of the subgroup with combined LVSD of circumferential and longitudinal (C+L) fibres; and the relationship between indexes of LV C+L function, which have not been previously investigated in this patient population.
METHODS
Study population
The SHORTening of midWall and longitudinAl left Ventricular fibers in diabEtes (SHORTWAVE) trial was a prospective, multicentre study evaluating clinical and echocardiographic characteristics of a large cohort of individuals with type 2 DM. Study participants were noninstitutionalized subjects >18 years of age with DM diagnosed according to WHO criteria (fasting serum glucose ≥7.0 mmol/L or 2 h postchallenge serum glucose ≥11.1mmol/L or use of hypoglycemic medication). All subjects were free from symptoms and clinical signs of cardiac disease and, in particular, of inducible myocardial ischemia evaluated by exercise electrocardiography/myocardial perfusion imaging on scintigraphy/echostress test performed within one year before enrollment. Exclusion criteria included a history of documented myocardial infarction, dilated cardiomyopathy or heart failure, primary hypertrophic cardiomyopathy, asymptomatic known LVSD, previous myocardial revascularization, significant heart valve disease (more than mild mitral and/or aortic regurgitation, and any degree of stenosis), atrial fibrillation, type 1 DM and chronic pulmonary disease.
After completion of clinical history, and physical and laboratory examination (hemoglobin, hematocrit, glycemia, C-reactive protein, serum creatinine, lipid profile, proteinuria), eligible subjects underwent echocardiographic examination. Participants were recruited from January 1 to October 31, 2011 at four Italian cardiovascular (CV) centres (Trento, Trieste, Peschiera and Brescia). A group of 120 normotensive healthy volunteers was also studied to assess the range of normal values of the shortening of LV C+L fibres for defining LV C+L dysfunction. These subjects were statistically comparable with a group of subjects enrolled into the study in terms of age, sex, body weight and body mass index. All provided written informed consent and the study was approved by the ethics committees of all participating centres.
Definitions
Concomitant hypertension was defined as a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg, and/or pharmacologically treated high blood pressure of unknown etiology. Well-controlled blood pressure was defined as systolic blood pressure <130 mmHg and diastolic blood pressure <80 mmHg. Obesity was diagnosed if patients had a body mass index ≥30 kg/m2. Dyslipidemia was defined as total serum cholesterol levels >4.9 mmol/L and or triglyceride levels ≥1.7 mmol/L or pharmacologically treated high lipid serum levels. Well-controlled dyslipidemia was defined as serum low-density lipoprotein cholesterol <2.6 mmol/L and tryglicerides <1.7 mmol/L. Metabolic syndrome was defined according to the the National Cholesterol Education Program Adult Treatment Panel III criteria. To assess renal function, the glomerular filtration rate was estimated using the simplified MDRD equation.
Echocardiography
Transthoracic Doppler echocardiography was performed following a standardized protocol. Images were stored on CD or MO disks and forwarded to the Echocardiography Core Laboratory at Villa Bianca Hospital of Trento, Italy, for final interpretation. Echocardiographers were blinded to all clinical participant information. LV chamber dimensions and wall thicknesses were measured using the American Society of Echocardiography guidelines, and LV mass was calculated using a necropsy-validated formula (7). LV mass was normalized for height to the power of 2.7, and LV hypertrophy was defined as LV mass >49.2 g/m2.7 for men and >46.7 g/m2.7 for women (8). Relative wall thickness was calculated as the 2 × end-diastolic ratio posterior wall thickness divided by LV diameter, and indicated concentric LV geometry if ≥0.43 (the 97.5th percentile in the normal population) (9). LV end-diastolic and end-systolic volumes and stroke volume were measured using the biplane method of disks from two-dimensional, apical four-chamber + two-chamber views and used to calculate ejection fraction. Stroke volume was used to estimate cardiac index and stroke work, according to the formula:
Circumferential LV systolic function was assessed at the midwall level and related to end-systolic circumferential stress, as previously described (10). Stress-corrected midwall shortening (sc-MS) <89% (10th percentile of the healthy controls) was indicative of systolic dysfunction of LV circumferential fibres. Tissue Doppler study (pulsed-wave spectral analysis) was used to measure peak mitral annular systolic velocity (S′ [mean of four measurements obtained in septal, lateral, inferior and anterior mitral annular position]), as an estimate of longitudinal LV function (11). Peak S′ <8.5 cm/s (10th percentile of the healthy controls) was considered to be compatible with systolic dysfunction of LV longitudinal fibres.
Transmitral and pulmonary vein pulsed-wave Doppler curves and early diastolic tissue Doppler velocity of mitral annulus (E′) were assessed according to the recommendations of the American Society of Echocardiography (12). Early diastolic velocity of transmitral flow (E) was divided by E′ and used to classify LV diastolic function together with other parameters (E/A ratio of transmitral flow, deceleration time of E and the difference in duration of atrial wave on pulmonary vein flow and atrial wave on transmitral flow) in 4° as proposed by Redfield et al (13): normal, mild dysfunction, moderate dysfunction and severe dysfunction. Pulmonary capillary wedge pressure was estimated by the formula validated by Nagueh et al (14):
Maximal left atrial volume was also computed from the two-dimensional, apical four-chamber view using the area minus length method and was normalized for body surface area. Mitral annular calcification (MAC) was considered to be present if an echodense band was visualized throughout systole and diastole, was distinguishable from the posterior mitral valve leaflet, and was located anterior and parallel to the posterior LV wall (15). Aortic valve sclerosis was defined as focal thickening and calcification of the aortic valve without obstruction of blood flow (16).
Statistical analysis
Data are reported as mean ± SD. SPSS version 19.0 (IBM Corporation, USA) was used for statistical analysis. The unpaired Student’s t test and χ2 statistics were used for descriptive statistics. Between-group comparisons of categorical and continuous variables were performed using the χ2 test and ANOVA with post hoc comparison between each group by Scheffè test and Tukey’s honestly significant difference (Spjotvoll/Stoline) test for unequal samples, as appropriate.
Multivariable analysis (logistic regression) was performed to identify the factors independently related to combined LVSD of C+L fibres. The following variables were included in the model: age, sex, body mass index, C-reactive protein level, glomerular filtration rate, presence of aortic valve sclerosis, presence of MAC, LV relative wall thickness, LV mass and pulmonary capillary wedge pressure. Metabolic syndrome and the number of CV risk factors (the calculation included the following conditions: active smoker, dyslipidemia, hypertension, obesity) were two additional variables forced into the analysis to verify whether ‘clustering effects’ of the traditional CV risk factors influenced the occurrence of combined LVSD of C+L fibres.
Curve fitting estimation analysis was performed to investigate the relationship between the C+L shortening of the myocardial fibres. The models considered for the estimation were: linear, quadratic, cubic, compound, growth, logarithmic, exponential, inverse, exponential, power and logistic. They were applied to the total population and to the subgroup of patients with combined LVSD of C+L fibres. To determine which model to use, scatterplots of the data were reviewed, the type of mathematical function was analyzed and data fitting the corresponding type of available model were transformed. The r2 coefficient was used as an indicator of goodness of fit for each model. The statistical difference between the models was measured by ANOVA. A two-tailed P<0.05 was considered to be statistically significant.
RESULTS
The study population consisted of 386 patients with type 2 DM whose clinical and echocardiographic characteristics are shown in Table 1 and Table 2, respectively. LVSD of C+L fibres coexisted in 66 patients (17% of the total study population). This group of patients was older, had worse renal function, increased LV volumes and mass, a higher prevalence of LV concentric geometry and diastolic dysfunction, MAC and aortic valve calcifications compared with the remaining patients (Tables 1 and 2).
TABLE 1.
Main clinical characteristics of the 386 study patients divided according to the presence of combined circumferential and longitudinal left ventricular dysfunction
| Variables | Combined circumferential and longitudinal dysfunction (n=66) | No combined dysfunction (n=320) | P | Total study population (n=386) |
|---|---|---|---|---|
| Clinical | ||||
| Age, years | 73±11 | 68±10 | <0.001 | 69±10 |
| Female sex, % | 37 | 44 | NS | 43 |
| Body mass index, kg/m2 | 27.5±4.6 | 28.9±5.3 | NS | 28.6±5.2 |
| Obesity, % | 33 | 38 | NS | 37 |
| Waist circumference, cm | 100±13 | 100±15 | NS | 100±15 |
| Hypertension, % | 77 | 82 | NS | 81 |
| Well-controlled hypertension, % | 36 | 46 | NS | 45 |
| Dyslipidemia, % | 56 | 46 | NS | 54 |
| Active smoker, % | 15 | 23 | NS | 22 |
| Duration of diabetes, years, mean (range) | 5 (3–13) | 5 (2–10) | NS | 5 (2–10) |
| Systolic blood pressure, mmHg | 142±25 | 138±18 | NS | 139±19 |
| Diastolic blood pressure, mmHg | 80±12 | 79±9 | NS | 79±10 |
| Pulse pressure, mmHg | 59±16 | 61±20 | NS | 60±17 |
| Heart rate, beats/min | 71±11 | 72±12 | NS | 71±11 |
| Laboratory | ||||
| Glycemia, mmol/L | 8.33±2.89 | 7.83±2.11 | NS | 7.94±2.28 |
| HbA1c, % | 7.2±1.2 | 7.1±1.3 | NS | 7.1±1.3 |
| HbA1c >7%, % | 52 | 41 | NS | 43 |
| Glomerular filtration rate, mL/min/1.73 m2 | 64±21 | 76±23 | <0.001 | 74±23 |
| Total cholesterol, mmol/L, mean (range) | 4.81 (4.39–5.43) | 4.75 (4.26–5.68) | NS | 4.78 (4.32–5.61) |
| LDL cholesterol, mmol/L, mean (range) | 2.64 (2.22–3.20) | 2.74 (2.25–3.3) | NS | 2.82 (2.22–3.33) |
| LDL cholesterol, >2.58 mmol/L, % | 53 | 53 | NS | |
| Triglycerides, mmol/L, mean (range) | 1.35 (1.11–2.01) | 1.45 (1.12–1.93) | NS | 1.42 (1.11–1.93) |
| Metabolic syndrome, % | 51 | 52 | NS | 52 |
| Macroalbuminuria >300 mg/g, % | 27 | 33 | NS | 32 |
| C-reactive protein, mg/L, mean (range) | 3.2 (2.0–8.5) | 1.8 (0.8–4.8) | <0.001 | 2.8 (1.6–6.8) |
| Pharmacological treatment | ||||
| Beta-blockers, % | 46 | 28 | 0.006 | 31 |
| ACEi/ARB, % | 64 | 73 | NS | 72 |
| Diuretics, % | 42 | 41 | NS | 42 |
| Calcium antagonists, % | 27 | 28 | NS | 28 |
| Antihypertension medications | 1.7±1.3 | 1.5±1.4 | NS | 1.5±1.2 |
| Antiplatelet agents, % | 46 | 38 | NS | 45 |
| Statins, % | 38 | 47 | NS | 45 |
| Metformin, % | 55 | 49 | NS | 50 |
| Other antidiabetic medications, % | 27 | 18 | NS | 19 |
| Insulin, % | 15 | 15 | NS | 15 |
Data presented as mean ± SD unless otherwise indicated. ACEi Angiotensin-converting enzyme inhibitors; ARB Angiotensin T1 receptor blockers; HbA1c Glycated hemoglobin; LDL Low-density lipoprotein; NS Not statistically significant
TABLE 2.
Echocardiographic characteristics
| Variable | Combined circumferential and longitudinal dysfunction (n=66) | No combined dysfunction (n=320) | P | Total study population (n=386) |
|---|---|---|---|---|
| LV EDD, mL/m2 | 2.7±0.4 | 2.6±0.3 | 0.01 | 2.6±0.3 |
| LV ESD, mL/m2 | 1.8±0.4 | 1.5±0.3 | <0.001 | 1.6±0.3 |
| LV EDV, mL/m2 | 57±20 | 51±13 | <0.001 | 52±15 |
| LV ESV, mL/m2 | 25±14 | 19±6 | <0.001 | 20±8 |
| Relative wall thickness | 0.44±0.08 | 0.42±0.08 | 0.04 | 0.43±0.08 |
| Concentric LV geometry, % | 59 | 44 | 0.02 | 47 |
| LV mass index, g/m2.7 | 54±12 | 47±12 | <0.001 | 48±12 |
| LV hypertrophy, % | 70 | 45 | <0.001 | 49 |
| LV stroke volume, mL | 60±17 | 61±19 | NS | 61±18 |
| LV stroke work, mmHg/mL | 123±42 | 122±41 | NS | 122±42 |
| Cardiac index, L/min/m2 | 2.3±0.6 | 2.3±0.6 | NS | 2.3±0.6 |
| LV ejection fraction, % | 59±9 | 63±6 | <0.001 | 62±7 |
| LV CESS, dynes/cm2 | 127±48 | 106±32 | <0.001 | 110±36 |
| LV midwall shortening, % | 14.3±2.1 | 17.8±2.6 | <0.001 | 17.2±2.9 |
| LV Sc- midwall shortening, % | 76.5±10.6 | 94.4±13.3 | <0.001 | 91.4±14.6 |
| Peak S′, cm/s | 7.3±1.1 | 10.2±1.9 | <0.001 | 9.7±2.1 |
| Peak E′, cm/s | 7.6±1.9 | 9.3±2.3 | <0.001 | 9.0±2.3 |
| E wave of transmitral flow, cm/s | 67.4±21.8 | 66.8±19.3 | NS | 66.9±19.7 |
| A wave of transmitral flow, cm/s | 78.6±24.9 | 80.5±20.1 | NS | 80.3±20.8 |
| E/A ratio | 0.85±0.45 | 0.84±0.31 | <0.0001 | 0.84±0.33 |
| E/E′ ratio | 9.4±4.1 | 7.5±2.7 | <0.0001 | 7.8±3.1 |
| LV diastolic dysfunction, % | 56 | 39 | <0.0001 | 42 |
| Grade I | 39 | 34 | 35 | |
| Grade II | 6 | 3 | 4 | |
| Grade III | 11 | 2 | 3 | |
| PCWP, mmHg | 13.6±5.1 | 11.2±3.4 | <0.0001 | 11.6±3.8 |
| Maximal left atrial volume, mL/m2 | 24.9±7.6 | 22.4±8.7 | 0.03 | 22.8±8.6 |
| Mitral valve calcification, % | 53 | 23 | <0.0001 | 28 |
| Aortic valve sclerosis, % | 52 | 33 | 0.004 | 36 |
Data presented as mean ± SD unless otherwise indicated. CESS Circumferential end-systolic stress; EDD End-diastolic diameter; EDV End-diastolic volume; ESD: end-systolic diameter; ESV End-systolic volume; LV Left ventricular; NS Not statistically significant; Peak E′ Peak early diastolic tissue Doppler velocity of mitral annulus; Peak S′ Peak mitral annular systolic velocity (tissue Doppler imaging); PCWP Pulmonary capillary wedge pressure; Sc Stress corrected
A logistic regression analysis was performed to assess the independent variables associated with combined LVSD of C+L fibres. The statistical model indicated four covariates: the presence of MAC (HR 3.68 [95% CI 1.93 to 7.00]; P<0.001), lower glomerular filtration rate (HR 0.98 [95% CI 0.96 to 0.99]; P=0.007), greater LV mass (HR 1.05 [95% CI 1.02 to 1.08]; P<0.001) and higher noninvasively estimated pulmonary capillary wedge pressure (HR 1.11 [95% CI 1.03 to 1.20]; P=0.009). Neither the metabolic syndrome nor the number of CV risk factors (included in the analysis as representations of possible ‘clustering effects’ of the traditional CV risk factors) were associated with combined LVSD of C+L fibres. The prevalence of the metabolic syndrome was 51% and 52% in patients who had and did not have combined LVSD of C+L fibres, respectively. Combined LVSD of C+L fibres was found in 13% of patients without CV risk factors (independent of DM), 25% in those with one factor, 19% in those with two factors, 10% in those with three factors and 19% in those with four factors (P not significant).
Considering the total population, curve fitting estimation analysis showed that the relationship between sc-MS and peak S′ was poor, although statistically significant, and the model was linear (Figure 1). Identical data resulted when patients with combined LVSD of C+L fibres were excluded. In contrast, this relationship was much closer and nonlinear in the subgroup of 66 patients with combined LVSD of C+L fibres, having the best fit by cubic function (Figure 2). The difference in r2 coefficient between the linear model (sc-MS = 30.5 + 6.22×peak S′; r2=0.32; P<0.0001) and the cubic model [sc-MS = 44.4× −2.8×(0.0001×peak S′ 3) −97]; r2=0.38; P<0.0001) was of 0.06 coefficient points corresponding to a 19% of relative increase in r2 (P=0.039).
Figure 1).
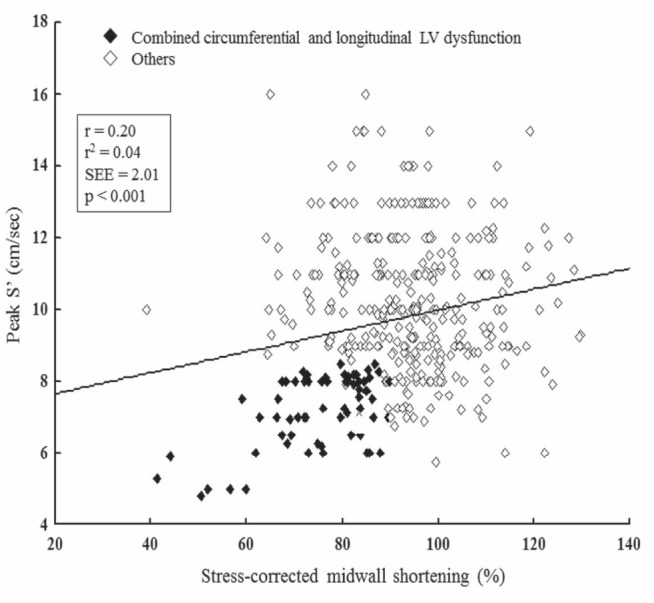
Relationship between stress-corrected midwall shortening, index of left ventricular (LV) circumferential function, and peak mitral annular systolic velocity (S′), index of LV longitudinal function in the entire study population. SEE Standard error of estimate
Figure 2).
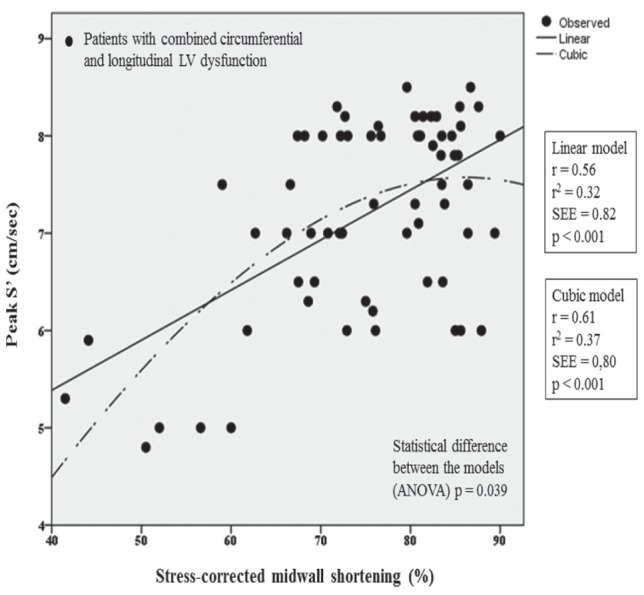
Plot of curve estimation fitting analysis. Cubic relationship between stress-corrected midwall shortening and peak mitral annular systolic velocity (S′) in the 66 patients with combined left ventricular (LV) systolic dysfunction of circumferential and longitudinal fibres. SEE Standard error of estimate
DISCUSSION
For the first time, the present prospective study demonstrated, in patients with type 2 DM without known heart disease or clinical evidence of CAD, that combined reduction of LV C+L shortening of myocardial fibres is not a sporadic state and was detectable in approximately one-sixth of subjects. This condition is strictly associated with abnormalities of LV geometry and diastolic function (represented by increased LV mass and pulmonary artery wedge pressure), with an impaired renal function and a higher prevalence of MAC. In these patients, the relationship between indexes of C+L function fit in a nonlinear cubic function.
The combination of LVSD of C+L myocardial fibres would theoretically be considered to be an uncommon condition in patients with type 2 DM without known heart disease or clinical evidence of CAD. However, although the condition was detected in a significant number of these patients, they were asymptomatic during the usual activities of daily living. Recently, we found a similar behaviour in clinically stable subjects with a history of heart failure and preserved LV ejection fraction (17).
In our experience, the presence of MAC was the strongest variable associated with combined LVSD of C+L fibres. Pathological studies from the 1980s theorized that MAC was associated with higher prevalence of atherosclerosis in multiple vascular beds including coronary and carotid arteries (15,18). This theory was supported by the results of several clinical investigations showing that MAC was independently associated with incident CV disease and death because all events occurred in a graded fashion according to MAC severity (15,18). Our findings add to previous knowledge and further illustrate the adverse prognostic role of MAC in patients at high risk for CV events by demonstrating that the presence of MAC corresponds to a DM phenotype with a worrisome subclinical LVSD (combined impairment of C+L fibres) in the absence of CAD or inducible myocardial ischemia. Two possible mechanisms may explain our result: the presence of undetectable myocardial ischemia at the small coronary artery level (morphofunctional cause), which often subsists in patients with DM, also favoured by the increased LV mass (the second predictor of the combined LVSD of C+L fibres emerged in our multivariate analysis); and the possible effect of unmeasured systemic factors (metabolic causes) including the derangement in parathormone/vitamin D and/or calcium-phosphorous metabolism, which are able to promote both the deposition of calcium in the form of hydroxyapatite crystals on the mitral apparatus and LVSD. It has been definitively established that arterial hypertension, myocardial ischemia, diabetic cardiomyopathy and heart failure may arise from a vitamin D deficiency, a largely underdiagnosed pathological condition by which the synthesis of various myocardial contractile proteins and activation of crucial intracellular mechanisms managing calcium metabolism and energy production are compromised (19). Regarding the derangement of calcium-phosphorous metabolism, it primarily depends on the coexistence of renal dysfunction (the third predictor of the combined LVSD of C+L fibres). It is well known that MAC is common in patients with chronic kidney disease, with a prevalence fivefold higher in diabetic patients than among nondiabetic patients, and is closely associated with findings of intimal arterial disease and inflammation (20). More importantly, the presence and magnitude of renal dysfunction is strongly related to an excessive increase in LV mass (21), a condition that is specifically driven by DM (22) and has emerged as predictor of adverse outcome in several studies recruiting patients with aortic stenosis (23) or arterial hypertension (24). All of these factors have led to speculation that MAC is a predictor of long-term exposure to CV risk factors and to DM itself, excessive LV mass and renal dysfunction.
Another condition independently associated with the combined LVSD of C+L fibres is higher pulmonary capillary wedge pressure, which indicates the coexistence of LV diastolic dysfunction. This finding is consistent with those reported by several studies involving patients with chronic heart failure and preserved LV ejection fraction (18,25), and may be interpreted as an example of the interdependence between LV systolic and diastolic function – two closely coupled, active processes requiring energy in the cardiac cycle that exert a mutual negative influence in the presence of excessive LV mass growth and/or myocardial ischemia (25).
An additional and relevant result of our study was the graphic interpretation of the relationship between circumferential midwall shortening and longitudinal shortening. Compared with patients without combined LVSD, in whom the relationship was poor and linear, in the subgroup of 66 patients with combined LVSD of C+L fibres, the relationship was much closer and demonstrated the best fit by a nonlinear cubic function. From a pathophysiological point of view, this relationship suggests that changes in the shortening of longitudinal fibres within the range of normal values are related to insignificant variations in the shortening of circumferential fibres, while marginal reductions in the shortening of longitudinal fibers within the range of its lowest values correspond to a considerable decrease in the shortening of circumferential fibres. Translating theory into practice, a 12% decrease in peak S′ from 8.5 cm/s to 7.5 cm/s (mild impairment) corresponded to a negligible decrease in sc-MS from 88% to 80% (−9%), while a comparable 12% decrease in peak S′ from 6.5 cm/s to 5.8 cm/s (moderate to severe impairment) corresponded to a significant (more than twofold higher) decrease in sc-MS from 62% to 50% (relative reduction of 20%) in our patients. This behaviour may stem from the physically separate work of LV C+L fibres in the human heart: LV circumferential fibres are primarily structured for generating cardiac output, while LV longitudinal fibres are organized to support circumferential fibres, which normalize systolic stress in all LV myocardial regions (18,26,27).
Given that patients with combined LVSD of C+L fibres experience increased (+17%) circumferential end-systolic stress, greater LV filling pressures and excessive LV mass, our findings suggest that a mild impairment in longitudinal function does not parallel a significant decrease in circumferential shortening, while higher degrees of impairment expose the circumferential fibres to increased stress and hasten dysfunction.
Study limitations
Limitations of the present study should be adressed. The mean duration of DM status (five years) would appear to be quite short to explain the existence of a combined LVSD of C+L myocardial fibres. DM may have been present and unrecognized for a longer time in many patients. Apparently, glycemic control was similar in patients with and without combined LVSD. However, during our study, we had no data regarding glycemic trends and, even more importantly, postprandial glycemic levels, a parameter strongly associated with adverse CV events (28). Furthermore, our results do not consider genetic polymorphisms and environmental factors, which, when combined, may have relevant clinical implications mainly due to impaired insulin sensitivity and a reduced effect of pharmacological therapy (29). All of these considerations may explain why DM patients with similar glycemic control and duration of disease manifest differences in renal function and inflammatory status represented by dissimilar serum C-reactive protein levels. Finally, although the findings reported in the present study demonstrate the close relationship among prognostically validated parameters of LVSD and reduced renal function, worse hemodynamic status and structural LV abnormalities – all of which indicate the vulnerability of these patients – we were not able to definitively demonstrate the clinical association between combined LVSD of C+L myocardial fibres and clinical heart failure. Follow-up studies will be necessary to confirm the significance of the results obtained in the present study.
CLINICAL IMPLICATIONS AND CONCLUSIONS
In a representative large sample of diabetic patients without overt cardiac disease, we found that the presence of combined LVSD of C+L fibres was not uncommon, and was associated with worse renal function, worse hemodynamic status and structural LV abnormalities (increased LV mass and MAC). This phenotype is surprisingly similar in clinical characteristics and function to the relationship between LV C+L fibres (nonlinear cubic) for the pheontype we recently found in patients with chronic heart failure and preserved LV ejection fraction (18); therefore, it may be considered a pre-clinical risk factor for heart failure. This speculation is supported by data showing that a low circumferential shortening measured at the midwall level is strongly related to an adverse prognosis both in patients with treated hypertension and in those with chronic heart failure and preserved ejection fraction (30,31). This information makes midwall shortening the most important and clinically relevant parameter of LV systolic function in patients with DM and preserved LV ejection fraction, and justifies our choice to assess LV circumferential function by midwall shortening with respect to newer techniques such as strain, strain rate and speckle tracking imaging, whose prognostic role remains unknown. Our experience strongly advocates the combined routine use of clinical information deriving by measurement of renal function and standard mono/two-dimensional echocardiography for measurement of LV mass and MAC (completed by the evaluation of LV diastolic function) for suspecting the presence of combined LVSD of C+L fibres. Although no prognostic information regarding this worrisome condition is presently available, we suggest that the coexistence of impaired longitudinal shortening worsens the prognosis of patients with DM and low circumferential shortening.
Acknowledgments
The authors thank Miss Cristina Battisti for her kind assistance in gathering the clinical data and supporting the organization of the study.
REFERENCES
- 1.Cioffi G, Giorda CB, Chinali M, et al. Analysis of midwall shortening reveals high prevalence of left ventricular myocardial dysfunction in patients with diabetes mellitus: The DYDA study. Eur J Cardiovasc Prev Rehabil. 2011 doi: 10.1177/1741826711417759. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 2.Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function. The Strong Heart Study. Circulation. 2000;101:2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 3.Ha JW, Lee HC, Kang ES, et al. Abnormal left ventricular longitudinal function reserve in patients with diabetes mellitus: Implication for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart. 2007;93:1571–6. doi: 10.1136/hrt.2006.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernande L, Rietzschel ER, Bergerot C, et al. Impaired myocardial radial function in asymptomatic patients with type 2 diabetes mellitus: A speckle-tracking imaging study. J Am Soc Echocardiogr. 2010;23:1266–72. doi: 10.1016/j.echo.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Andersen NH, Poulsen SH, Eiskjaer H, et al. Decreased left ventricular longitudinal contraction in normotensive and normoalbuminuric patients with Type II diabetes mellitus: A Doppler tissue tracking and strain rate echocardiography study. Clin Sci. 2003;105:59–66. doi: 10.1042/CS20020303. [DOI] [PubMed] [Google Scholar]
- 6.Rademakers FE, Rogers WJ, Guier WH, et al. Relation of regional cross-fiber shortening to wall thickening in the intact heart. Three-dimensional strain analysis by NMR tagging. Circulation. 1994;89:1174–82. doi: 10.1161/01.cir.89.3.1174. [DOI] [PubMed] [Google Scholar]
- 7.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 8.de Simone G, Devereux RB, Daniels SR, et al. Effect of growth on variability of left ventricular mass: Assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–62. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 9.de Simone G, Daniels SR, Kimball TR, et al. Evaluation of concentric left ventricular geometry in humans: Evidence for age-related systematic underestimation. Hypertension. 2005;45:64–8. doi: 10.1161/01.HYP.0000150108.37527.57. [DOI] [PubMed] [Google Scholar]
- 10.de Simone G, Devereux RB, Roman MJ, et al. Assessment of left ventricular function by the midwall fractional shortening/end systolic stress relation in human hypertension. J Am Coll Cardiol. 1994;23:1444–51. doi: 10.1016/0735-1097(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 11.Sohn DW, Chai HI, Lee DJ, et al. Assessment of mitral annulus velocity by tissue Doppler imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–80. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Redfield MM, Jacobsen SJ, Burnett JC, Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 14.Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 15.Fox CS, Vasan RS, Parise H, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality. The Framingham Heart Study. Circulation. 2003;107:1492–6. doi: 10.1161/01.cir.0000058168.26163.bc. [DOI] [PubMed] [Google Scholar]
- 16.Gharacholou SM, Karon BL, Shub C, et al. Aortic valve sclerosis and clinical outcomes: Moving towards a definition. Am J Med. 2011;124:103–10. doi: 10.1016/j.amjmed.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Cioffi G, Senni M, Tarantini L, et al. Analysis of circumferential and longitudinal left ventricular systolic function in patients with non-ischemic chronic heart failure and preserved ejection fraction (from the CARRY-IN-HFpEF Study) Am J Cardiol. 2012;109:383–9. doi: 10.1016/j.amjcard.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Nair CK, Sudhakaran C, Aronow WS, et al. Clinical characteristics of patients younger than 60 years with mitral anular calcium: Comparison with age- and sex-matched control subjects. Am J Cardiol. 1984;54:1286–7. doi: 10.1016/s0002-9149(84)80082-x. [DOI] [PubMed] [Google Scholar]
- 19.Cioffi G, Gatti D, Adami S. Vitamin D deficiency, left ventricular dysfunction and heart failure. G Ital Cardiol. 2010;11:645–53. [PubMed] [Google Scholar]
- 20.Fox CS, Larson MG, Vasan RS, et al. Cross-sectional association of kidney function with valvular and annular calcification: The Framingham heart study. J Am Soc Nephrol. 2006;17:521–7. doi: 10.1681/ASN.2005060627. [DOI] [PubMed] [Google Scholar]
- 21.Cioffi G, Tarantini L, Frizzi R, et al. Chronic kidney disease elicits excessive increase in left ventricular mass growth in patients at increased risk for cardiovascular events. J Hypertens. 2011;29:565–73. doi: 10.1097/HJH.0b013e3283424188. [DOI] [PubMed] [Google Scholar]
- 22.Cioffi G, Faggiano P, Lucci D, et al. DYDA Investigators Inappropriately high left ventricular mass in patients with type 2 diabetes mellitus and no overt cardiac disease. The DYDA study. J Hypertens. 2011;29:1994–2003. doi: 10.1097/HJH.0b013e32834acc6d. [DOI] [PubMed] [Google Scholar]
- 23.Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic value of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97:301–7. doi: 10.1136/hrt.2010.192997. [DOI] [PubMed] [Google Scholar]
- 24.de Simone G, Verdecchia P, Pede S, et al. Prognosis of inappropriate left ventricular mass in hypertension: The MAVI Study. Hypertension. 2002;40:470–6. doi: 10.1161/01.hyp.0000034740.99323.8a. [DOI] [PubMed] [Google Scholar]
- 25.Yu C, Lin H, Yang H, et al. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 26.Helm RH, Leclercq C, Faris OP, et al. Cardiac dyssyncrhrony analysis using circumferential versus longitudinal strain: Implications for assessing cardiac resynchronization. Circulation. 2005;111:2760–7. doi: 10.1161/CIRCULATIONAHA.104.508457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DH Maclver The reactive impact of circumferential and longitudinal shortening on left ventricular ejection fraction and stroke volume. Exp Clin Cardiol. 2012;17:5–11. [PMC free article] [PubMed] [Google Scholar]
- 28.O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100:899–904. doi: 10.1016/j.amjcard.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 29.Latini G, Marcovecchio ML, Del Vecchio A, et al. Influence of environment on insulin sensitivity. Environ Int. 2009;35:987–93. doi: 10.1016/j.envint.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Wachtell K, Gerdts E, Palmieri V, et al. In-treatment midwall and endocardial fractional shortening predict cardiovascular outcome in hypertensive patients with preserved baseline systolic ventricular function: The Losartan Intervention For Endpoint reduction study. J Hypertens. 2010;28:1541–6. doi: 10.1097/HJH.0b013e328339f943. [DOI] [PubMed] [Google Scholar]
- 31.Borlaug BA, Lam CS, Roger VL, et al. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–8. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


