Abstract
OBJECTIVE:
To explore the effects of atorvastatin on the migration and adhesion of endothelial progenitor cells (EPCs) and on pulmonary artery pressure (PAP) in patients with chronic pulmonary heart disease.
METHODS:
A total of 68 patients with chronic pulmonary heart disease were randomly assigned to either a control group (n=35) or a treatment group (n=33). In addition, 30 healthy volunteers (17 male, 13 female) were enrolled as healthy controls. Atorvastatin (20 mg per day) was administered to the treatment group. The migration and adhesion activities of EPCs in peripheral blood were assessed before and six months after the treatment. PAP was measured using echocardiography before and after the treatment.
RESULTS:
EPC number, migration ability and adhesion activity in the peripheral blood of patients in the control and treatment groups were lower than in patients in the healthy control group at baseline (all P<0.05). After six months of atorvastatin therapy, the number of EPCs in the treatment group was greater than in the control group (P<0.05). Migration and adhesion functions of EPCs in the treatment group were greater than in the control group (all P<0.05). The reduction in PAP in the treatment group was greater than in the untreated control group following six months of therapy (P<0.05).
CONCLUSION:
Atorvastatin therapy increased the migration and adhesion activities of EPCs in patients with chronic pulmonary heart disease. Atorvastatin treatment was also associated with a reduction in PAP in these patients.
Keywords: Atorvastatin, Chronic pulmonary heart disease, Endothelial progenitor cells, Pulmonary hypertension
Chronic pulmonary heart disease usually derives from chronic obstructive pulmonary disease (COPD), which involves the pulmonary circulation and is associated with a high rate of mortality (1,2). Pulmonary artery hypertension (PAH) is prevalent in patients with COPD (3,4). Although the pathophysiological mechanisms of PAH in COPD are not entirely clear, vasoconstriction and remodelling of pulmonary arteries are believed to play a major role (3,4). Hypoxia, pulmonary dysfunction with air trapping, inflammation and toxic effects of cigarette smoking are important factors that lead to PAH in COPD patients (3,4). Pulmonary arteriole endothelial injury and lumen stenosis or clogging also play important roles in the pathogenesis of PAH (5).
Endothelial progenitor cells (EPCs) have been shown to be involved in COPD pathogenesis, and the number of circulating EPCs has been correlated with disease severity (6). The number of circulating EPCs are lower in COPD patients as a result of excessive lung use in response to cell apoptosis and hypoxemia (6,7). EPCs may be a novel therapeutic target for the treatment of chronic pulmonary heart disease because mounting evidence suggests that early EPC transplantation significantly improves exercise tolerance and pulmonary hemodynamics in PAH patients (8,9).
Statins are known to have various pleiotropic effects that are independent of their lipid-lowering functions, including anti-inflammatory and antioxidant effects, suppression of cell proliferation and induction of apoptosis. They have also been shown to enhance EPC counts and improve endothelial function in patients with stable coronary artery disease (10), acute myocardial infarction (11), chronic heart failure (12) and other cardiovascular diseases (13). However, little is known regarding the effect of statins on EPC counts and function in patients with chronic pulmonary heart disease.
The aim of the present study was to explore the effect of atorvastatin on peripheral blood EPC numbers and functions, as well as its effects on pulmonary hypertension, in patients with chronic pulmonary heart disease.
METHODS
Patient selection
A total of 68 patients with pulmonary heart disease, diagnosed between September 2008 and December 2009 at Liaocheng People’s Hospital of Taishan, Taishan, China, were enrolled in the present study. Patients were randomly assigned to either a control group (n=35) or a treatment group (n=33). In addition, 30 healthy volunteers (17 male and 13 female) were enrolled as control subjects.
Chronic pulmonary heart disease was diagnosed according to current guidelines (1). Patients were eligible for inclusion if they met the following criteria: 60 to 85 years of age; in the stable phase of chronic pulmonary heart disease, in which the forced expiratory volume in 1 s (FEV1) was ≤65% of the predicted value, the ratio of FEV1 to the forced vital capacity was ≤70% and the pulmonary hypertension systolic pressure was >30 mmHg; and New York Heart Association (NYHA) functional class I or II. Patients with any one of the following conditions were excluded from the present study: a history of valvular heart disease, congenital heart disease, trauma, ulcer, retinopathy, surgery in the previous three months, acute inflammation, tumour, chronic liver or kidney disease, and acute myocardial infarction in the previous three months.
The present study was approved by the Human Research Ethics Committee of Liaocheng People’s Hospital, Taishan, China. Informed written consent was obtained from all participants.
Intervention
The management strategy for COPD was identical between the control and treatment groups, and included administration of oxygen, bronchodilation and control of infections if present. Atorvastatin (20 mg/day) was administered to the treatment group for six months.
Cell culture and identification of EPCs
Mononuclear cells were isolated from peripheral venous blood using density gradient centrifugation, according to the method described by Vasa et al (10). Mononuclear cells were plated on 24-well culture dishes coated with human fibronectin (Gibco, USA) and maintained in basal medium (Endothelial Basal Medium-2 [EMB-2], Clonetics Corporation, USA) for four days. The liquid medium was changed and cells were cultured for an additional seven days. Adherent cells were detached using EDTA after seven days of culture.
After detachment, adherent cells were incubated with 1,1-dioctad ecyl-3,3,3,3-tetramethylindocarbocyanine-labelled acetylated low-density lipoprotein (DiLDL) (Invitrogen, USA) for 1 h at 37°C. Cells were fixed in paraformaldehyde, washed in phosphate-buffered saline and incubated for 1 h with lectin-conjugated fluorescein isothiocyan-ate (FITC)-labelled Ulex europaeus agglutinin (UEA)-1 (Vector Laboratories, USA). EPCs were characterized by double positivity for the uptake of DiLDL and binding of FIFC-UEA-1 lectin when examined using inverted fluorescence microscopy.
Colony forming assay
Mononuclear cells (5×106) were seeded in a six-well plate with 20 mg/L human fibronectin. After incubation for two days, nonadherent cells (ie, in the supernatant) were collected by gentle aspiration and counted for each subject’s sample. These cells were replated onto new fibronectin-coated plates at a concentration of 1×106 cells/well for the final assessment of the number of colony-forming units per well. EPC colonies, which were characterized by the appearance of multiple thin, flattened cells projecting from a central cluster of rounded cells, were counted by a single-blinded observer after five days of culture.
Assessment of adhesion activity
After digestion and collection of the cultured adherent cells, equal numbers of EPCs were seeded in 24-well culture plates coated with human fibronectin and cultured for 30 min. Adhesion activity was assessed by counting adherent cells after the nonadherent cells were removed by washing with phosphate buffer solution.
Assessment of migration activity
EBM-2 culture solutions were added into the lower chamber of an improved Boyden chamber (Jiangsu Haimen Qilin Medical Instrument Plant, China) and 2×104 EPCs were injected into the upper chamber after being suspended in EBM-2 culture medium for 24 h at 37°C following the digestion and collection of the cultured adherent cells. The nonmobile cells above the filtration membrane were scraped, and cells were fixed with methanol and stained with Giemsa stain. The cells migrating to the bottom chamber were counted using three randomly selected microscopic views.
Transthoracic echocardiography
Pulmonary arterial pressure (PAP) was evaluated using colour Doppler echocardiography (iE33, Philips, Netherlands) and was calculated from the velocity of regurgitant blood flow through the tricuspid valve (14). Echocardiography was performed before and six months after atorvastatin treatment. In the healthy control group, echocardiography was performed one day after enrollment in the study.
Statistical analysis
Continuous variables were presented as mean ± SD, and were compared using two-tailed Student’s t tests or one-way ANOVA for multiple variables. Categorical data were presented as n and %, and compared using the χ2 test or Fisher’s exact test. All data were analyzed using SPSS version 11.0 (IBM Corporation, USA). A two-sided P≤0.05 was considered to be statistically significant.
RESULTS
General findings
At baseline, there were no statistically significant differences in age, sex, NYHA class, PaO2, PCO2 or medications between the treatment group and the control group (Table 1). PAP was similar between the two groups (P>0.05). All patients completed the six-month follow-up.
TABLE 1.
Baseline characteristics of the two groups with chronic pulmonary heart disease
| Variable |
Group
|
P | |
|---|---|---|---|
| Treatment (n=33) | Untreated control (n=35) | ||
| Age, mean ± SD | 66.2±7.4 | 64.9±8.2 | NS |
| Female sex | 13 (39.4) | 12 (34.2) | NS |
| Heart rate, beats/min, mean ± SD | 110.3±9.6 | 107.7±8.2 | NS |
| NYHA class | |||
| I | 0 (0) | 0 (0) | NS |
| II | 15 (45.5) | 16 (45.7) | NS |
| III | 17 (51.5) | 18 (51.4) | NS |
| IV | 1 (3.0) | 1 (2.9) | NS |
| Arterial blood gas, mean ± SD | |||
| PaO2, kPa | 7.42±0.73 | 7.33±0.69 | NS |
| PCO2, kPa | 8.35±0.41 | 8.24±0.38 | NS |
| PAP, mmHg, mean ± SD | 52.7±8.1 | 51.7±7.9 | NS |
| Medications | |||
| Oxygen supplementation | 33 (100.0) | 35 (100.0) | NS |
| IV or oral glucocorticoids | 20 (60.6) | 22 (65.7) | NS |
| Beta2-receptor agonists | 28 (84.8) | 29 (82.9) | NS |
| IV frusemide | 21 (63.6) | 18 (51.4) | NS |
| Spirolactone | 19 (57.6) | 20 (57.1) | NS |
| Digoxin | 12 (36.4) | 10 (28.5) | NS |
Data presented as n (%) unless otherwise indicated. IV Intravenous; NS Not statistically significant; NYHA New York Heart Association; PaO2 Arterial oxygen pressure; PAP Pulmonary arterial pressure; PCO2 Arterial carbon dioxide pressure
Characteristics of EPCs
Mononuclear cells transformed into fusiform endothelial cells after seven days of culture. EPCs possessed the characteristics of endothelial cells and appeared red after uptake of DiLDL, green after binding lectin and yellow after double staining with DiLDL and lectin (Figure 1).
Figure 1).
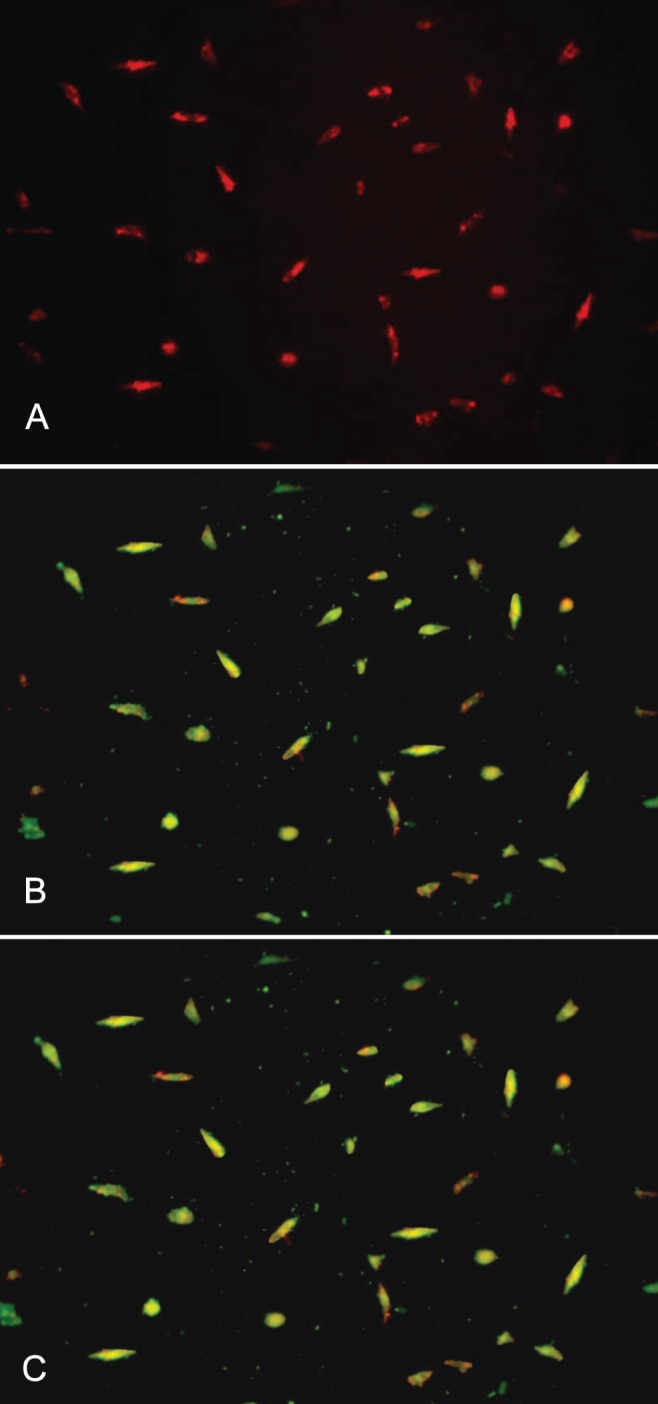
Endothelial progenitor cells appear red after the uptake of dioctadecyl-tetramethylindocarbocyanine-labelled acetylated low-density lipoprotein (A), green after lectin binding (B), and yellow after double staining (C). Images were produced using inverted fluorescence microscopy (original magnification × 200)
EPC counts
The number of EPCs was similar in the treatment and control groups before therapy (P>0.05), but was lower than in the healthy control group (P<0.05). After six months of therapy, the number of EPCs was increased in both patient groups, but the increase reached statistical significance only in the treatment group (P<0.05). The number of EPCs in the treatment group were higher than in the control group after six months’ therapy (P<0.05) (Table 2).
TABLE 2.
Comparison of EPC and PAP before and post-treatment
| Variable | Group | ||||
|---|---|---|---|---|---|
|
| |||||
| Treatment | Untreated control | Healthy control | |||
|
|
|
||||
| Baseline | Post-treatment | Baseline | Post-treatment | ||
| EPC counts (number of CFUs) | 13.9±3.8* | 18.2±4.7† | 14.6±4.3* | 15.2±5.1 | 25.7±5.3 |
| EPC migration | 10.4±3.2* | 15.4±3.8† | 11.3±3.7* | 11.0±3.6 | 23.9±4.8 |
| EPC adhesion | 11.3±2.3* | 16.9±4.5† | 10.8±3.1* | 11.7±4.6 | 24.6±5.4 |
| PAP, mmHg | 52.7±8.1* | 45.4±6.8† | 51.7±7.9* | 49.1±7.3 | 15.3±3.8 |
Data presented as mean ± SD.
P<0.01 versus healthy control group;
P<0.05 versus baseline values in the same group. CFU Colony-forming unit; EPC Endothelial progenitor cell; PAP Pulmonary arterial pressure
Migration and adhesion activity
At baseline, the migration and adhesion activities of EPCs were comparable between the two treatment groups (P>0.05) but were lower than in the healthy control group (P<0.05). After six months of therapy, the migration and adhesion activities of EPCs in the treatment and control groups were increased compared with baseline values (P<0.05). However, the migration and adhesion activities of EPCs in the treatment group were higher than in the control group after six months (P<0.05, Table 2).
DISCUSSION
The present study showed that the numbers and migration and adhesion activities of peripheral blood EPCs were decreased in patients with chronic pulmonary artery disease. However, these parameters were improved after six months of atorvastatin therapy. In addition, atorvastatin treatment was associated with a greater reduction in PAP compared with the untreated control group. These results indicate that atorvastatin may help improve impaired endothelial function and reduce PAP in patients with chronic pulmonary artery disease.
Recent studies have demonstrated that endothelial dysfunction plays an important role in the pathogenesis of PAH in patients with COPD (5). EPCs are precursor cells that can proliferate and differentiate to become vascular endothelial cells, participating in the repair processes after endothelial injury (5,15). After vascular injury, circulating EPCs can return to the injured vessel and differentiate to become vascular endothelial cells, accelerating the repair of the injured vascular endothelium (16). Recent research demonstrated that the number of circulating EPCs in COPD patients was reduced by 30% to 50%, and this reduction was associated with the severity of hypoxemia and airway obstruction (6). Consistent with these results, we found that EPC counts in patients with chronic pulmonary artery disease were lower than in healthy controls. Furthermore, our study showed that adhesion and migration activities of EPCs were reduced in patients with chronic pulmonary artery disease, which is also consistent with other reports in which reduction of adhesion and migration activities was associated with increased PAP in COPD patients (7).
A major finding of our study was that atorvastatin increased the number of EPCs and improved EPC migration and adhesion activities in patients with chronic pulmonary artery disease. Evidence has shown that statins (3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors) exhibit a number of vascular protective effects such as vessel dilation, attenuation of smooth muscle cell proliferation, restoration of endothelial functions, angiogenesis and inflammatory reaction control (17–19). These beneficial vascular effects appear to be independent of the lipid-regulating effects of statins (17–19). A previous study also found that atorvastatin and mevastatin promote EPC proliferation and suppress the aging of circulating EPCs (20). In patients with acute myocardial infarction, the numbers of circulating EPCs in the peripheral blood positively correlated with statin treatment (21). However, most studies have focused on coronary artery disease, heart failure or patients with multiple cardiovascular risk factors (22,23). In the present study, we demonstrated that statins may also facilitate the repair of endothelial function in chronic pulmonary artery disease.
We also found that PAP was decreased after six months of atorvastatin therapy in patients with chronic pulmonary artery disease, although the exact mechanisms remain unclear. In a rat model of severe pulmonary hypertension, statins induced apoptosis in proliferative lesions in the pulmonary arteries (17). In a study involving a rat model of pulmonary hypertension, simvastatin suppressed the expression of inflammatory factors in the lungs by limiting the secretion of monocrotaline, interleukin-6 and interleukin-8 by inflammatory cells, ultimately reducing pulmonary hypertension (24). Whether attenuation of inflammation is one of the mechanisms leading to the reduction in PAP in our patients remains to be determined.
CONCLUSIONS
EPC counts, migration and adhesion activities were decreased in patients with chronic pulmonary heart disease. Atorvastatin therapy elevated the number of EPCs and improved the migration and adhesion activities of the circulating EPCs in these patients. Furthermore, atorvastatin therapy for six months resulted in a reduction in PAP. These results suggest that statins may help to restore endothelial function and reduce PAP in patients with chronic pulmonary heart disease.
REFERENCES
- 1.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: Gold executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Glaab T, Vogelmeier C, Hellmann A, Buhl R. Guideline-based survey of outpatient copd management by pulmonary specialists in germany. Int J Chron Obstruct Pulmon Dis. 2012;7:101–8. doi: 10.2147/COPD.S27887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkins MR. Pulmonary hypertension: The science behind the disease spectrum. Eur Respir Rev. 2012;21:19–26. doi: 10.1183/09059180.00008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zakynthinos E, Daniil Z, Papanikolaou J, Makris D. Pulmonary hypertension in COPD: Pathophysiology and therapeutic targets. Curr Drug Targets. 2011;12:501–13. doi: 10.2174/138945011794751483. [DOI] [PubMed] [Google Scholar]
- 5.Fadini GP, Avogaro A, Ferraccioli G, Agostini C. Endothelial progenitors in pulmonary hypertension: New pathophysiology and therapeutic implications. Eur Respir J. 2010;35:418–25. doi: 10.1183/09031936.00112809. [DOI] [PubMed] [Google Scholar]
- 6.Palange P, Testa U, Huertas A, et al. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J. 2006;27:529–41. doi: 10.1183/09031936.06.00120604. [DOI] [PubMed] [Google Scholar]
- 7.Fadini GP, Schiavon M, Cantini M, et al. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells. 2006;24:1806–13. doi: 10.1634/stemcells.2005-0440. [DOI] [PubMed] [Google Scholar]
- 8.Wang XX, Zhang FR, Shang YP, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: A pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–71. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Ghofrani HA, Barst RJ, Benza RL, et al. Future perspectives for the treatment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S108–117. doi: 10.1016/j.jacc.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasa M, Fichtlscherer S, Adler K, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–90. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 11.Leone AM, Rutella S, Giannico MB, et al. Effect of intensive vs standard statin therapy on endothelial progenitor cells and left ventricular function in patients with acute myocardial infarction: Statins for regeneration after acute myocardial infarction and PCI (strap) trial. Int J Cardiol. 2008;130:457–62. doi: 10.1016/j.ijcard.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 12.Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: Pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111:2356–63. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- 13.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 14.Ma ZS, Ma SJ, Dong MF, Wang JT, Wang LX. Effect of captopril on pulmonary artery pressure following corrective surgery for tetralogy of Fallot. J Card Surg. 2009;24:553–7. doi: 10.1111/j.1540-8191.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 15.Allegra A, Coppolino G, Bolignano D, et al. Endothelial progenitor cells: Pathogenetic role and therapeutic perspectives. J Nephrol. 2009;22:463–75. [PubMed] [Google Scholar]
- 16.Bailey AS, Jiang S, Afentoulis M, et al. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood. 2004;103:13–9. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 17.Taraseviciene-Stewart L, Scerbavicius R, et al. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L668–76. doi: 10.1152/ajplung.00491.2005. [DOI] [PubMed] [Google Scholar]
- 18.Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerates reendothelialization: A novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–24. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 19.Spyridopoulos I, Haendeler J, Urbich C, et al. Statins enhance migratory capacity by upregulation of the telomere repeat-binding factor trf2 in endothelial progenitor cells. Circulation. 2004;110:3136–42. doi: 10.1161/01.CIR.0000142866.50300.EB. [DOI] [PubMed] [Google Scholar]
- 20.Assmus B, Urbich C, Aicher A, et al. Hmg-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res. 2003;92:1049–55. doi: 10.1161/01.RES.0000070067.64040.7C. [DOI] [PubMed] [Google Scholar]
- 21.Schomig K, Busch G, Steppich B, et al. Interleukin-8 is associated with circulating cd133+ progenitor cells in acute myocardial infarction. Eur Heart J. 2006;27:1032–7. doi: 10.1093/eurheartj/ehi761. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt-Lucke C, Fichtlscherer S, Rossig L, Kamper U, Dimmeler S. Improvement of endothelial damage and regeneration indexes in patients with coronary artery disease after 4 weeks of statin therapy. Atherosclerosis. 2010;211:249–54. doi: 10.1016/j.atherosclerosis.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Tousoulis D, Andreou I, Tsiatas M, et al. Effects of rosuvastatin and allopurinol on circulating endothelial progenitor cells in patients with congestive heart failure: The impact of inflammatory process and oxidative stress. Atherosclerosis. 2011;214:151–7. doi: 10.1016/j.atherosclerosis.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang WH, Zhang YJ, Liu CP, Yu BX, Lu WX. Simvastatin protects against the development of monocrotaline-induced pulmonary hypertension in rats via a heme oxygenase-1-dependent pathway. Exp Lung Res. 2011;37:492–9. doi: 10.3109/01902148.2011.591892. [DOI] [PubMed] [Google Scholar]


