Abstract
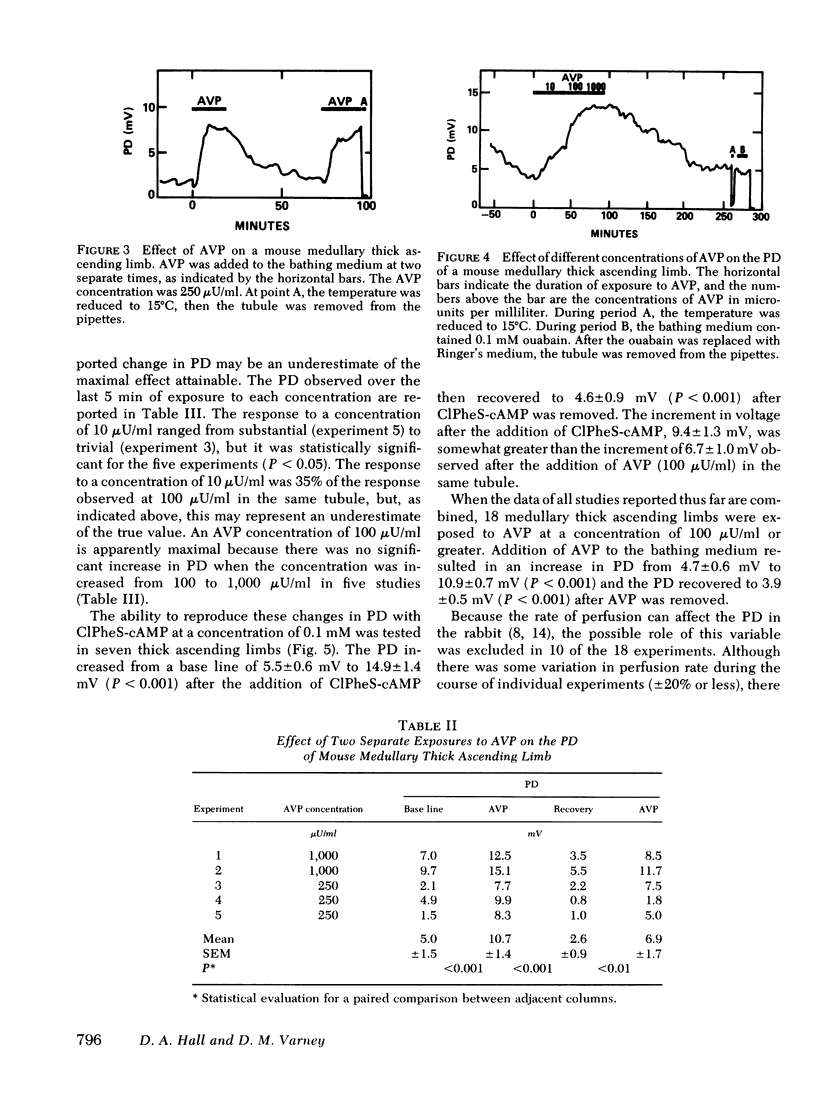
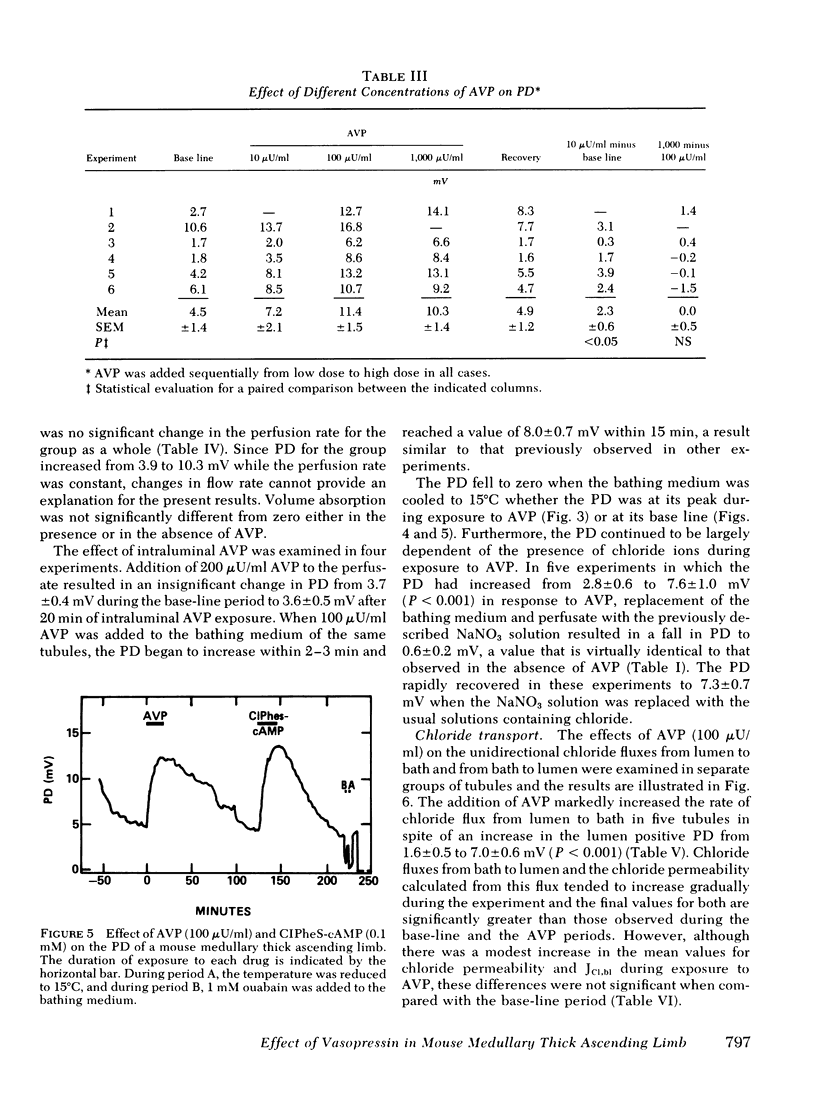
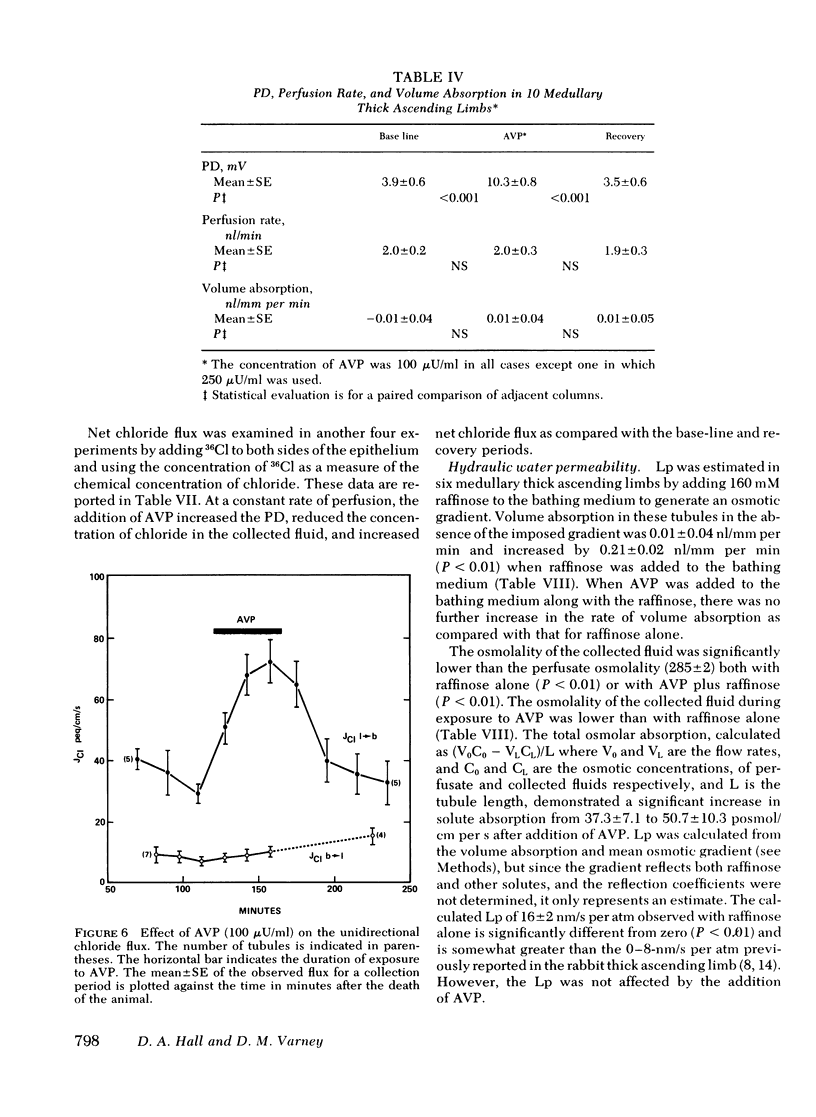
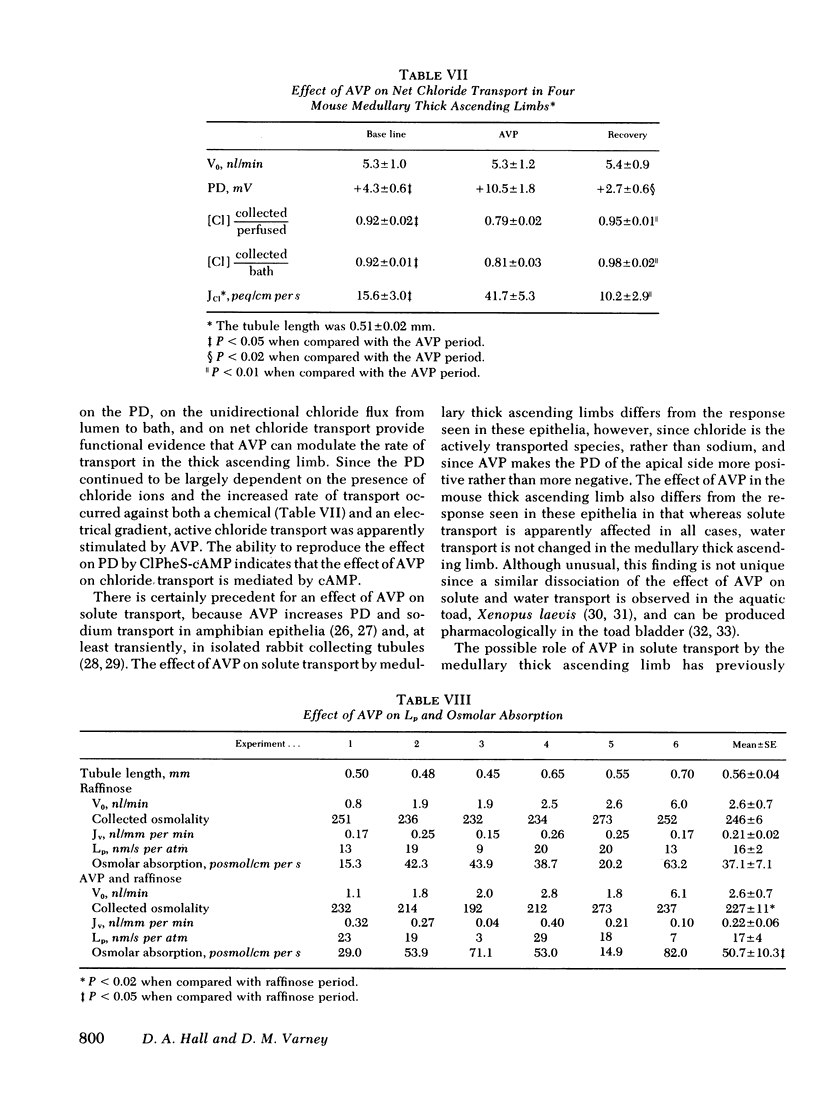
Medullary thick ascending limbs of Henle's loop of the Swiss-Webster mouse were perfused in vitro with an isotonic perfusate and a Ringer's bathing medium. In five studies, addition of a supramaximal concentration of synthetic arginine vasopressin (AVP) to the bathing medium resulted in an increase in electrical potential difference (PD) from 5.0 +/- 1.5 mV, lumen positive, to 10.7 +/- 1.4 mV (P < 0.001). When AVP was removed, the PD returned to 2.6 +/- 0.9 mV (P < 0.001), then increased again to 6.9 +/- 1.7 mV (P < 0.01) when AVP was added a second time. A significant, but submaximal, increase in PD of 2.3 +/- 0.6 MV (P < 0.05) was observed in five medullary thick ascending limbs when AVP was added to the bathing medium at a concentration of 10 microunits/ml. This increase was approximately one-third of the response observed at a concentration of 100 microunits/ml in the same tubule. No further increment in PD was observed in five medullary thick ascending limbs when the AVP concentration was increased from 100 to 1,000 microunits/ml. In seven thick ascendcing limbs, the effect of AVP on PD was reproduced by the addition of 8-[p-chlorophenylthio]-cyclic 3',5'-adenosine monophosphate to the bathing medium at a final concentration of 0.1 mM. AVP increased unidirectional chloride flux from lumen to bath from 29.3 +/- 3.2 to 69.8 +/- 6.2 peq/cm per s (P < 0.001) in spite of an increase in the lumen positive PD from 1.6 +/- 0.5 mV to 7.0 +/- 0.6 mV (P < 0.001). Unidirectional chloride flux from bath to lumen was not affected by AVP. In another series of experiments, net chloride flux increased from 15.6 +/- 3.0 to 41.7 +/- 5.3 peq/cm per s (P < 0.05) after addition of AVP. The effect of AVP on hydraulic water permeability (Lp) was examined by adding raffinose to the bathing medium in both the presence and the absence of AVP. The calculated Lp of 16 +/- 2 nm/s per atm in the absence of AVP, although very low, was significantly different from zero (P < 0.01). However, the Lp did not increase significantly when AVP was added to the bathing medium. These results suggest that AVP has a second site of action in the kidney to increase chloride transport by the medullary thick ascending limb in addition to its well-known effect on the water permeability of the collecting tubule. The former effect would contribute to urinary concentrating ability by increasing the axial osmotic gradient in the renal medulla.
Full text
PDF


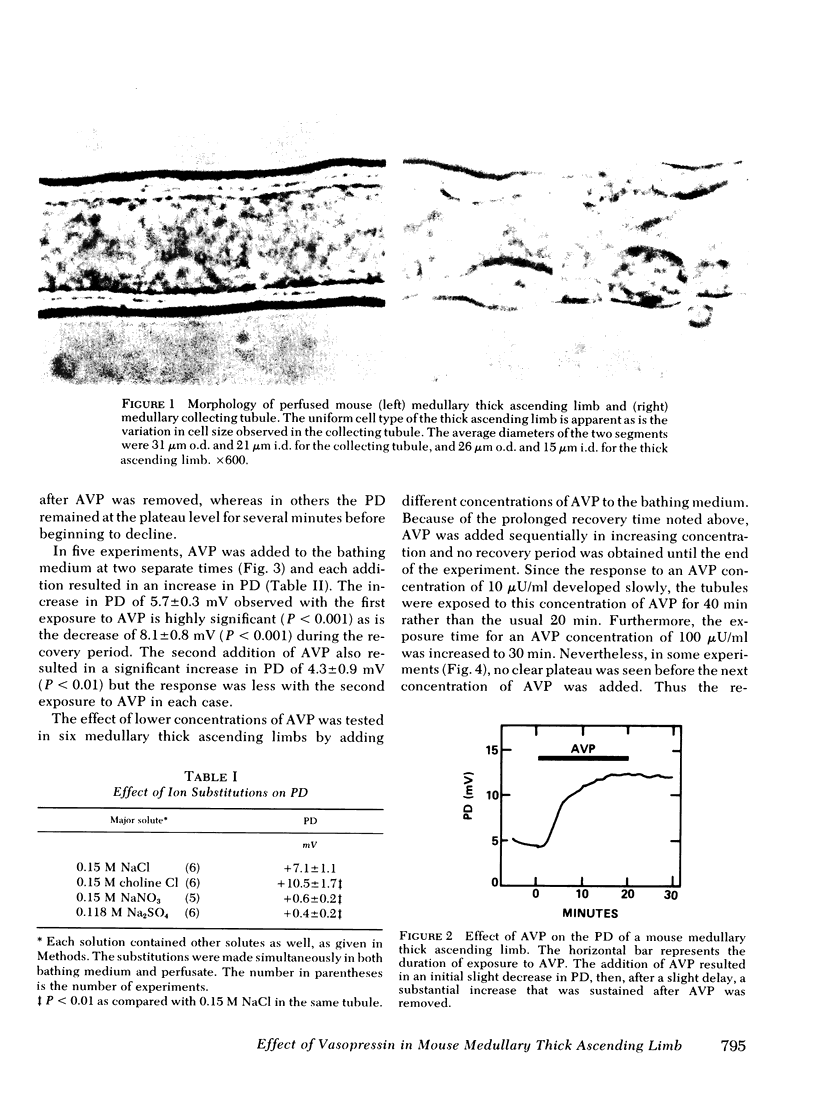



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Zahid G., Schafer J. A., Troutman S. L., Andreoli T. E. Effect of antidiuretic hormone on water and solute permeation, and the activation energies for these processes, in mammalian cortical collecting tubules: evidence for parallel ADH-sensitive pathways for water and solute diffusion in luminal plasma membranes. J Membr Biol. 1977 Feb 24;31(1-2):103–129. doi: 10.1007/BF01869401. [DOI] [PubMed] [Google Scholar]
- Antoniou L. D., Burke T. J., Robinson R. R., Clapp J. R. Vasopressin-related alterations of sodium reabsorption in the loop of Henle. Kidney Int. 1973 Jan;3(1):6–13. doi: 10.1038/ki.1973.2. [DOI] [PubMed] [Google Scholar]
- Atherton J. C., Green R., Thomas S. Influence of lysine-vasopressin dosage on the time course of changes in renal tissue and urinary composition in the conscious rat. J Physiol. 1971 Mar;213(2):291–309. doi: 10.1113/jphysiol.1971.sp009383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P. J. Neurohypophyseal hormones in amphibia: a comparison of their actions and storage. Gen Comp Endocrinol. 1969 Aug;13(1):39–44. doi: 10.1016/0016-6480(69)90219-6. [DOI] [PubMed] [Google Scholar]
- Burg M. B., Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol. 1973 Mar;224(3):659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Burg M., Stoner L., Cardinal J., Green N. Furosemide effect on isolated perfused tubules. Am J Physiol. 1973 Jul;225(1):119–124. doi: 10.1152/ajplegacy.1973.225.1.119. [DOI] [PubMed] [Google Scholar]
- Chabardès D., Gagnan-Brunette M., Imbert-Teboul M., Gontcharevskaia O., Montégut M., Clique A., Morel F. Adenylate cyclase responsiveness to hormones in various portions of the human nephron. J Clin Invest. 1980 Feb;65(2):439–448. doi: 10.1172/JCI109687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., DiBona D. Pathways for movement of ions and water across toad urinary bladder. II. Site and mode of action of vasopressin. J Membr Biol. 1974;19(3):195–220. doi: 10.1007/BF01869978. [DOI] [PubMed] [Google Scholar]
- Du Bois R., Vernoiry A., Abramow M. Computation of the osmotic water permeability of perfused tubule segments. Kidney Int. 1976 Dec;10(6):478–479. doi: 10.1038/ki.1976.135. [DOI] [PubMed] [Google Scholar]
- FUHRMAN F. A., USSING H. H. A characteristic response of the isolated frog skin potential to neurohypophysial principles and its relation to the transport of sodium and water. J Cell Physiol. 1951 Aug;38(1):109–130. doi: 10.1002/jcp.1030380109. [DOI] [PubMed] [Google Scholar]
- Frindt G., Burg M. B. Effect of vasopressin on sodium transport in renal cortical collecting tubules. Kidney Int. 1972 Apr;1(4):224–231. doi: 10.1038/ki.1972.32. [DOI] [PubMed] [Google Scholar]
- Gardner K. D., Jr, Vierling J. M. Solids, water, and solutes in papillary region of the rat kidney. Am J Physiol. 1969 Jul;217(1):58–64. doi: 10.1152/ajplegacy.1969.217.1.58. [DOI] [PubMed] [Google Scholar]
- Gross J. B., Imai M., Kokko J. P. A functional comparison of the cortical collecting tubule and the distal convoluted tubule. J Clin Invest. 1975 Jun;55(6):1284–1294. doi: 10.1172/JCI108048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai M. A., Thomas S. The time-course of changes in renal tissue composition during lysine vasopressin infusion in the rat. Pflugers Arch. 1969;310(4):297–317. doi: 10.1007/BF00587241. [DOI] [PubMed] [Google Scholar]
- Helman S. I., Grantham J. J., Burg M. B. Effect of vasopressin on electrical resistance of renal cortical collecting tubules. Am J Physiol. 1971 Jun;220(6):1825–1832. doi: 10.1152/ajplegacy.1971.220.6.1825. [DOI] [PubMed] [Google Scholar]
- Imbert-Teboul M., Chabardès D., Montégut M., Clique A., Morel F. Vasopressin-dependent adenylate cyclase activities in the rat kidney medulla: evidence for two separate sites of action. Endocrinology. 1978 Apr;102(4):1254–1261. doi: 10.1210/endo-102-4-1254. [DOI] [PubMed] [Google Scholar]
- Jamison R. L., Buerkert J., Lacy F. A micropuncture study of collecting tubule function in rats with hereditary diabetes insipidus. J Clin Invest. 1971 Nov;50(11):2444–2452. doi: 10.1172/JCI106743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. A., Lacy F. B., Jamison R. L. Effect of antidiuretic hormone-induced antidiuresis on water reabsorption by the superficial loop of Henle in Brattleboro rats. J Lab Clin Med. 1977 Dec;90(6):1004–1011. [PubMed] [Google Scholar]
- Kokko J. P. Membrane characteristics governing salt and water transport in the loop of Henle. Fed Proc. 1974 Jan;33(1):25–30. [PubMed] [Google Scholar]
- Kokko J. P., Rector F. C., Jr Countercurrent multiplication system without active transport in inner medulla. Kidney Int. 1972 Oct;2(4):214–223. doi: 10.1038/ki.1972.97. [DOI] [PubMed] [Google Scholar]
- LEAF A., DEMPSEY E. Some effects of mammalian neurohypophyseal hormones on metabolism and active transport of sodium by the isolated toad bladder. J Biol Chem. 1960 Jul;235:2160–2163. [PubMed] [Google Scholar]
- LEVITIN H., GOODMAN A., PIGEON G., EPSTEIN F. H. Composition of the renal medulla during water diuresis. J Clin Invest. 1962 May;41:1145–1151. doi: 10.1172/JCI104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERLMUTT J. H. Influence of hydration on renal function and medullary sodium during vasopressin infusion. Am J Physiol. 1962 Jun;202:1098–1104. doi: 10.1152/ajplegacy.1962.202.6.1098. [DOI] [PubMed] [Google Scholar]
- PETERSEN M. J., EDELMAN I. S. CALCIUM INHIBITION OF THE ACTION OF VASOPRESSIN ON THE URINARY BLADDER OF THE TOAD. J Clin Invest. 1964 Apr;43:583–594. doi: 10.1172/JCI104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Permeability of medullary nephron segments to urea and water: Effect of vasopressin. Kidney Int. 1974 Dec;6(6):379–387. doi: 10.1038/ki.1974.123. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973 Mar;52(3):612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Guiñazú A., Arrizurieta E. E., Yelinek L. Electrolyte, water, and urea content in dog kidneys in different states of diuresis. Am J Physiol. 1964 Apr;206(4):725–730. doi: 10.1152/ajplegacy.1964.206.4.725. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Valtin H., Thurau K., Nagel W., Horster M., Fischbach H., Wahl M., Liebau G. Micropuncture studies on the influence of antidiuretic hormone on tubular fluid reabsorption in rats with hereditary diabetes insipidus. Pflugers Arch. 1969;306(2):103–118. doi: 10.1007/BF00586878. [DOI] [PubMed] [Google Scholar]
- Stephenson J. L. Concentration of urine in a central core model of the renal counterflow system. Kidney Int. 1972 Aug;2(2):85–94. doi: 10.1038/ki.1972.75. [DOI] [PubMed] [Google Scholar]
- Stokes J. B. Effect of prostaglandin E2 on chloride transport across the rabbit thick ascending limb of Henle. Selective inhibitions of the medullary portion. J Clin Invest. 1979 Aug;64(2):495–502. doi: 10.1172/JCI109487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A., Maffly R., Wilson L., Reaven E. Evidence for involvement of microtubules in the action of vasopressin. Ann N Y Acad Sci. 1975 Jun 30;253:723–737. doi: 10.1111/j.1749-6632.1975.tb19241.x. [DOI] [PubMed] [Google Scholar]
- Zain-ul-Abedin Effects of vasopressin upon the composition of rat's kidney. Q J Exp Physiol Cogn Med Sci. 1967 Jul;52(3):285–292. doi: 10.1113/expphysiol.1967.sp001914. [DOI] [PubMed] [Google Scholar]



