Abstract
Adhesions can form after almost any type of abdominal surgery. Postoperative adhesions can be prevented by improved surgical techniques, such as reducing surgical trauma, preventing ischemia, and avoiding exposure of the peritoneal cavity to foreign materials. Although improved surgical techniques can potentially reduce formation of adhesions, they cannot be eliminated completely. Therefore, finding more effective methods to prevent postoperative adhesions is imperative. Recently, we found that a novel thermosensitive hydrogel, ie, poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCEC) had the potential to prevent postoperative adhesions. Using the ring-opening polymerization method we prepared a PCEC copolymer which could be dissolved and assembled at 55°C into PCEC micelles with mean size of 25 nm. At body temperature, a solution containing PCEC micelles could convert into a hydrogel. The PCEC copolymer was biodegradable and had low toxicity in vitro and in vivo. We found that most animals in a hydrogel-treated group (n = 10) did not develop adhesions. In contrast, 10 untreated animals developed adhesions that could only be separated by sharp dissection (P < 0.001). The hydrogel could adhere to peritoneal wounds and degraded gradually over 7–9 days, transforming into a viscous fuid that was completely absorbed within 12 days. The injured parietal and visceral peritoneum remesothelialized over about seven and nine days, respectively. This study confirms that PCEC hydrogel has potential application in the prevention of postoperative adhesions.
Keywords: poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone), thermosensitive, biodegradable, hydrogel, postoperative adhesions
Introduction
Formation of adhesions generally occurs after abdominal and pelvic surgery,1,2 in particular after cholecystectomy, gastrectomy, appendectomy, hysterectomy, colostomy, abdominoperineal resection, and abdominal vascular procedures.3–9 Intraperitoneal adhesions arise after 67%–93% of general surgical procedures and after up to 97% of open pelvic gynecologic procedures.2,10 Adhesions are the result of peritoneal injury during abdominal or pelvic surgery, abdominal inflammatory disease, and infection. Diseases caused by adhesions include adhesive small bowel obstruction,11,12 acquired female infertility,13 chronic abdominal pain,6,14 and inadvertent organ injury after repeated surgeries.15
Formation of adhesions carries a number of risks, so finding a method of preventing them is very important. A variety of surgical techniques and agents have been suggested for the prevention of occurrence and recurrence of postoperative intra-abdominal adhesions.16 Agents such as corticosteroids17 and antihistamines18 have been evaluatedin experimental trials. Researchers have investigated abdominal prostheses with different adhesion properties,19 but these have rarely been successful in preventing surgical adhesions. Surgical techniques to prevent postoperative adhesions include reducing surgical trauma, preventing ischemia, and avoiding exposure of the peritoneal cavity to foreign materials.20 Although the risk of adhesions can potentially be reduced by improved surgical techniques, they cannot be completely avoided.
Antiadhesive strategies can be classified as pharmacologic agents or as solid or liquid intraperitoneal barriers. Pharmacologic agents target inflammatory reactions by limiting fibrin deposition, thereby improving fibrin absorption and suppressing activation of fibroblasts. Barriers are used to prevent apposition of traumatized peritoneal surfaces during the healing process so as to avoid tissue adherence. However, these agents might have disadvantages, including side effects. For example, one clinical study showed that solid material left in the body for a long time can cause significant inflammation and new lesions.21
Poly(ε-caprolactone) (PCL)/poly(ethylene glycol) (PEG) copolymers are biodegradable, amphiphilic, and easy to produce, and have demonstrated promise in the treatment of a number of major diseases.21–24 Poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCEC) is one of the PCL-PEG copolymers. Previously, we found that PCEC could be used in drug delivery systems and also that it worked well as an immune adjuvant.25,26 In this work, we investigated a novel strategy for preventing postoperative peritoneal adhesions by using PCEC copolymers. PCEC copolymers could form micelles at room temperature and be transformed rapidly into a gel when the temperature was increased. The biocompatibility, cytotoxicity, and degradability of these PCEC hydrogels were characterized and their effectiveness in preventing adhesions in vivo was evaluated in animal models.
Materials and methods
Materials
PEG (molecular weight 1,000 Da), ε-caprolactone, and stannous octoate were sourced from Sigma-Aldrich (St Louis, MO, USA). Roswell Park Memorial Institute (RPMI)-1640 medium was supplied by Gibco (Grand Island, NY, USA). All reagents used were analytic reagent grade and used as received. All animal experiments were approved by the institutional animal care and use committee and were in compliance with all regulatory guidelines. Female Wistar albino rats, weighing 240–260 g, were purchased from theLaboratory Animal Center of Sichuan University, Chengdu, People’s Republic of China. All animals were in quarantine for a week before treatment.
Preparation of PCEC triblock copolymer
PCEC triblock copolymers were prepared by ring-opening copolymerization of ε-caprolactone initiated by PEG using stannous octoate as a catalyst, as reported elsewhere.27–30 Briefy, 5 g each of ε-caprolactone and PEG were introduced into a dry glass ampoule under a nitrogen atmosphere, and 0.05 g of stannous octoate was then added to the reaction vessel under mild agitation. The reaction system was kept at 130°C for 6 hours. After the mixture was degassed under vacuum for another 1 hour, the resulting PCEC block copolymer was cooled to room temperature, dissolved in dichloromethane, and reprecipitated from the filtrate using excess cold petroleum ether (boiling range 30°C–60°C). The mixture was then filtered and vacuum-dried to a constant weight. The purified PCEC copolymers were kept in desiccators until further use.
Preparation of PCEC micelles and PCEC hydrogel
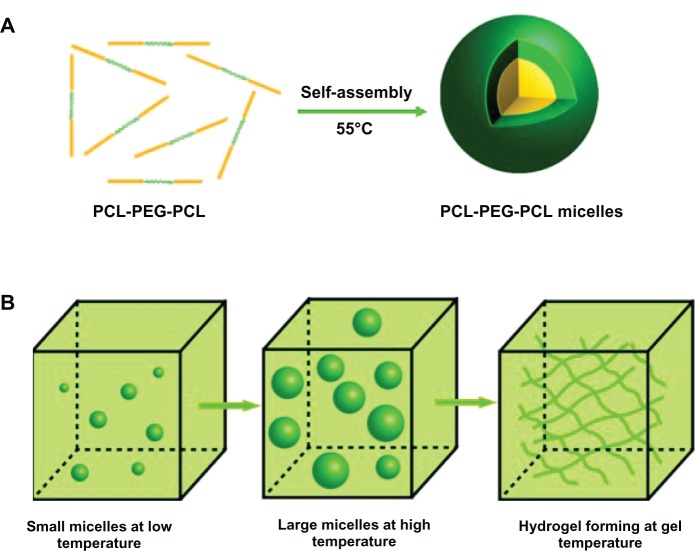
The PCEC hydrogel was prepared in two stages (Figure 1). First, PCEC micelles were made by the self-assembly method. Briefly, PCEC copolymer was dissolved in normal saline solution at 55°C, with PCEC assembling into micelles. These micelles had a small particle size at low temperature, but became larger as the temperature increased. If the concentration of PCEC solution was higher than the critical strength needed for gelation (>20 wt%), the PCEC would transform into hydrogel when the temperature increased. The prepared PCEC triblock copolymer was dissolved in normal saline solution at a concentration of 20 wt% and the temperature was then increased to 55°C, allowing assembly of the copolymer into micelles. The PCEC micelles were kept at 4°C until needed.
Figure 1.
Preparation of thermosensitive PCEC hydrogel. (A) First, PCEC triblock copolymers were assembled into PCEC micelles at 55°C. These micelles were then stored at 4°C. (B) When PCEC micelles were used, the micelles became larger with increasing temperature, and the hydrogel formed when the temperature reached gelation temperature.
Abbreviation: PCEC, poly(ε-caprolactone)(PCL)-poly(ethylene glycol)(PEG)-poly(ε-caprolactone).
Characterization of PCEC micelles
The particle size of the micelles was determined by dynamic light scattering (Nano-ZS 90, Malvern, Worcestershire, UK) at temperatures of 4°C, 10°C, 20°C, 30°C, 37°C, and 42°C. All results shown are the mean of three test runs. The morphology of the PCEC micelles was observed by transmission electron microscopy (H-6009IV, Hitachi, Tokyo, Japan), for which the micelles were diluted with distilled water and placed on a copper grid covered with nitrocellulose. Samples werenegatively stained with phosphotungstic acid and dried at room temperature.
Rheologic analysis
The sol-gel-sol phase transition behavior of PCEC hydrogel was investigated by rheometry (AR Rheometer 2000ex, TA Instruments, New Castle, DE, USA). The hydrogel was placed between parallel plates 40 mm in diameter with a gap of 31 μm. The data were collected under controlled stress (0.5 dyne/cm2) at a frequency of 1.0 radian per second. The heating rate was 2°C per minute. Gelation times for the PCEC hydrogel at 25°C and 37°C were also investigated by rheometry. The morphology of the PCEC hydrogel was observed using a scanning electron microscope (Inspect F, FEI Company, Eindhoven, The Netherlands).
Cytotoxicity assay
Cell viability in the presence of PCEC was investigated in vitro using the MTT assay and murine L929 fibroblasts and HEK293 cells (American Type Culture Collection, Manassas, VA, USA). The cell lines were cultured in RPMI-1640 and Dulbecco’s Modified Eagle’s medium supplemented with 10% fetal bovine serum at 37°C in 5% CO2. Cells (5 × 103) were placed in each well of 96-well plates, and incubated at 37°C in 5% CO2 overnight. The medium was then replacedwith fresh medium at a different PCEC concentration. MTT assays were performed at 24, 48, and 72 hours after adding the PCEC. Only data from the 48-hour time point are shown. Absorbance values were normalized to wells in which cells were not treated with PCEC.
In vivo degradation assay
BALB/c mice were used in this experiment. Each animal was injected subcutaneously through the ventral skin with 1 mL of 20% PCEC hydrogel. The animals were sacrificed after 1–6 weeks to observe degradation of PCEC.
In vivo application of PCEC hydrogel
A rat sidewall defect-cecum abrasion model was established as described previously.31 As a standardized qualitative and quantitative model mimicking reproductive surgery, the sidewall defect-cecum abrasion model has been shown to provide reproducible results and consistent formation of adhesions. Twenty albino Wistar rats were used, and all were treated humanely during the study. The animals were allocated to receive either no treatment or to covering of the injured area with 1 mL of PCEC hydrogel.
Aseptic techniques were used throughout the study. The rats were anesthetized with 3% sodium pentobarbital 30 mg/kg and placed in the supine position, upon whichthe abdomen was exposed by a ventral midline incision. Abdominal adhesions were induced according to the method described by Kosaka et al.32 First, 1 cm × 1 cm of the cecal serosa was abraded with a bone burr until the serosal surface was disrupted and hemorrhagic but not perforated. Second, a 1 cm × 1 cm defect comprising parietal peritoneum and a layer of muscle on the right lateral abdominal wall was excised. Next, 1 mL of 20 wt% PCEC hydrogel stored at 4°C or 1 mL of normal saline was sprayed on the defective sites. Finally, the incision was closed in two layers with 3/0 surgical silk.
The animals were sacrificed on day 14, and the extent of adhesions on the cecal defect was scored as follows: score 0, no adhesion; score 1, one thin filmy adhesion; score 2, more than one thin adhesion; score 3, thick adhesion with focal point; score 4, thick adhesion with plantar attachment or more than one thick adhesion with focal point; score 5, very thick vascularized adhesion or more than one plantar adhesion.
Further, we explored tissue remodeling and repair in peritoneal wounds treated with the hydrogel. We repeated the above procedures and sacrificed the hydrogel-treated rats at different time points to observe the dynamics of hydrogel degradation and healing of peritoneal wounds.
Tissue preparation
We took specimens from the damaged cecum, damaged abdominal wall, and adhesion tissues in the abdominal wall and cecum. The specimens obtained were fixed in 4% paraformaldehyde in phosphate-buffered solution for 48 hours and then embedded in paraffin.
Rats in the hydrogel-treated group were sacrificed on the days indicated, and histologic sections were prepared from injured abdominal wall tissue. The sections were then stained with hematoxylin and eosin. The presence of collagen was detected by Masson staining to assay for fibroblast activity.
The peritoneum is composed of a single layer of mesothelial cells. The surfaces of these cells show typical microvilli on scanning electron microscopy, which was used to observe repair of the peritoneal defect and morphologic changes in mesothelial cells in the group treated with PCEC hydrogel.
The specimens were dehydrated in a critical point device and examined by scanning electron microscopy after gold sputter coating. The tissues were serially sectioned, then stained with hematoxylin and eosin and Masson trichrome for histopathologic examination. All slides were analyzed using light microscopy (Olympus BX 45, Olympus, Hamburg, Germany) by two pathologists in a blinded manner.
Toxicity assessment
To assess for possible side effects, all rats were observed after administration of PCEC hydrogel (including their hair, feces, behavioral patterns, and other clinical symptoms, as well as body weight and mortality). Various organs (heart, lungs, liver, spleen, kidney, stomach, small intestine, and brain) were harvested after sacrifice and fixed in 4% paraformaldehyde in phosphate-buffered solution. Two pathologists observed sections of these tissues stained with hematoxylin and eosin in a blinded manner.
Statistical analysis
Adhesion scores did not always follow a normal distribution. Statistical inferences were made using the Mann–Whitney U test or Fisher’s exact test and the Statistical Package for the Social Sciences version 17.0 software (SPSS Inc, Chicago, IL, USA). P < 0.05 on a two-tailed test was considered to be statistically significant.
Results
Preparation and characterization of PCEC hydrogel
The PCEC micelles were uniform in size, had a very narrow particle size distribution, and had a particle size of 42.5 ± 1.25 nm on transmission electron microscopy (Figure 2A). The relationship between particle size and temperature was also investigated. In a weak concentration of PCEC solution, the size of the PCEC micelles was 38.6 ± 3.2 nm at 4°C, 43.6 ± 3.8 nm at 10°C, 47.9 ± 3.9 nm at 20°C, 135 ± 14.9 nm at 30°C, 417.9 ± 28.7 nm at 37°C, and 822.2 ± 66.5 nm at 42°C. Figure 2B shows that the size of the PCEC micelles became larger with increasing temperature.
Figure 2.
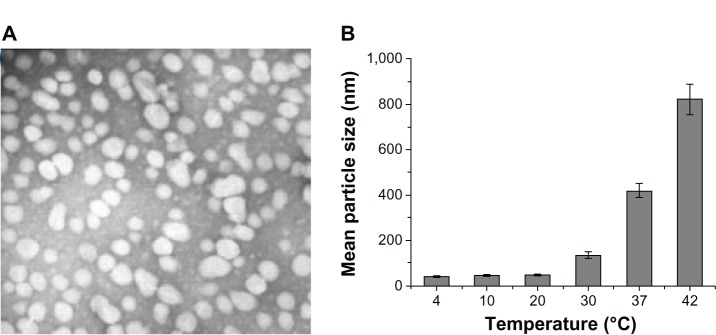
(A) Transmission electron micrograph of PCEC micelles. (B) Relationship between size of micelles and temperature, with the mean size of the PCEC micelles becoming larger with increasing temperature.
Abbreviation: PCEC, poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone).
Rheologic analysis
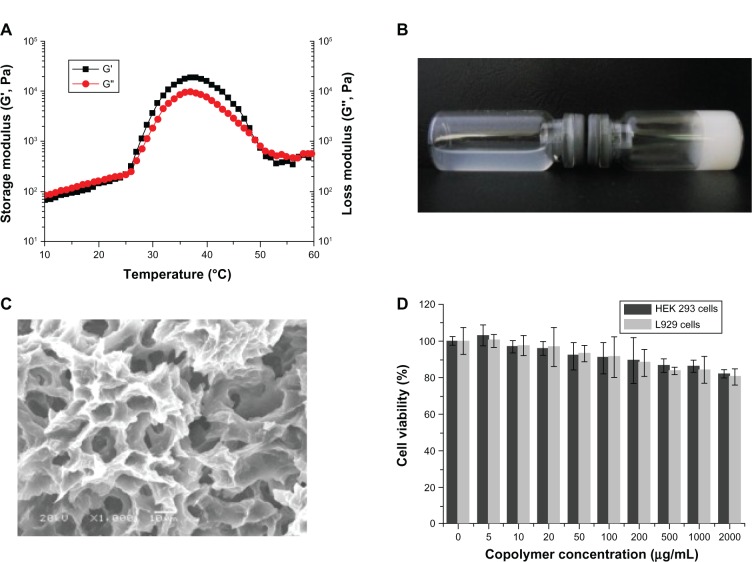
Gel temperature is defined as the temperature at which the storage modulus (G′) and loss modulus (G″) are equal. At 26°C, the PCEC solution started to form a gel (Figure 3A). When the temperature decreased the gels formed solutions. The process was reversible, such that when the temperature was increased again, more solution was transformed into hydrogel (20 wt%). At a temperature of 49°C–57°C, the hydrogel transformed into solution.
Figure 3.
Characterization of PCEC hydrogel. (A) Rheologic analysis of PCEC-micelles-PCEC-hydrogel dual-drug delivery system as a function of temperature. (B) Morphology of blank hydrogel (20 wt%) at 4°C and 37°C. (C) Scanning electron micrograph of PCEC hydrogel. (D) Cytotoxicity of the PCEC was determined by MTT assay.
Abbreviations: PCEC, poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone); MTT, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; HEK, human embryonic kidney.
Figure 3A shows the change in storage modulus (G′) and loss modulus (G″) of PCEC hydrogel (20 wt%) as a function of temperature. G′ in the sol state was less than 249 Pa and increased abruptly during sol-gel transition at 27°C. When the temperature was 37°C, G′ reached almost 30,000 Pa. The dramatic decrease in G′ at about 47°C demonstrates the gel-sol transition of PCEC hydrogel.
The PCEC triblock copolymer was dissolved in normal saline solution at the designated temperature and a concentration of 20 wt% to form a PCEC hydrogel, which was kept at 4°C until use. The PCEC hydrogel showed reversible temperature-dependent sol-gel-sol transition. The hydrogel was a transparent flowing sol at 4°C and formed a solid-like gel at 37°C (Figure 3B). On scanning electron microscopy, it was found that the gel had a three-dimensional structure cross-linked into a network (Figure 3C).
Cytotoxicity of PCEC
Minimal cytotoxicity was seen in cultures containing PCEC solution at concentrations up to 2 mg/mL and for an incubation period of 48 hours. Figure 3D shows cell viability as a function of polymer concentration at 48 hours.
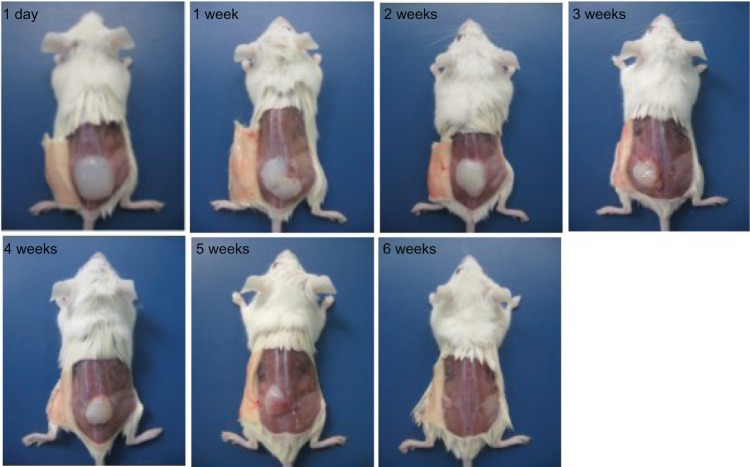
In vivo degradation assay
PCEC solution was injected subcutaneously to assess degradation of the PCEC hydrogel in vivo. When injected via this route at body temperature, the PCEC solution rapidly formed a PCEC gel in situ (Figure 4). On visual observation, the PCEC hydrogel became smaller with time, and had disappeared completely by the end of six weeks.
Figure 4.
Degradation of PCEC hydrogel in vivo, with the hydrogel becoming smaller with time and completely disappearing by week 6, indicating that PCEC is biodegradable.
Abbreviation: PCEC, poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone).
Prevention of abdominal adhesions
Adhesions were formed by abrasion of the cecum and excision of a section of the adjacent abdominal wall in rats (Figure 5A and B). In the untreated group, adhesions were formed between the injured cecum and the abdominal wall after two weeks (Figure 5C and Table 1). In contrast, adhesions scoring no more than 3 were present in the group treated with PCEC hydrogel. In the treated group, the injured cecum and abdominal wall were clearly separated from each other (Figure 5D). PCEC hydrogel formed a protective membrane over the injured cecum with increasing temperature, providing a spacer layer between the cecum and adjacent tissues.
Figure 5.
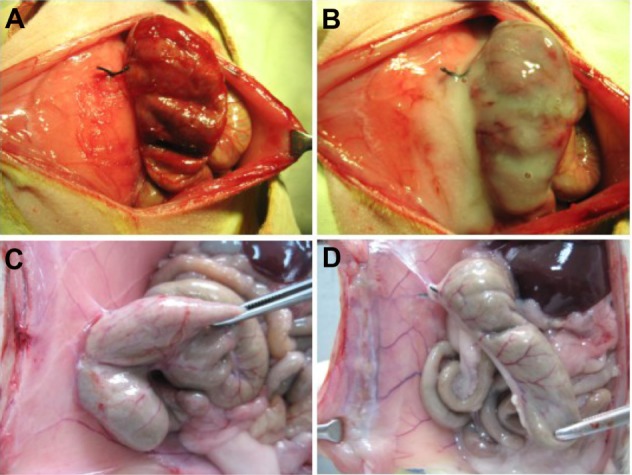
Creation and treatment in the rat abdominal surgery adhesion model. (A) Rat model of abdominal wall defect-cecum. (B) PCEC hydrogel applied to the injured abdominal wall and cecum. (C) Score 4 adhesion between the injured abdominal wall and cecum in a control rat, with adhesion of an intestine segment to the injured abdominal wall and adhesion of the omentum to the sutured midline incision. (D) No adhesions were observed in the group treated with the PCEC gel.
Abbreviation: PCEC, poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone).
Table 1.
Evaluation of adhesion formation in the rat model
| Score | Control group (n = 10) | Percentage | Hydrogel group (n = 10) | Percentage |
|---|---|---|---|---|
| 0 | 0 | 0 | 7 | 70% |
| 1 | 0 | 0 | 2 | 20% |
| 2 | 0 | 0 | 1 | 10% |
| 3 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 9 | 90% | 0 | 0 |
| Death | 1 | 10% | 0 | 0 |
Histologic analysis of adhesion sites in the untreated animals showed fibroblasts and inflammatory cells(Figure 6C). After two weeks, the smooth muscles were fused to the muscles of the abdominal wall (Figure 6C). Compared with the untreated animals, the injured cecum had completely regenerated and the histologic structure was normal in the PCEC hydrogel group after two weeks (Figure 6A and B).
Figure 6.
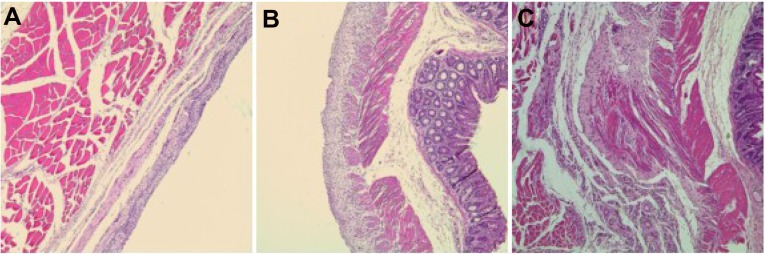
Prevention of abdominal adhesions in a rat abrasion model. (A and B) No adhesions were formed between the abdominal wall and cecum after two weeks of treatment with poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCEC). (C) Adhesions between the defective cecum and surrounding tissue after two weeks in the untreated group.
On gross examination, none of the 10 rats in the PCEC hydrogel-treated group had developed score 5 adhesions. The flowing water gel was transformed into a solid film layer at body temperature on the first day after treatment with PCEC hydrogel. This solid film adhered to the surface of the injured abdominal wall and wrapped around the injured cecum (Figure 7). The traumatic area on the cecum could still be observed after partially stripping the hydrogel membrane. The hydrogel gradually disappeared from the injured surfaces of the abdominal wall and the cecum within seven days and five days, respectively, transforming into a viscous liquid. Upon degradation and absorption of the hydrogel, the injured parietal and visceral peritoneum started to heal.
Figure 7.
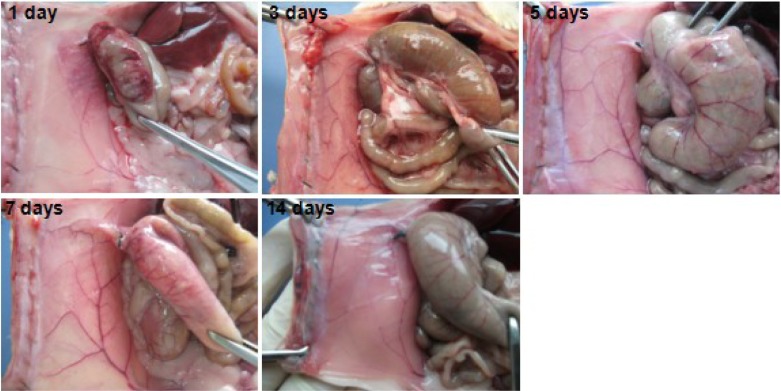
Photographs taken after using dry gauze to absorb viscous liquid in the abdominal cavity in hydrogel-treated rats in the days following treatment. The poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCEC) hydrogel transformed into a solid-like membrane adhering to the injured surfaces of the abdominal wall and wrapping around the injured cecum. The injured surfaces of the cecum could still be observed after partially stripping the hydrogel membrane. With the passage of time, the hydrogel covering on the injured surfaces of the abdominal wall and cecum gradually disappeared and transformed into a viscous liquid, which was completely absorbed by 14 days, with gradual healing of the peritoneal wounds.
Space-time relationship between fibrosis and remesothelialization
We found that the PCEC hydrogel was still covering the surface of the abdominal wall on days 1, 3, and 5. When the rats had been treated with PCEC hydrogel for five days, a layer was found on the surface of the damaged abdominal wall muscles, which was composed of residual water gel and eosinophilic cells, macrophages, and foamy macrophages (Figure 8). However, little collagen deposition was apparenton the surface of the abdominal muscles (Figure 9). Those foamy macrophages may have been derived from phagocytosis of polymerization debris. On day 7 after treatment with the PCEC hydrogel, the hydrogel had disappeared and a layer of mesothelial cells had appeared on the surface of the abdominal wall muscles. Under the mesothelial cell layer was a layer of inflammatory cells (Figure 8). At this time, we also found a large amount of collagen in the inflammatory cell layer (Figure 9). Since the mesothelial cell layer emerged, they can prevent adhesions. In the following week, the collagen was replaced by inflammatory cell layer basally, and a thick layer of mesothelial cells was formed in the upper. The pathologic changes in the damaged cecal surface were similar to those in the parietal peritoneum (data not shown).
Figure 8.
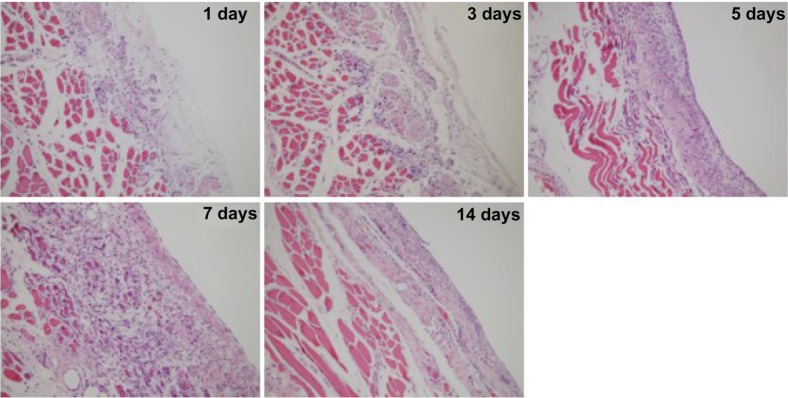
Histologic observations on hematoxylin and eosin staining.
Figure 9.
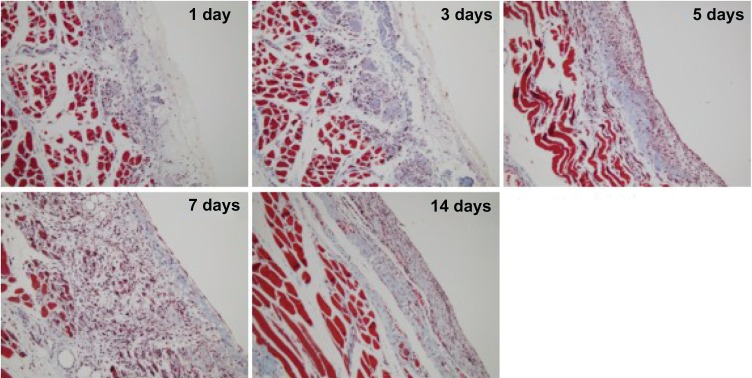
Histologic observations on Masson trichrome staining.
Electron microscopic examination
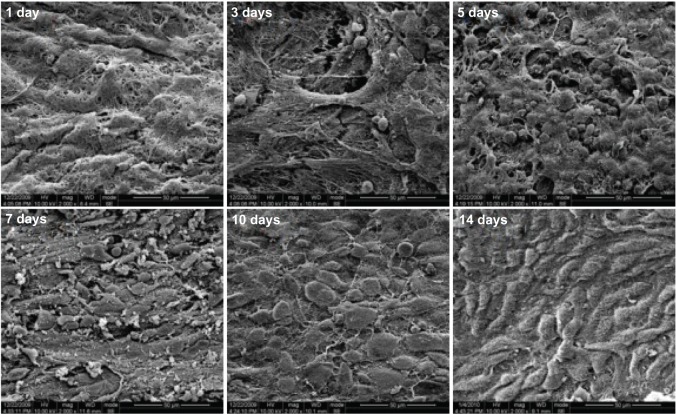
The peritoneal surface was observed by scanning electron microscopy in the PCEC hydrogel-treated group on days 1,3, and 5 (Figure 10). During this time, the peritoneal surface was covered with a layer of hydrogel. Spherical inflammatory cells appeared on the surface of the peritoneum on day 3, but no mesothelial cells were found. The number of spherical inflammatory cells increased during the following 2 days and reached a maximum on day 5. On day 7, the spherical cells become elongated and flattened, and increased in number over the following 3 days, reaching a maximum by day 10. We also found that the amount of hydrogel covering the surface of the peritoneum reduced with time. By day 7, the hydrogel was completely degraded, and had disappeared by day 10. On scanning electron microscopy, repair of the damaged visceral peritoneum treated with PCEC hydrogel was similar to that of the parietal peritoneum at day 14 (data not shown).
Figure 10.
Scanning electron micrographs of the surface of the stripped peritoneum treated with hydrogel on the days indicated. Spherical inflammatory cells appeared on the surface of the peritoneum on day 3 that increased in number during the following two days and reached a maximum on day 5. On day 7, the spherical cells became elongated and flattened, and increased in number during the following three days, reaching a maximum on day 7. At the same time, the hydrogel covering on the surface of the peritoneum reduced with time. On day 7, the hydrogel was degraded and had completely disappeared by day 10.
Toxicity
No pathologic changes in heart, liver, spleen, lung and kidney tissues were seen on histologic examination in the group treated with PCEC hydrogel when compared with the control group. No anomalies in the organs of the abdominal cavity were seen in the PCEC group.
Discussion
Rheologic analysis showed that the physical characteristics of PCEC copolymers could contribute to prevention of postoperative adhesions. Potential advantages of PCEC hydrogels when used to prevent postoperative adhesions in comparison with existing methods are outlined as follows. First, the PCEC copolymers are easy and cost-effective to produce, and the PCEC hydrogel is easy to handle. The PCEC copolymer is applied as a mildly viscous solution, which readily forms a hydrogel when the temperature increases. Because the solution forms at room temperature, PCEC can cover a wide range of affected sites upon application. Further, PCEC can form a durable physical membrane over affected sites with changes in temperature, making PCEC suitable for the treatment of celiac disease. The thermosensitivity of the polymer also makes its transformation from solution to hydrogel a reversible process. Gelation occurs at a low concentration and in a reasonably short time period, and is initiated by an increase in temperature. The gelling process did not require other cross-linking agents, such as initiators or light sources.
PCEC hydrogel can exist for several weeks as a durable physical membrane, given that it forms and degrades gradually, and eventually disappears at the end of the healing process. The residence time can be adjusted by varying the concentration of PCEC solution (data not shown). Moreover, the PCEC polymer is biodegradable and has low cytotoxicity, indicating that PCEC might be biocompatible both in vitro and in vivo.
It is widely acknowledged that abdominal adhesions are inevitable complications associated with surgical trauma to the peritoneum. Trauma is often associated with general physiologic reactions, particularly inflammation, which results in increased vascular permeability and secretion of plasma proteins, especially fibrous proteins.33,34 Activation of the coagulation process leads to synthesis of thrombin, which reforms fibrinogen into a fibrin matrix.34 Inaddition, the viscous fibrin matrix on the peritoneal surface induces adhesions between adjacent tissues and damaged serosal surfaces.35 The fibrin matrix that forms in the process of normal wound healing is usually degraded by the local fiber protease system within 72 hours.36 However, the process of fibrinolysis also provides the opportunity for mesothelial cells to proliferate, with the aim of reversing the peritoneal defect within 4–5 days to prevent permanent attachment of adjacent surfaces.36–38 Fibroblasts invade fibrinous adhesions in the event of reduced fibrinolysis in the plasminogen-plasmincascade. Subsequently, collagen is deposited, which leads to formation of permanent fibrous adhesions.39,40
Although a lot of anti-adhesion studies have been reported in the past few decades, none has identified pharmaceutical agents that are effective in dealing with abdominal adhesions.13,41 Use of barrier agents in the first few days following surgery has been reported to be effective.41–43 Barrier agents designed to prevent adhesions can be grouped into three types, ie, sticky polymer solutions, solid sheet materials, and cross-linkable in situ hydrogels. Use of solid sheet materials to inhibit adhesions is a complex process. Also, there are many difficulties in preparing such materials, ie, fixing tissue with stitches and achieving hemostasis.13,41 Further, leaving solid materials in the body for a long time (over one month) is risky.21 In contrast, there has been a report of a polymer solution remaining in the body for too short a time.44 Also, some of the cross-linkable hydrogels may need more gelation time or an initiator, such as ultraviolet light. Such maneuvers can be difficult to perform during surgery, so use of the currently available barrier systems is limited.
Compared with current barrier systems, hydrogels41–47 have several unique physicochemical features. They can gelate in response to increasing temperature, transforming from a flowing solution at room temperature to a gel at body temperature, and then rapidly form a protective covering the surface of peritoneal wounds to avoid formation of adhesions in the abdominal cavity. Further, the barrier formed can help in rebuilding the mesothelial cell layer and prevent invasion by fibroblasts. Moreover, PCEC is biodegradable and had almost no toxicity as assessed in vitro and in vivo. Overall, the physicochemical characteristics and distinctive degradation kinetics of PCEC make it a promising material for preventing postoperative adhesions in clinical practice.
Conclusion
We have successfully developed a novel thermosensitive PCEC hydrogel. The results of the study reported here indicate that this hydrogel can prevent formation of abdominal adhesions effectively and that it is degradable in vivo. Our findings suggest that PCEC hydrogel might have potential in the prevention of postoperative adhesions in the clinical setting.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (NSFC81201724), Specialized Research Fund for the Doctoral Program of Higher Education (20120181120044), Sichuan SupportProject of Science and Technology (2013SZ0018), National Natural Science Foundation of China (81101603) and the National Key Basic Research Program (973 Program) of China (2010CB529900).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Trew G. Postoperative adhesions and their prevention. Rev Gynaecol Pract. 2006;6:47–56. [Google Scholar]
- 2.Menzies D, Ellis H. Intestinal obstruction from adhesions – how big is the problem? Ann R Coll Surg Engl. 1990;72:60–63. [PMC free article] [PubMed] [Google Scholar]
- 3.Szabó G, Mikó I, Nagy P, et al. Adhesion formation with open versus laparoscopic cholecystectomy: an immunologic and histologic study. Surg Endosc. 2007;21:253–257. doi: 10.1007/s00464-005-0015-y. [DOI] [PubMed] [Google Scholar]
- 4.Menzies D. Postoperative adhesions: their treatment and relevance in clinical practice. Ann R Coll Surg Engl. 1993;75:147–153. [PMC free article] [PubMed] [Google Scholar]
- 5.Schnüriger B, Barmparas G, Branco BC, Lustenberger T, Inaba K, Demetriades D. Prevention of postoperative peritoneal adhesions: a review of the literature. Am J Surg. 2011;201:111–121. doi: 10.1016/j.amjsurg.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann-Willenbrock EL, Mecke H, Riedel HH. Sequelae of appendectomy, with special reference to intra-abdominal adhesions, chronic abdominal pain, and infertility. Gynecol Obstet Invest. 1990;29:241–245. doi: 10.1159/000293326. [DOI] [PubMed] [Google Scholar]
- 7.Hauters P, Cardin JL, Lepere M, Valverde A, Cossa J P, Auvray S. Prevention of parastomal hernia by intraperitoneal onlay mesh reinforcement at the time of stoma formation. Hernia. 2012;16:655–660. doi: 10.1007/s10029-012-0947-9. [DOI] [PubMed] [Google Scholar]
- 8.Tang CL, Seow-Choen F, Fook-Chong S, Eu KW. Bioresorbable adhesion barrier facilitates early closure of the defunctioning ileostomy after rectal excision. Dis Colon Rectum. 2003;46:1200–1207. doi: 10.1007/s10350-004-6716-9. [DOI] [PubMed] [Google Scholar]
- 9.Esposito AJ, Heydrick SJ, Cassidy MR, Gallant J, Stucchi AF, Becker JM. Substance P is an early mediator of peritoneal fibrinolytic pathway genes and promotes intra-abdominal adhesion formation. J Surg Res. 2013;181:25–31. doi: 10.1016/j.jss.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 10.Operative Laparoscopy Study Group Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700–704. [PubMed] [Google Scholar]
- 11.Lim JK, Kim J Y, Kim GC, et al. Detection of the transition zone and adhesions in the diagnosis of adhesive small-bowel obstruction: the added value of ultrasonography (US) in comparison with only CT imaging. J Korean Soc Ultrasound Med. 2009;28:43–50. [Google Scholar]
- 12.Catena F, Ansaloni L, Di Saverio S, Pinna AD, World Society of Emergency Surgery P.O.P.A. study: prevention of postoperative abdominal adhesions by icodextrin 4% solution after laparotomy for adhesive small bowel obstruction. A prospective randomized controlled trial. 2012;16:382–388. doi: 10.1007/s11605-011-1736-y. [DOI] [PubMed] [Google Scholar]
- 13.Liakakos T, Thomakos N, Fine PM, et al. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260–273. doi: 10.1159/000050149. [DOI] [PubMed] [Google Scholar]
- 14.Mueller MD, Tschudi J, Herrmann U, Klaiber C, et al. An evaluation of laparoscopic adhesiolysis in patients with chronic abdominal pain. Surg Endosc. 1995;9:802–804. doi: 10.1007/BF00190085. [DOI] [PubMed] [Google Scholar]
- 15.Ten Broek RP, Kok-Krant N, Bakkum EA, Bleichrodt RP, van Goor H. Different surgical techniques to reduce post-operative adhesion formation: a systematic review and meta-analysis. Hum Reprod Update. 2013;19:12–25. doi: 10.1093/humupd/dms032. [DOI] [PubMed] [Google Scholar]
- 16.Kamel RM. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150:111–118. doi: 10.1016/j.ejogrb.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Racz GB, Day M, Heavner JE, Smith JP. The Racz procedure: lysis of epidural adhesions (percutaneous neuroplasty). Comprehensive treatment of chronic pain by medical, interventional, and integrative approaches. American Academy of Pain Medicine. 2013:521–534. [Google Scholar]
- 18.Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. J Surg Res. 2011;165:91–111. doi: 10.1016/j.jss.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Sannino A, Conversano F, Esposito A, Maffezzoli A. Polymeric meshes for internal sutures with differentiated adhesion on the two sides. J Mater Sci Mater Med. 2005;16:289–296. doi: 10.1007/s10856-005-0626-9. [DOI] [PubMed] [Google Scholar]
- 20.Somigliana E, Vigano P, Benaglia L, Busnelli A, Vercellini P, Fedele L. Adhesion prevention in endometriosis: a neglected critical challenge. J Minim Invasive Gynecol. 2012;19:415–421. doi: 10.1016/j.jmig.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Wallwiener M, Brucker S, Hierlemann H, et al. Innovative barriers for peritoneal adhesion prevention: liquid or solid? A rat uterine horn model. Fertil Steril. 2006;86:1266–1276. doi: 10.1016/j.fertnstert.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Gong C, Shi S, Dong P, et al. Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int J Pharm. 2009;365:89–99. doi: 10.1016/j.ijpharm.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Gong C, Deng S, Wu Q, et al. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials. 2013;34:1413–1432. doi: 10.1016/j.biomaterials.2012.10.068. [DOI] [PubMed] [Google Scholar]
- 24.Yang B, Gong CY, Zhao X, et al. Preventing postoperative abdominal adhesions in a rat model with PEG-PCL-PEG hydrogel. Int J Nanomedicine. 2012;7:547–557. doi: 10.2147/IJN.S26141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gou ML, Zheng XL, Men K, et al. Poly(ε-caprolactone)/poly(ethylene glycol)/poly(ε-caprolactone) nanoparticles: preparation, characterization, and application in doxorubicin delivery. J Phys Chem B. 2009;113:12928–12933. doi: 10.1021/jp905781g. [DOI] [PubMed] [Google Scholar]
- 26.Luo Z, Peng X, Shi H, et al. Comparison of the protective effects of truncated bFGF and native bFGF against murine lung carcinoma. Int J Mol Med. 2011;28:3–8. doi: 10.3892/ijmm.2011.676. [DOI] [PubMed] [Google Scholar]
- 27.Gou M, Gong C, Zhang J, et al. Polymeric matrix for drug delivery: honokiol-loaded PCL-PEG-PCL nanoparticles in PEG-PCL-PEG thermo-sensitive hydrogel. J Biomed Mater Res Part A. 2010;93A:219–226. doi: 10.1002/jbm.a.32546. [DOI] [PubMed] [Google Scholar]
- 28.Wei X, Gong C, Gou M, et al. Biodegradable poly(ε-caprolactone)-poly(ethylene glycol) copolymers as drug delivery system. Int J Pharm. 2009;381:1–18. doi: 10.1016/j.ijpharm.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 29.Gao X, Kan B, Gou M, et al. Preparation of anti-CD40 antibody modified magnetic PCL-PEG-PCL microspheres. J Biomed Nanotechnol. 2011;7:285–291. doi: 10.1166/jbn.2011.1280. [DOI] [PubMed] [Google Scholar]
- 30.Wei X, Gong C, Shi S, et al. Self-assembled honokiol-loaded micelles based on poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) copolymer. Int J Pharm. 2009;369:170–175. doi: 10.1016/j.ijpharm.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Gong C, Yang B, Qian Z, et al. Improving intraperitoneal chemotherapeutic effect and preventing postsurgical adhesions simultaneously with biodegradable micelles. Nanomedicine. 2012;8:963–973. doi: 10.1016/j.nano.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Kosaka H, Yoshimoto T, Yoshimoto T, Fujimoto J, Nakanishi K. Interferon-γ is a therapeutic target molecule for prevention of postoperative adhesion formation. Nat Med. 2008;14:437–441. doi: 10.1038/nm1733. [DOI] [PubMed] [Google Scholar]
- 33.Dijkstra FR, Nieuwenhuijzen M, Reijnen MM, van Goor H. Recent clinical developments in pathophysiology, epidemiology, diagnosis and treatment of intra-abdominal adhesions. Scand J Gastroenterol Suppl. 2000;232:52–59. [PubMed] [Google Scholar]
- 34.Reijnen MM, Bleichrodt RP, van Goor H. Pathophysiology of intraabdominal adhesion and abscess formation, and the effect of hyaluronan. Br J Surg. 2003;90:533–541. doi: 10.1002/bjs.4141. [DOI] [PubMed] [Google Scholar]
- 35.Holmdahl L. Making and covering of surgical footprints. Lancet. 1999;353:1456–1457. doi: 10.1016/S0140-6736(99)90061-2. [DOI] [PubMed] [Google Scholar]
- 36.Sulaiman H, Dawson L, Laurent GJ, Bellingan GJ, Herrick SE. Role of plasminogen activators in peritoneal adhesion formation. Biochem Soc Trans. 2002;30:126–131. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 37.Holmdahl L, Ivarsson ML. The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. Eur J Surg. 1999;165:1012–1019. doi: 10.1080/110241599750007810. [DOI] [PubMed] [Google Scholar]
- 38.Rout UK, Diamond MP. Role of plasminogen activators during healing after uterine serosal lesioning in the rat. Fertil Steril. 2003;79:138–145. doi: 10.1016/s0015-0282(02)04569-7. [DOI] [PubMed] [Google Scholar]
- 39.De Iaco PA, Stefanetti M, Pressato D, et al. A novel hyaluronan-based gel in laparoscopic adhesion prevention: preclinical evaluation in an animal model. Fertil Steril. 1998;69:318–323. doi: 10.1016/s0015-0282(98)00496-8. [DOI] [PubMed] [Google Scholar]
- 40.Ustun C, Yanik FF, Kocak I, Canbaz MA, Cayli R. Effects of Ringer’s lactate, medroxyprogesterone acetate, gonadotropin-releasing hormone analogue and its diluent on the prevention of postsurgical adhesion formation in rat models. Gynecol Obstet Invest. 1998;46:202–205. doi: 10.1159/000010034. [DOI] [PubMed] [Google Scholar]
- 41.Attard JA, MacLean AR. Adhesive small bowel obstruction: epidemiology, biology and prevention. Can J Surg. 2007;50:291–300. [PMC free article] [PubMed] [Google Scholar]
- 42.Becker JM, Dayton MT, Fazio VW, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183:297–306. [PubMed] [Google Scholar]
- 43.Marana R, Catalano GF, Caruana P, Margutti F, Muzii L, Mancuso S. Postoperative adhesion formation and reproductive outcome using interceed after ovarian surgery: a randomized trial in the rabbit model. Hum Reprod. 1997;12:1935–1938. doi: 10.1093/humrep/12.9.1935. [DOI] [PubMed] [Google Scholar]
- 44.Diamond MP. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. The Sepracoat Adhesion Study Group. Fertil Steril. 1998;69:1067–1074. doi: 10.1016/s0015-0282(98)00057-0. [DOI] [PubMed] [Google Scholar]
- 45.Peyton CC, Keys T, Tomblyn S, et al. Halofuginone infused keratin hydrogel attenuates adhesions in a rodent cecal abrasion model. J Surg Res. 2012;178:545–552. doi: 10.1016/j.jss.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Ni J, Chen L, Yu L, Xu J, Ding J. Encapsulation of cell-adhesive RGD peptides into a polymeric physical hydrogel to prevent postoperative tissue adhesion. J Biomed Mater Res B. 2012;100:1599–1609. doi: 10.1002/jbm.b.32728. [DOI] [PubMed] [Google Scholar]
- 47.Yang B, Yang Gong C, Yong Qian Z, et al. Prevention of abdominal adhesion formation by thermosensitive PECE-hydrogel in a rat uterine horn model. J Biomed Mater Res B. 2011;96:57–66. doi: 10.1002/jbm.b.31739. [DOI] [PubMed] [Google Scholar]