Abstract
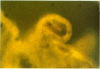
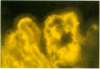
The relationship of riboflavin transport to the transport of other substances including drugs in rabbit choroid plexus, the anatomical locus of the blood-cerebrospinal fluid barrier, and brain cells were studied in vivo and in vitro. In vitro, the ability of rabbit choroid plexus to transport riboflavin from the medium (cerebrospinal fluid surface) through the choroid plexus epithelial cells into the extracellular and vascular spaces of the choroid plexus was documented using fluorescence microscopy. These studies provided further evidence that riboflavin is transported from cerebrospinal fluid to blood via the choroid plexus. The transport of [14C]riboflavin by the isolated choroid plexus was inhibited by thiol agents, ouabain, theophylline, various flavins (lumiflavin and lumichrome > sugar containing flavins), and cyclic organic acids including penicillin and fluorescein. Riboflavin inhibited [14C]penicillin transport competitively and the inhibition constant (K1) for riboflavin equaled the concentration of riboflavin at which the saturable transport system for riboflavin is 50% saturated (KT). These and other data suggest that riboflavin, penicillin, and possibly fluorescein are transported by the same transport system in choroid plexus. In vivo, the intra-ventricular injection or riboflavin and [14C]penicillin inhibited [14C]penicillin transport from cerebrospinal fluid. In vitro, various flavins (riboflavin > other sugar-containing flavins > lumiflavin > lumichrome) inhibited [14C]riboflavin accumulation by brain slices. These studies support the notions that: (a) riboflavin accumulation by choroid plexus (active transport) is quite different from that in brain cells (facilitated diffusion and intracellular trapping), and (b) therapeutically important cyclic organic acids (e.g., penicillin) are transported fom cerebrospinal fluid by the riboflavin transport system in choroid plexus.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bresler S. E., Bresler V. M., Kazbekov E. N., Nikiforov A. A., Vasilieva N. N. On the active transport of organic acids (fluorescein) in the choroid plexus of the rabbit. Biochim Biophys Acta. 1979 Jan 5;550(1):110–119. doi: 10.1016/0005-2736(79)90119-6. [DOI] [PubMed] [Google Scholar]
- Hieber J. P., Nelson J. D. A pharmacologic evaluation of penicillin in children with purulent meningitis. N Engl J Med. 1977 Aug 25;297(8):410–413. doi: 10.1056/NEJM197708252970802. [DOI] [PubMed] [Google Scholar]
- Jusko W. J., Rennick B. R., Levy G. Renal exretion of riboflavin in the dog. Am J Physiol. 1970 Apr;218(4):1046–1053. doi: 10.1152/ajplegacy.1970.218.4.1046. [DOI] [PubMed] [Google Scholar]
- LOVTRUP S. A COMPARATIVE STUDY OF THE INFLUENCE OF CHLORPROMAZINE AND IMIPRAMINE ON MITOCHONDRIAL ACTIVITY; CYTOCHROME OXIDASE AND NADH2-CYTOCHROME C REDUCTASE. J Neurochem. 1964 May;11:377–386. doi: 10.1111/j.1471-4159.1964.tb11931.x. [DOI] [PubMed] [Google Scholar]
- Lorenzo A. V., Spector R. Transport of salicylic acid by the choroid plexus in vitro. J Pharmacol Exp Ther. 1973 Feb;184(2):465–471. [PubMed] [Google Scholar]
- MCCORMICK D. B. INHIBITION OF FLAVIN ADENINE DINUCLEOTIDE PYROPHOSPHORYLASE BY ISORIBOFLAVIN. Nature. 1964 Feb 29;201:925–926. doi: 10.1038/201925a0. [DOI] [PubMed] [Google Scholar]
- Madinaveitia J. The antagonism of some antimalarial drugs by riboflavin. Biochem J. 1946;40(3):373–375. doi: 10.1042/bj0400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T., Nagatsu-Ishibashi I., Okuda J., Yagi K. Incorporation of peripherally administered riboflavine into flavine nucleotides in the brain. J Neurochem. 1967 Feb;14(2):207–210. doi: 10.1111/j.1471-4159.1967.tb05895.x. [DOI] [PubMed] [Google Scholar]
- Nikiforov A. A., Bresler V. M. Double dependence of organic acid active transport in proximal tubules of surviving frog kidney on sodium ions. II. Relationship between counter-flows of fluorescein and sodium ion across cell layer. Biochim Biophys Acta. 1977 Jul 4;468(1):100–113. doi: 10.1016/0005-2736(77)90154-7. [DOI] [PubMed] [Google Scholar]
- Nogami H., Hanano M., Awazu S., Iga T. Pharmacokinetic aspects of biliary excretion. Dose dependency of riboflavin in rat. Chem Pharm Bull (Tokyo) 1970 Feb;18(2):228–234. doi: 10.1248/cpb.18.228. [DOI] [PubMed] [Google Scholar]
- PROSKY L., BURCH H. B., BEJRABLAYA D., LOWRY O. H., COMBS A. M. THE EFFECTS OF GALACTOFLAVIN ON RIBOFLAVIN ENZYMES AND COENZYMES. J Biol Chem. 1964 Aug;239:2691–2695. [PubMed] [Google Scholar]
- Rivlin R. S. Hormones, drugs and riboflavin. Nutr Rev. 1979 Aug;37(8):241–245. doi: 10.1111/j.1753-4887.1979.tb06678.x. [DOI] [PubMed] [Google Scholar]
- Robinson R. J., Cutler R. W., Lorenzo A. V., Barlow C. F. Transport of sulphate, thiosulphate and iodide by choroid plexus in vitro. J Neurochem. 1968 Oct;15(10):1169–1179. doi: 10.1111/j.1471-4159.1968.tb06834.x. [DOI] [PubMed] [Google Scholar]
- Spector R., Boose B. Active transport of riboflavin by the isolated choroid plexus in vitro. J Biol Chem. 1979 Oct 25;254(20):10286–10289. [PubMed] [Google Scholar]
- Spector R., Greene L. A. Ascorbic acid transport by a clonal line of pheochromocytoma cells. Brain Res. 1977 Nov 4;136(1):131–140. doi: 10.1016/0006-8993(77)90137-8. [DOI] [PubMed] [Google Scholar]
- Spector R., Kelley P. Niacin and niacinamide accumulation by rabbit brain slices and choroid plexus in vitro. J Neurochem. 1979 Jul;33(1):291–298. doi: 10.1111/j.1471-4159.1979.tb11731.x. [DOI] [PubMed] [Google Scholar]
- Spector R., Lorenzo A. V. Folate transport by the choroid plexus in vitro. Science. 1975 Feb 14;187(4176):540–542. doi: 10.1126/science.1167256. [DOI] [PubMed] [Google Scholar]
- Spector R., Lorenzo A. V. Inhibition of penicillin transport from the cerebrospinal fluid after intracisternal inoculation of bacteria. J Clin Invest. 1974 Aug;54(2):316–325. doi: 10.1172/JCI107767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector R. Riboflavin accumulation by rabbit brain slices in vitro. J Neurochem. 1980 Jun;34(6):1768–1771. doi: 10.1111/j.1471-4159.1980.tb11274.x. [DOI] [PubMed] [Google Scholar]
- Spector R. Riboflavin homeostasis in the central nervous system. J Neurochem. 1980 Jul;35(1):202–209. doi: 10.1111/j.1471-4159.1980.tb12507.x. [DOI] [PubMed] [Google Scholar]
- Spector R. Thiamine transport in the central nervous system. Am J Physiol. 1976 Apr;230(4):1101–1107. doi: 10.1152/ajplegacy.1976.230.4.1101. [DOI] [PubMed] [Google Scholar]
- Spector R. Vitamin B6 transport in the central nervous system: in vitro studies. J Neurochem. 1978 Apr;30(4):889–897. doi: 10.1111/j.1471-4159.1978.tb10798.x. [DOI] [PubMed] [Google Scholar]
- YAGI K., OZAWA T., NAGATSU T. Mechanism of inhibition of D-amino acid oxidase. IV. Inhibitory action of chlorpromazine. Biochim Biophys Acta. 1960 Sep 23;43:310–317. doi: 10.1016/0006-3002(60)90441-8. [DOI] [PubMed] [Google Scholar]