It is crucial for physicians to identify sleep apnea when it co-exists with common neurologic conditions and to be prepared to offer effective interventions.
Obstructive sleep apnea (OSA) is an extremely common public health problem manifested by sleep-disordered breathing, daytime hypersomnia and poor sleep quality, adverse neurocognitive sequelae, and hypoxia. OSA occurs in about two to four percent of the general population, or an estimated 18 million Americans.1 Co-morbid OSA is even more frequent in neurological patients, affecting at least one-third of those with epilepsy and about two-thirds of stroke survivors. Just as effective treatment of OSA may improve hypertensive control and reduce risk of cardiovascular complications, there is now growing evidence that treating co-morbid OSA also improves neurological outcomes such as cognitive functioning and seizure control. Since neurologists frequently serve as principal care providers for those with epilepsy, stroke, multiple sclerosis, and migraine, (see Part I, available online at PracticalNeurology.net also available as: PMID:22298957, http://www.ncbi.nlm.nih.gov/pubmed/22298957) it is crucial for neurologic physicians to be familiar with the identification of sleep apnea early in its presentation to provide optimal care for their patients.
Diagnosis and Treatment
Prompt diagnosis of OSA enables expeditious treatment that improves daytime alertness, neurocognitive functioning, and quality of life. A history should be sought from all patients for typical clinical symptoms of hypersomnia, loud disruptive snoring, snort or gasp arousals, witnessed pauses in breathing, and habitual morning xerostomia or headache. Screening with the simple, brief, and efficient Epworth Sleepiness Scale, a well validated tool that provides a subjective rating of drowsiness and tendency to doze in sedentary situations, can be very helpful in identifying subtle hypersomnia in many patients (a convenient, online version is readily available at: http://www.stanford.edu/~dement/epworth.html).40 Suspicious physical examination attributes accompanying OSA may include unexplained hypertension, obesity, prominent oropharyngeal airway narrowing, a large tongue and dependent uvula, thickened neck circumference (greater than 17 inches/43 centimeters in men and 16 inches/41 centimeters in women), and hypognathia.
Portable overnight oximetry may be a particularly helpful adjunct to screen patients who are especially likely to benefit from polysomnography, showing frequent oscillatory desaturations consistent with sleep disordered breathing. It is particularly helpful in planning for appropriate in lab titration by revealing otherwise unexpected hypoventilation or likely central apnea mechanisms in some patients (see Figures 1–3).
Figure 1.

Portable overnight oximetry in a 77-year-old man with obstructive sleep apnea. Numerous oscillatory desaturations are seen, especially between 2–3 AM and 4–5 AM, consistent with obstructive sleep disordered breathing. The oxygen desaturation index (hourly rate of 4% or greater oxyhemoglobin desaturations) was 15/hour, and subsequent polysomnography confirmed moderately severe obstructive sleep apnea, with apnea-hypopnea index of 24/hour, with predominant obstructive apneic events occurring during supine position sleep. Nasal continuous positive airway pressure therapy at 15 centimeters of water optimal pressure effectively treated his apnea, and one month later his sleepiness had resolved after adherence with treatment.
Figure 3.
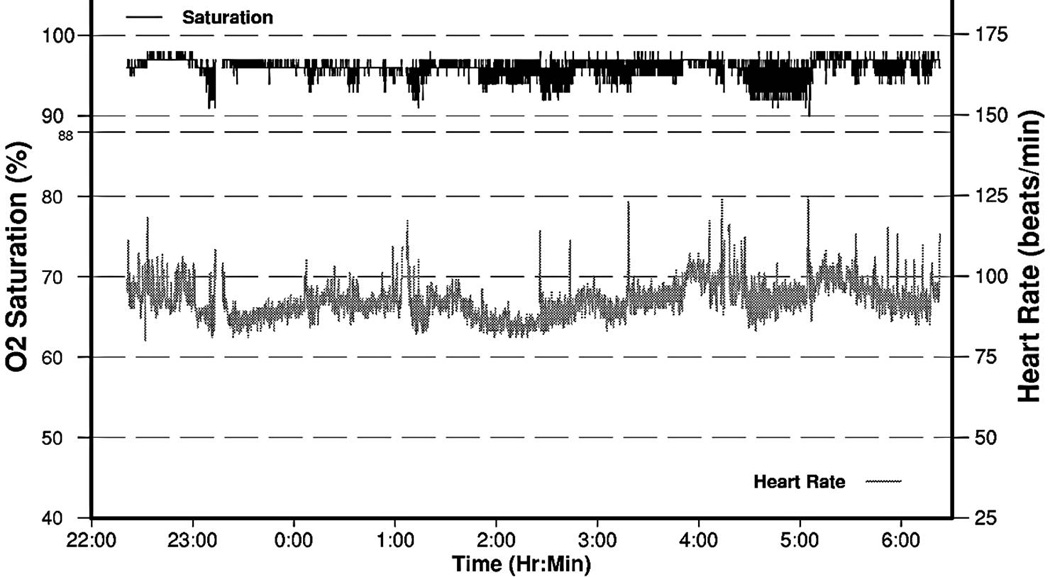
Portable overnight oximetry in a 25-year-old woman with central sleep apnea. Numerous oscillatory desaturations are seen, especially 01:00 AM to 05:00 AM, consistent with sleep disordered breathing. The oxygen desaturation index was 14/hour. Note that many of the oxyhemoglobin desaturations have an extremely confluent or “box car” type morphology, especially those near 05:00 AM, a finding highly suspicious although not in itself diagnostic of central sleep apnea. Subsequent polysomnography confirmed moderately severe exclusively central sleep apnea, with apnea-hypopnea index of 155/hour, with frequent central and rare obstructive apneic events occurring especially during NREM sleep. CPAP was ineffective without any impact on apnea event frequency, but bilevel positive airway pressure with an assisted servo-ventilator (ASV) device led to complete resolution of sleep disordered breathing. Three months later sleepiness had resolved, and the ASV device download showed excellent treatment effect without evidence for residual apneic events.
However, polysomnography remains the “gold standard” for formal assessment of suspected sleep disordered breathing and hypersomnia. Polysomnography combines evaluation of sleep, breathing, and movement by offering analysis of polygraphic physiologic variables including electroencephalography (EEG), chin electromyography (EMG), limb EMG leads to analyze periodic leg movements, oronasal airflow via thermistor and nasal pressure sensors, electrocardiogram (ECG), and respiratory effort as measured by inductance plethysmography or piezo crystal sensors (see Figures 4–5). Body position is also analyzed to delineate effects of sleeping position on breathing. Arousals from sleep and their mechanisms, whether due to breathing, movement, or spontaneous causes, are then determined. The advantages of in-lab, attended polysomnography include the ability to directly observe sleep and relevant cardiorespiratory and neurological behaviors and the opportunity to offer intervention with therapeutic trials of positive airway pressure treatment when indicated, while disadvantages include a less natural sleep environment and expense.
Figure 4.
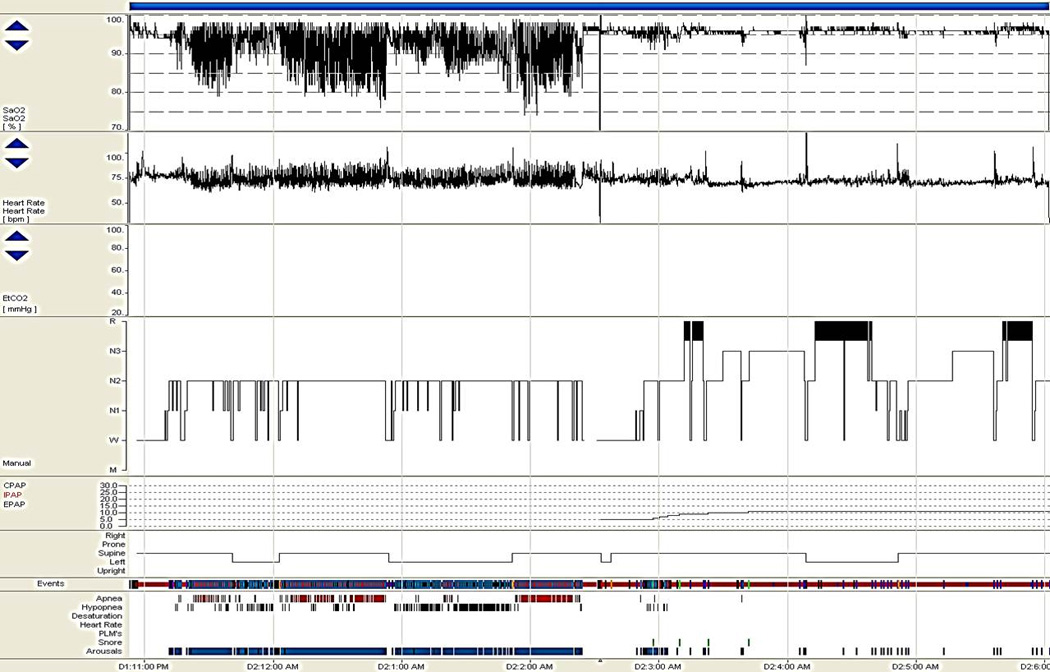
Split night polysomnogram. Hyponogram and graphic data displayed represents a split-night polysomnogram study in a 55-year-old man. Severe obstructive sleep apnea was diagnosed during the first half of a split-night polysomnogram, with an apnea-hypopnea index of 65/hour. Note the repetitive oscillatory oxyhemoglobin desaturations shown in the top row on the trended pulse oximetry display, the related repetitive tachy-brady cardiac dysrhythmia shown on the ECG rhythm strip typical of OSA, the disturbed sleep architecture with only light NREM sleep obtained in the first half of the night and considerable N3 (slow wave) and REM sleep rebound occurring during effective nasal CPAP treatment. The graphs below represent the CPAP pressure levels during titration, body position, and respiratory events and related arousals on the bottom.
Figure 5.
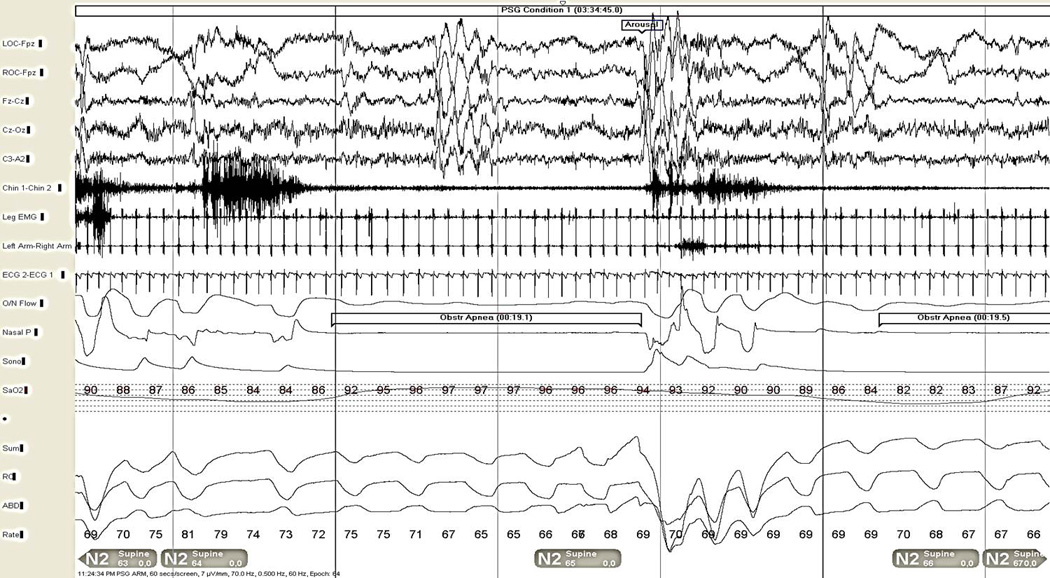
Severe obstructive sleep apnea in a 55-year-old man (shown also in Figure 4), 30 second epoch. The patient is in light NREM (N2) stage sleep. Note the arousal shown in the electroencephalogram channels (top 5 channels), snoring artifact evident in the chin EMG (channel 6), obstructive apnea with cessation of airflow in the oronasal thermistor and nasal pressure sensor channels (channels 10 and 11, respectively), despite continued respiratory effort in the summed, chest, and abdominal respiratory effort channels (channels 14–16). Bradycardia during the apnea is shown on the ECG rhythm strip, with relative tachycardia upon arousal (channel 9, also visible on limb electromyogram leads in channels 7–8). The oxyhemoglobin desaturation seen in the pulse oximetry display (channel 13) in the first half of the epoch is due to another apnea event on the preceding page, while the related desaturation to the apneic event in the first half of this epoch is seen toward the end of the epoch as the next episode of apnea is occurring.
The rationale for treating OSA is to improve symptoms and neurobehavioral sequelae and to decrease cardiovascular and cerebrovascular risks. OSA treatment improves symptoms and quality of life. While the influence of OSA treatment on cardiovascular risks are less clear, expert consensus favors treatment of moderate and severe OSA.
While snoring may be a socially objectionable symptom or disruptive to the patient’s sleep partner, primary snoring (i.e., snoring in isolation, without OSA) is not abnormal per se, and patients may simply be reassured if bed partners are not disturbed by their snoring. If snoring is disruptive or socially embarrassing and treatment is desired, options include relieving nasal obstruction, commercially available lubricant throat sprays, maxillary-mandibular advancement devices, and nasal or upper airway surgical approaches such as nasal septal repair or uvulopalatopharyngoplasty (UPPP, or UP3 procedure). An otorhinolaryngology consultation may be helpful in determining which surgical approaches may be of most benefit for the individual.
Treatment
Therapeutic options for snoring and sleep apnea include reducing nasal congestion or obstruction, positional therapy, nasal continuous positive airway pressure (CPAP), oral appliances, or surgical management (see Table). Among lifestyle or behavioral changes, positional therapy involves employing one or more simple strategies to enforce sleep only in non-supine body positions, usually on one or both sides. One method is the “tennis ball t-shirt” approach, where a longitudinally extensive pocket is sewn onto the back of a snug fitting t-shirt between the shoulder blades, and two to three tennis balls are then inserted to provide a mechanical stimulus to the patient to discourage supine sleep. Other options include body pillows wedged behind the patient. Unfortunately, long-term adherence remains poor, with only about one-third of patients able to maintain positional therapy strategies in long-term follow-up. Patients with position-dependent OSA should be counseled to be cautious for development of severe OSA problems during any future anticipated prolonged periods of supine position sleep, such as postoperative recovery periods following surgery, or following a major injury.
Table.
Treatment Options for Obstructive Sleep Apnea
| Treatment Type | Treatment Modality |
|---|---|
| Behavioral Modification |
|
| Positive Airway Pressure |
|
| Oral Appliances |
|
| Surgery |
|
Other conservative non-PAP treatments for OSA include recommending weight loss when appropriate, which may reduce soft tissue in the neck, rendering the oropharynx less compressible, although many OSA remissions by weight loss are only temporary with the influences of further weight gain or aging. OSA patients should also be cautioned about the potential influence of alcohol and certain prescription medications such as benzodiazepines and barbiturates that can reduce upper airway tone or cause sedation and reduce respiratory drive, thereby worsening OSA.
The mainstay of treatment for OSA is positive airway therapy (PAP). PAP machines include a blower unit delivering calibrated pressures to maintain airway patency, tubing that connects the blower to an interface to the patient, and the nasal or oronasal mask (or nasal pillows) and associated headgear. Continuous PAP (CPAP) delivers a continuous set pressure between 5–20cm H2O, is usually offered during the second half night of split-night polysomnography following initial diagnostic evaluation. If an optimal pressure is not determined by laboratory titration, an auto-titrating PAP device may be offered, offering flexibility for delivering a wide range of self-adjusting treatment pressures as the patient’s apnea severity varies during changes in sleep stage and body position.
Most new PAP machines offer an expiratory pressure relief feature, which may aid patient tolerability. Considerable patience on the part of the patient and treating sleep physician is often necessary to optimize PAP therapy for an individual, since interface comfort and fit are significant reasons for poor tolerability of treatment, and PAP treatment may fail when leak rates are too high, requiring dedicated longitudinal follow-up and refitting of interfaces over time. Early adaption to PAP and attempts by the sleep physician to optimize patient tolerability lead to improved patient adherence and outcomes.
Non-PAP treatment alternatives include positional therapy, oral appliance therapy, and surgery. Oral appliances that advance the mandible are an alternative for mild to moderate sleep apnea and require dedicated fitting by a dental sleep specialist. Surgical therapies for OSA include palatal approaches (uvulopalatopharangoplasty, also known as UPPP or “UP3”) and tongue based procedures (genioglossus advancement, hyoid myotomy and suspension, lingualplasty), or maxillary-mandibular advancement (MMA). UPPP is performed by otorhinolaryngologists, and while effective for snoring relief, it is mostly ineffective for OSA treatment, especially for moderate or severe OSA. MMA is quite effective in mild to moderate OSA and selected severe cases, but perioperative pain is considerable and long-term outcomes remain unclear.
Conclusions
OSA is a vital public health problem that is increasingly recognized to impact overall well being, general health including stroke risk, neurocognitive functioning, and seizure and headache control in neurological patients. As principal care providers, neurologists should remain vigilant toward detection of likely co-morbid OSA in their patients, since prompt identification and treatment of OSA may reduce health risk while improving quality of life and neurological functioning.
Figure 2.

Portable overnight oximetry in a 69-year-old woman with amyotrophic lateral sclerosis and co-morbid obstructive sleep apnea. Numerous oscillatory desaturations are seen, especially near 11:00 PM, between 12:30 AM to 01:00 AM, following 02:00 AM, and between 03:30 AM and 04:00 AM, consistent with obstructive sleep disordered breathing. The oxygen desaturation index was only 7/hour. Note also the steep descent and oxyhemoglobin saturation nadir of 70%, due to likely superimposed REM-sleep related hypoventilation. Subsequent polysomnography confirmed moderately severe obstructive sleep apnea, with apnea-hypopnea index of 17/hour, with predominant obstructive apneic events occurring during supine and REM sleep. There was also prominent persisting oxyhemoglobin desaturation during CPAP trial during REM sleep due to hypoventilation, requiring conversion to bilevel positive airway pressure treatment with a timed back-up rate.
Acknowledgments
This publication was made possible by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the author and do not necessarily represent the official view of NCRR or NIH. IInformation on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Verstraeten E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep. 2007;vol. 7(no. 2):161–166. doi: 10.1007/s11910-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 3.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome. J Intl Neuropsych Soc. 2004;10:772–785. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 4.St. Louis EK. Error detection is impaired in obstructive sleep apnea syndrome. Sleep. 2009;32(Suppl):A420. [Google Scholar]
- 5.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26(3):298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 6.Quan SF, Wright R, Baldwin CM, Kaemingk KL, Goodwin JL, Kuo TF, Kaszniak A, Boland LL, Caccappolo E, Bootzin RR. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med. 2006;7:498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Powell NB, Schechtman KB, Riley RW. The road to danger: the comparative risks of driving while sleepy. Laryngoscope. 2001;111:887–893. doi: 10.1097/00005537-200105000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Boyle LN, Tippin J, Paul A, Rizzo M. Driver performance in the moments surrounding a microsleep. Transp Res Part F Traffic Psychol Behav. 2008;11(2):126–136. doi: 10.1016/j.trf.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellen RLB, Marshall SC, Palayew M, Molnar FJ, Wilson KG, Man-Son-Hing M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;2:193. [PubMed] [Google Scholar]
- 10.Tregear S. Obstructive sleep apnea and commercial motor vehicle driver safety: findings of evidence report. 2007 accessed on world wide web at: http://www.mrb.fmcsa.dot.gov/documents/PPP/OSA_Evidence_Report_Tregear.pdf.
- 11.Ayalon L, Ancoli-Israel S, Aka AA, McKenna BS, Drummond SP. Relationship between obstructive sleep apnea severity and brain activation during a sustained attention task. Sleep. 2009;32(3):373–381. doi: 10.1093/sleep/32.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayalon L, Peterson S. Functional central nervous system imaging in the investigation of obstructive sleep apnea. Curr Opin Pulm Med. 2007;13(6):479–483. doi: 10.1097/MCP.0b013e3282f0e9fb. [DOI] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, Liu L, Ayalon L, He F, Loredo JS. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56(11):2076–2081. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malow BA, Foldvary-Schaefer N, Vaughn BV, Selwa LM, Chervin RD, Weatherwax KJ, Wang L, Song Y. Treating obstructive sleep apnea in adults with epilepsy: a randomized pilot trial. Neurology. 2008;71(8):572–577. doi: 10.1212/01.wnl.0000323927.13250.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nath Zallek S, Chervin RD. Improvement in cluster headache after treatment for obstructive sleep apnea’. Sleep Med. 2000;1(2):135–138. doi: 10.1016/s1389-9457(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 16.Abrams B. Add Alzheimer's to the list of sleep apnea consequences. Med Hypotheses. 2005;65(6):1201–1202. doi: 10.1016/j.mehy.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Sforza E, Roche F, Thomas-Anterion C, Kerleroux J, Beauchet O, Celle S, Maudoux D, Pichot V, Laurent B, Barthélémy JC. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33(4):515–521. doi: 10.1093/sleep/33.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bliwise DL. Sleep apnea, APOE4 and Alzheimer's disease 20 years and counting? J Psychosom Res. 2002;53(1):539–546. doi: 10.1016/s0022-3999(02)00436-1. [DOI] [PubMed] [Google Scholar]
- 19.Gottlieb DJ, DeStefano AL, Foley DJ, Mignot E, Redline S, Givelber RJ, Young T. APOE E4 is associated with obstructive sleep apnea/hypopnea. Neurology. 2004;63:664–668. doi: 10.1212/01.wnl.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- 20.O'Hara R, Schröder CM, Kraemer HC, Kryla N, Cao C, Miller E, Schatzberg AF, Yesavage JA, Murphy GM., Jr Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurology. 2005;65(4):642–644. doi: 10.1212/01.wnl.0000173055.75950.bf. [DOI] [PubMed] [Google Scholar]
- 21.Moraes W, Poyares D, Sukys-Claudino L, Guilleminault C, Tufik S. Donepezil improves obstructive sleep apnea in Alzheimer disease: a double-blind, placebo-controlled study. Chest. 2008;133(3):677–683. doi: 10.1378/chest.07-1446. [DOI] [PubMed] [Google Scholar]
- 22.Rongve A, Boeve BF, Aarsland D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc. 2010;58(3):480–486. doi: 10.1111/j.1532-5415.2010.02733.x. [DOI] [PubMed] [Google Scholar]
- 23.Chong MS, Ayalon L, Marler M, Loredo JS, Corey-Bloom J, Palmer BW, Liu L, Ancoli Israel S. Continuous positive airway pressure reduces subjective daytime sleepiness in patients with mild to moderate Alzheimer's disease with sleep disordered breathing. J Am Geriatr Soc. 2006;54(5):777–781. doi: 10.1111/j.1532-5415.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 24.Cooke JR, Ancoli-Israel S, Liu L, Loredo JS, Natarajan L, Palmer BS, He F, Corey-Bloom J. Continuous positive airway pressure deepens sleep in patients with Alzheimer's disease and obstructive sleep apnea. Sleep Med. 2009;10(10):1101–1106. doi: 10.1016/j.sleep.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, Liu L, Ancoli-Israel S. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer's disease and obstructive sleep apnea: preliminary study. J Clin Sleep Med. 2009;5(4):305–309. [PMC free article] [PubMed] [Google Scholar]
- 26.Frohnhofen H, Heuer HC, Kanzia A, Firat A. Influence of type of treatment for sleep apnea on activities of daily living in a sample of elderly patients with severe sleep apnea. J Physiol Pharmacol. 2009;60(Suppl 5):51–55. [PubMed] [Google Scholar]
- 27.Ayalon L, Ancoli-Israel S, Stepnowsky C, Marler M, Palmer BW, Liu L, Loredo JS, Corey-Bloom J, Greenfield D, Cooke J. Adherence to continuous positive airway pressure treatment in patients with Alzheimer's disease and obstructive sleep apnea. Am J Geriatr Psychiatry. 2006 Feb;14(2):176–180. doi: 10.1097/01.JGP.0000192484.12684.cd. [DOI] [PubMed] [Google Scholar]
- 28.Arnulf I. Excessive daytime sleepiness in parkinsonism. Sleep Med Rev. 2005;9(3):185–200. doi: 10.1016/j.smrv.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Rye DB. Excessive daytime sleepiness and unintended sleep in Parkinson's disease. Curr Neurol Neurosci Rep. 2006 Mar;6(2):169–176. doi: 10.1007/s11910-996-0041-8. [DOI] [PubMed] [Google Scholar]
- 30.Dyken ME, Im KB. Obstructive sleep apnea and stroke. Chest. 2009;136:1668–1677. doi: 10.1378/chest.08-1512. [DOI] [PubMed] [Google Scholar]
- 31.Brown DL, Lisabeth LD, Zupancic MJ, Concannon M, Martin C, Chervin RD. High prevalence of supine sleep in ischemic stroke patients. Stroke. 2008;39:2511–2514. doi: 10.1161/STROKEAHA.107.513572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malow BA, Levy K, Maturen K, Bowes R. Obstructive sleep apnea is common in medically refractory epilepsy patients. Neurology. 2000;55(7):1002–1007. doi: 10.1212/wnl.55.7.1002. [DOI] [PubMed] [Google Scholar]
- 33.Manni R, Terzaghi M, Arbasino C, Sartori I, Galimberti CA, Tartara A. Obstructive sleep apnea in a clinical series of adult epilepsy patients: Frequency and features of comorbidity. Epilepsia. 2003;44(6):836–840. doi: 10.1046/j.1528-1157.2003.55702.x. [DOI] [PubMed] [Google Scholar]
- 34.Malow BA, Foldvary-Schaefer N, Vaughn BV, Selwa LM, Chervin RD, Weatherwax KJ, Wang L, Song Y. Treating obstructive sleep apnea in adults with epilepsy: a randomized pilot trial. Neurology. 2008;71(8):572–577. doi: 10.1212/01.wnl.0000323927.13250.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alberti A. Headache and sleep. Sleep Med Rev. 2006;10:431–437. doi: 10.1016/j.smrv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Rains JC, Poceta JS. Headache and sleep disorders: review and clinical implications for headache management. Headache. 2006;46:1344–1363. doi: 10.1111/j.1526-4610.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- 37.Laberge L, et al. Sleep Complaints in Patients with Myotonic Dystrophy. J. Sleep Res. 2004;13:95–100. doi: 10.1111/j.1365-2869.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 38.Laberge L, et al. A Polysomnographic Study of Daytime Sleepiness in Myotonic Dystrophy Type 1. JNNP. 2009;80:642–646. doi: 10.1136/jnnp.2008.165035. [DOI] [PubMed] [Google Scholar]
- 39.Phillips MF, et al. Daytime Somnolence in Myotonic Dystrophy. J. Neurol. 1999;246:275–282. doi: 10.1007/s004150050346. [DOI] [PubMed] [Google Scholar]
- 40.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]


