Abstract
In this study, in vitro cytotoxicity of nickel zinc (NiZn) ferrite nanoparticles against human colon cancer HT29, breast cancer MCF7, and liver cancer HepG2 cells was examined. The morphology, homogeneity, and elemental composition of NiZn ferrite nanoparticles were investigated by scanning electron microscopy, transmission electron microscopy, and energy dispersive X-ray spectroscopy, respectively. The exposure of cancer cells to NiZn ferrite nanoparticles (15.6–1,000 μg/mL; 72 hours) has resulted in a dose-dependent inhibition of cell growth determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The quantification of caspase-3 and -9 activities and DNA fragmentation to assess the cell death pathway of the treated cells showed that both were stimulated when exposed to NiZn ferrite nanoparticles. Light microscopy examination of the cells exposed to NiZn ferrite nanoparticles demonstrated significant changes in cellular morphology. The HepG2 cells were most prone to apoptosis among the three cells lines examined, as the result of treatment with NiZn nanoparticles. In conclusion, NiZn ferrite nanoparticles are suggested to have potential cytotoxicity against cancer cells.
Keywords: NiZn ferrite nanoparticles, cancer cells lines, anticancer, apoptosis
Introduction
Magnetic nanoparticles are used in many biological and medical applications due to their interesting properties such as superparamagnetic behavior, high surface-to-volume ratio, and external magnetic force.1 For instance, their high surface area and ability to bind with suspended antibiotic-resistant bacteria has encouraged environmental researchers to use them in the treatment of polluted waste water.2
Magnetic nanoparticles also represent a new era of promising applications in counteracting nosocomial infections, where microorganisms tend to attach and subsequently grow on solid surfaces, including medical devices, and form biofilms.3 The presence of these microbial biofilms is a critical problem in the biomedical field. Microbial biofilms act as barriers against the action of antimicrobial agents, which become refractory to antimicrobial therapy. Different approaches have been applied to protect solid surfaces against colonization and biofilm formation, such as the use of nanoparticle-coated surfaces.4
In anticancer studies, magnetic nanoparticles are widely used in medical examinations, targeting, and treatment.5 For detection purposes, incorporating magnetic nanoparticles into imaging modalities, such as magnetic resonance imaging (MRI), confers enhanced performance to cancer diagnosis.6 In another advanced study, magnetic nanoparticles were utilized to detect tumors with diameters as small as 10 mm, which enabled medical doctors to discover cancers at early stages of malignancy.7 This diagnosis became known as the targeted-detection technique.8,9
Magnetic fluid hyperthermia is another medical application intended to inhibit tumor cell growth.10 This application allows the insertion of magnetic nanoparticles into solid tumors followed by exposure to an alternating current (AC) magnetic field, which increases the temperature and subsequently kills the cancer cells with fewer side effects on normal cells.11
Magnetic nanoparticles are considered good carriers for many chemotherapeutic agents.12,13 For example, in vivo injection of doxorubicin-loaded magnetic nanoparticles intratumorally into mice implanted subcutaneously with lung carcinoma has resulted in an increase in the efficacy of doxorubicin against tumors.14
HepG2, HT29, and MCF7 are three cell lines of epithelial origin that were isolated from the hepatocellular carcinoma of a 15-year-old male adolescent, the colorectal adenocarcinoma of a 44-year-old female adult, and the breast carcinoma of a 69-year-old female adult, respectively. MCF10a is a nontumorigenic epithelial cell line isolated from the mammary gland of a 36-year-old female adult (American Type Culture Collection [ATCC], Rockville, MD, USA).
Doxorubicin is one of the best drugs for systemic chemotherapy, which works against breast cancer. For colon cancer treatment, oxaliplatin is commonly used, while tamoxifen is the most common drug used for liver cancer. Most chemotherapies are expensive and cause serious side effects ranging from nausea, vomiting, mucositis, ulceration and necrosis of the colon to acute myeloid leukemia with a preleukemic phase and heart failure.15
Several reports clearly show that different types of magnetic nanoparticles are toxic to different types of cancer cells cultured in vitro including human SK-MEL-37 melanoma cells,16 human osteosarcoma Saos-2 cells,17 and cervical adenocarcinoma HeLa cells.18 Current paradigms of apoptosis suggest that in mammalian cancer cells, the magnetic nanoparticles activate the oxidative stress by increasing reactive oxygen species (ROS) levels and depleting the antioxidant glutathione, which result in the induction of apoptosis via p53, survivin, bax/bcl-2, and caspase pathways.19
Very few studies have reported on the role of magnetic nanoparticles as anticancer materials. To our knowledge, this is the first report of the in vitro anticancer effect of nickel zinc (NiZn) ferrite nanoparticles against three cancer cell lines. The magnetic, structural, and morphological characteristics of NiZn ferrite nanoparticles were revealed. Further, the toxicity of NiZn ferrite nanoparticles against human colon cancer HT29, human breast cancer MCF7, and human liver cancer HepG2 cell lines was assessed and compared with the effects of oxaliplatin, doxorubicin, and tamoxifen, respectively. The mechanism of cytotoxicity of NiZn ferrite nanoparticles in these cancer cell lines is also discussed.
Methodology
Materials and preparation
Trypsin/ethylenediamine tetracetic acid (EDTA) solution was purchased from Invitrogen (Carlsbad CA, USA). Dimethylsulfoxide (DMSO), phosphate-buffered saline (PBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Dulbecco’s modified Eagle’s medium (DMEM), diphenylamine (DPA) reagent (100 mL glacial acetic acid 1.5 g diphenylamine, 1.5 mL concentrated sulfuric acid 0.5 mL and 16 mg/mL acetaldehyde stock) and trypan blue dye were purchased from Sigma Chemical Company (Perth, WA, Australia). NiZn ferrite nanoparticle powder of the chemical formula Ni0.5Zn0.5Fe2O4 with 98.5% purity was procured from Nanostructured and Amorphous Materials Inc (Houston, TX, USA). The powder was formulated following techniques published previously20
Characterization
Structure and morphology
Powder X-ray diffraction (XRD) patterns of NiZn ferrite nanoparticles were recorded with a Shimadzu XRD-6000 instrument (Shimadzu Corporation, Kyoto, Japan) in the range of 10°–70° using CuKα as a radiation source (λ = 1.5418 Å) generated at 30 kV and 30 mA. Fourier transform infrared (FTIR) spectra of NiZn ferrite nanoparticle powder were recorded over the range of 400–4000 cm−1 on a Thermo Nicolet Nexus, Smart Orbit spectrometer using a sample of approximately 1% in 200 mg of spectroscopic-grade potassium bromide (KBr) with 10 tons of pressure. Scanning electron microscopy (SEM, Model LEO 1450VP [LEO Electron Microscopy Ltd Cambridge, UK]), with an accelerating voltage of 30 kV, and energy dispersive X-ray spectroscopy (EDX) were used in order to investigate the morphology and elemental composition of NiZn ferrite nanoparticles, respectively. In addition, transmission electron microscopy (TEM, Model CM12; Philips, Eindhoven, The Netherlands) with an accelerating voltage of 120 kV and a maximum magnification limit of 660 k times, was used to determine the homogeneity of the nanoparticles.
Cell culture
Three human cell lines were obtained from ATCC. The cell lines comprise human breast cancer (MCF7), human colon cancer (HT29), human liver cancer (HepG2), and normal breast (MCF10a) cells, which are characterized as virus-negative. They grow as an adherent monolayer of tightly knit epithelial cells.
Cytotoxicity MTT assay
HT29, MCF7, and HepG2 cells lines were plated at 2 × 103 cells/well by adding 200 μL of a 1 × 104 cells/mL suspension to each well of a 96-well tissue culture plate. The plates were incubated for sufficient time to ensure attachment at 30% to 40% confluency. The media was aspirated off and replaced with fresh media (200 μL) containing NiZn ferrite of different concentrations (15.6 to 1,000 μg/mL) and chemotherapeutic agents at 0.156 to 10.0 μg/mL (oxaliplatin for HT29 cells, doxorubicin for both MCF7 and MCF10a cells, and tamoxifen for HepG2 cells). The last row was left as an untreated control. The plates were incubated at 37°C, 5% CO2, for 72 hours. After incubation with the compounds, the media was aspirated off and the cells were washed by PBS buffer three times to ensure that all NiZn ferrite nanoparticles were removed, and then replaced with a fresh media. MTT solution (20 μL) in a total volume of 200 μL was added to every well and mixed gently with the media, which was later incubated for 4 to 6 hours at 37°C with 5% CO2. The MTT-containing medium was then removed carefully and replaced with DMSO (200 μL per well) to dissolve the formazin crystals. The plates were read in a microtiter plate reader at 570 nm. The concentration of drug needed to inhibit cell growth by 50% (IC50) was generated from the dose-response curves for each compound and each cell line.
Bromodeoxyuridine (BrdU) cell proliferation assay
To confirm antiproliferative effects in NiZn ferrite-treated cells, a BrdU cell proliferation kit was used. HepG2, MCF7, MCF10a, and HT29 cells were seeded at 1.5 × 105 cells/mL in a final volume of 100 μL in a 96-well culture dish and incubated overnight. The cells were treated with NiZn ferrite for 24, 48, and 72 hours, respectively. Then, BrdU label (20 μL, diluted by 1/2,000 in media) was added to each well and incubated for 24 hours at 37°C. The media was discarded and replaced by fix/denaturing solution (200 μL). The plate was incubated for 30 minutes at room temperature. The fix/denaturing solution was removed by inverting the plate. Then, BrdU antibody (100 μL, diluted by 1/100 in dilution buffer) was added to each well and left for 1 hour at room temperature. The cells were washed three times with wash buffer. After that, secondary antibody (100 μL, anti-mouse immunoglobulin G horseradish peroxidase-conjugated) was added to each well for 30 minutes. The washing step was done three times with wash buffer and once with distilled water. The distilled water was removed and substrate solution (100 μL) was added to each well. The plate was left for 15 minutes at room temperature in the dark. Finally, a stop solution (100 μL) was added to the wells and absorbance measured at 450–540 nm.
Microscopic examination of cell morphology
HepG2, HT29, and MCF7 cells (1 × 104) were seeded in the 6-well plates and incubated overnight to allow the cells to adhere to the dish. The cells were exposed to NiZn ferrite nanoparticles for 72 hours at IC50 concentrations (calculated from MTT results). General morphology and membrane changes were examined via light-inverted microscope.
Caspase-3 and -9 assay
The extent of caspase-3 and -9 activation in HepG2, HT29, MCF10a, and MCF7 cells treated with 10, 50, 100, and 1000 μg/mL NiZn ferrite nanoparticle was assessed using a commercially available colorimetric assay kit in accordance with the protocol supplied by the manufacturer (Promega, Madison, WI, USA). The caspase activity in a sample is proportional to the amount of p-nitroaniline (pNA) product detected spectrophotometrically. This assay makes use of a caspase-specific substrate L-aspartic-L-glutamic-L-valyl-L-aspartic acid para-nitroaniline (DEVD-pNA) and L-leucine-L-glutamyl-L-histidyl-L-aspartic-p-nitroaniline acid amide (LEHD-pNA) labeled with pNA for caspase-3 and -9, respectively. Cleavage of the substrate by the specific cellular caspase yields free pNA that can be detected by spectrophotometer at 405 nm.
The cells were plated at a density of 1 × 106 cells/culture dish. After treatment with nanoparticles, the cells were harvested by centrifugation. The pellets were washed with PBS and lysed in 50 μL of chilled cell lyses buffer and left on ice for 10 minutes. The lysate was centrifuged at 50 g in Eppendorf Centrifuge 5810R (Hamburg, Germany) at 4°C for 5 minutes, and the supernatant was used for caspase assay. The caspase-3 and -9 activities were measured by the cleavage of colorimetric DEVD-pNA and LEHD-pNA. The production of pNA was estimated at 405 nm.
DNA fragmentation
DNA fragmentation was quantitatively determined using diphenylamine reagent. The cells were treated with NiZn ferrite nanoparticles at different concentrations, then harvested 12 and 24 hours after treatment. One hundred eight microliters of 5-molar perchloric acid was added to the samples, which were then heated at 70°C for 15 minutes. Later, two volumes of a solution containing diphenylamine reagent were added, and then the samples were stored at 4°C for 48 hours. The colorimetric reaction was quantified at 575 nm using an ultraviolet-visible Thermo Smart Orbit spectrophotometer (Perkin Elmer, Waltham, MA, USA). DNA from both pellet and supernatant were quantified. The degree of DNA fragmentation is given by the following equation:
| (1) |
Statistical analysis
All experiments were done at least three times unless otherwise indicated. Data are expressed as means ± standard deviation (SD). All statistical analyses were performed using Minitab statistical software (Minitab Inc, State College, PA, USA). Treatment effects were determined using one-way analysis of variance followed by Tukey’s post hoc analysis. A value of P < 0.05 was considered significant unless indicated otherwise.
Results
XRD analysis
The XRD diffraction patterns of NiZn ferrite nanoparticles are depicted in Figure 1. In the studied angle range of 2θ = 10°–70°, it is clearly seen that the Ni0.5Zn0.5Fe2O4 powder has a crystalline phase with nine intense peaks which correspond to diffractions due to (111), (220), (311), (222), (400), (422), (511), (440), and (531) planes. These planes are well indexed to a cubic spinel structure of a lattice parameter 8.4 Å with no impurity phase detected. The most intense peak of pure NiZn ferrite powder is assigned to the (311) index plane at 2θ = 35.4°. The particle size was calculated to be 12 nm using Debye–Scherrer’s equation,
Figure 1.
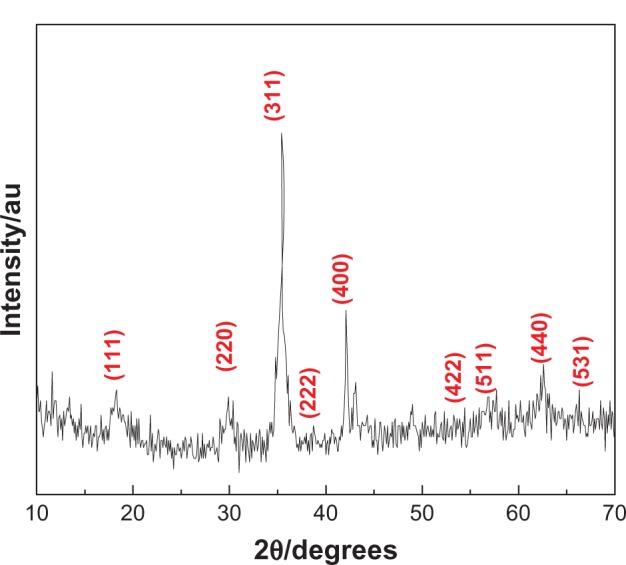
X-ray diffraction pattern of Ni0.5Zn0.5Fe2O4 powder.
Abbreviation: au, arbitrary unit.
| (2) |
where D is the crystallite size, λ is the incident X-ray wavelength, β is the full width at half-maximum, and θ is the diffraction angle.
Infrared spectroscopy
The FTIR spectrum for NiZn ferrite nanoparticles is shown in Figure 2. The spectrum was determined at a range of 400–4,000 cm−1 using a KBr pellet method. The strong absorption peak at 540 cm−1 is due to the characteristic stretching of NiZn ferrite nanoparticles. The absorption peaks at 3,344 and 1,630 cm−1 for urea materials are attributed to stretching of the N—H (group NH2) and C=O amide groups, respectively These bands indicate that the urea may not be completely removed from the resulting material during the preparation process.
Figure 2.
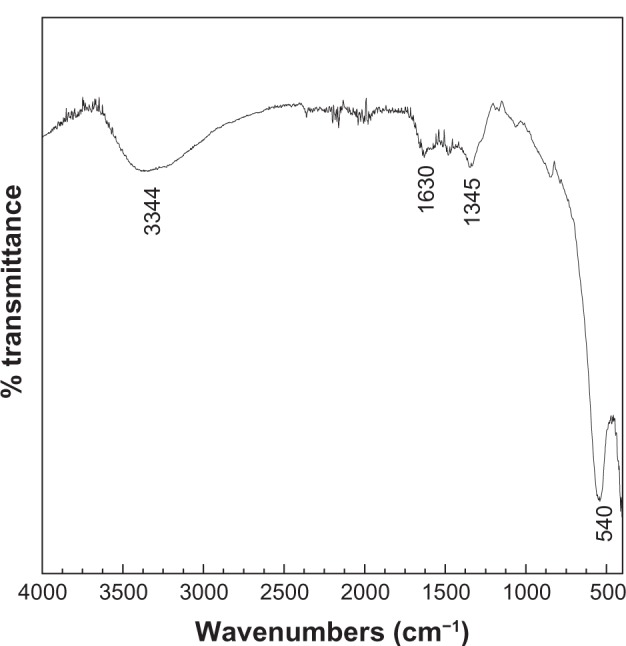
Fourier transform infrared spectra of Ni0.5Zn0.5Fe2O4.
Morphological and elemental analysis of nanoparticles
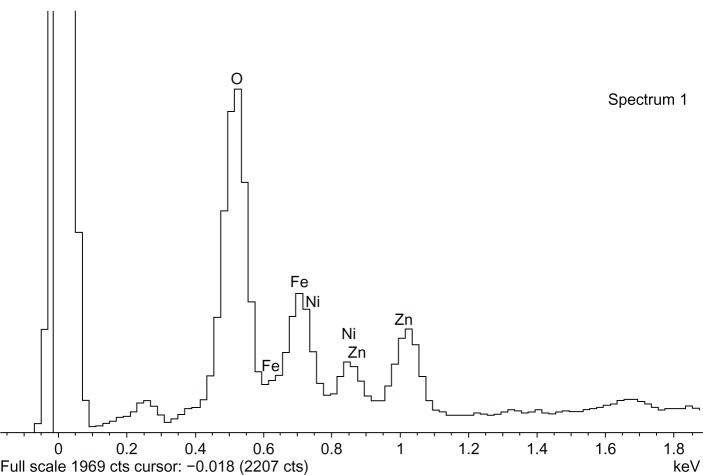
The morphology of NiZn ferrite nanoparticles was examined with SEM. EDX was performed to confirm the elemental composition of the pure sample. SEM and EDX micrographs for the pure sample are shown in Figures 3 and 4, respectively. It is clear that NiZn ferrite nanoparticles are agglomerated by forming bigger clusters, which is ascribed to their strong magnetic dipole–dipole interaction amongst the single-domain particles. It is believed that homogeneity could be enhanced, either by adding an appropriate agent or by using a proper homogenizing tool (eg, sonication, mechanical stirring, magnetic stirring etc), which would improve the overall physical properties of NiZn ferrite nanoparticles.
Figure 3.

Scanning electron micrograph of pure NiZn ferrite nanoparticles.
Figure 4.
Energy dispersive X-ray spectroscopy of pure NiZn ferrite nanoparticles.
Abbreviations: keV, kiloelectronvolt; Fe, iron; Ni, nickel; Zn, zinc; O, oxygen.
The morphology and the extent of dispersion of NiZn ferrite nanoparticles were determined with TEM. The TEM images of pure NiZn ferrite nanoparticles are shown in Figure 5. It is apparent that the nanoparticles are approximately spherical in shape with diameters ranging from 10 to 30 nm. In addition, most of the nanoparticles are agglomerated, and few are detached. This behavior suggests the presence of high magnetic-dipole interparticle interactions among the NiZn ferrite nanoparticles.
Figure 5.
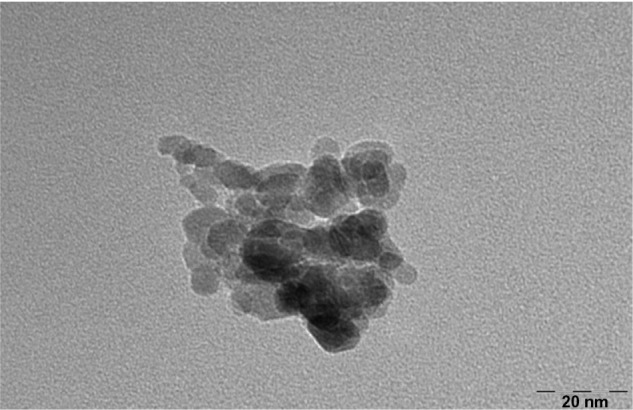
Transmission electron microscopy of pure NiZn ferrite nanoparticles.
Cellular sensitivity of cells to NiZn ferrite nanoparticles
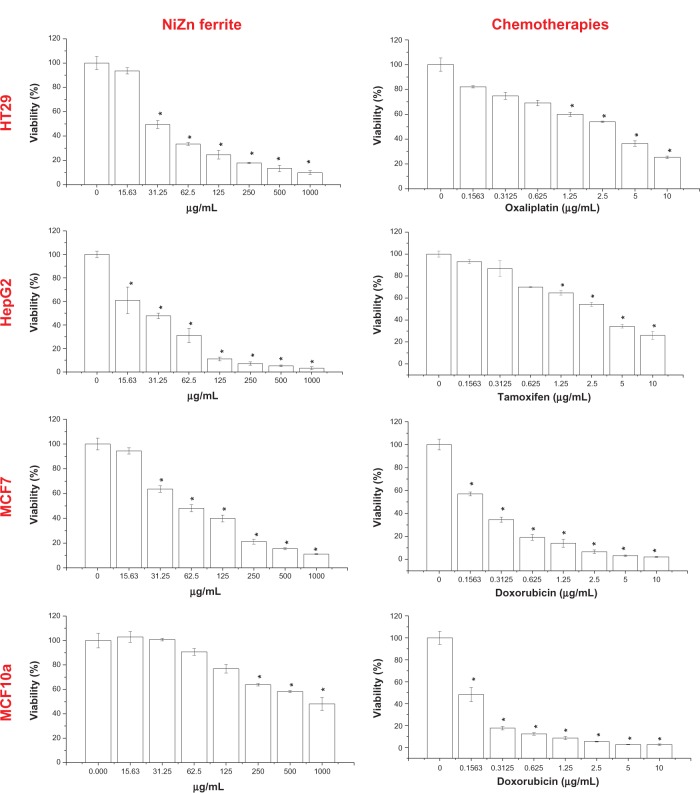
The cytotoxicity of NiZn ferrite nanoparticles on the MCF7, HT29, and HepG2 cells was determined by MTT assay. Figure 6 shows that NiZn ferrite nanoparticles have significant cytotoxic effects against liver cancer HepG2 cells at all concentrations tested (P < 0.05). In the case of MCF7, the lowest concentration at 15.6 μg/L had no significant effect on cell growth, where the nanoparticles inhibited the growth of all cell lines tested in a dose-dependent manner.
Figure 6.
NiZn ferrite nanoparticles and chemotherapeutic effects on the viability of treated cells, which were evaluated through mitochondrial activity using MTT assay.
Notes: Mean ± SD (n = 3 wells/treatment); *P < 0.05 compared with the untreated cells.
Abbreviations: SD, standard deviation; MTT, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide); NiZn, nickel zinc.
The IC50 values of NiZn ferrite nanoparticles calculated from the dose-response curves are shown in Table 1. The results obtained from MTT assay showed significant change in the viability from HT29 cells treated with magnetic nanoparticles at concentrations of 31.3 and 62.5 μg/mL for 72 hours when compared to untreated cells. On the other hand, the normal breast MCF10a cells were the least sensitive to NiZn ferrite nanoparticles, with an IC50 value of approximately 915 μg/mL, while the breast cancer MCF7 cells were found to be more sensitive with an IC50 value of 58.7 μg/mL. The IC50 value of NiZn ferrite nanoparticles in normal breast MCF10 cells was almost 15 times higher than that in breast cancer MCF7 cells lines (Table 1). Furthermore, compared with NiZn ferrite nanoparticles, doxorubicin showed higher cytotoxicity in the normal breast MCF10a cell line.
Table 1.
IC50 of NiZn ferrite nanoparticles, oxalipatin, doxorubicin, and tamoxifen on HT29, MCF7, HepG2, and MCF10a cells after 72 hours
| Treatment | IC50 (μg/mL)
|
|||
|---|---|---|---|---|
| HT29 | MCF7 | HepG2 | MCF10a | |
| NiZn ferrite nanoparticles | 31.1 ± 1.2 | 58.7 ± 9.1 | 28.5 ± 1.4 | 915.0 ± 2.9 |
| Oxaliplatin | 3.06 ± 0.3 | – | – | – |
| Doxorubicin | – | 0.21 ± 0.1 | – | 0.15 ± 0.2 |
| Tamoxifen | – | – | 3.04 ± 1.1 | – |
Note: Mean ± SD.
Abbreviations: IC50, concentration of drug needed to inhibit cell growth by 50%; SD, standard deviation.
Antiproliferative effect of NiZn ferrite nanoparticles
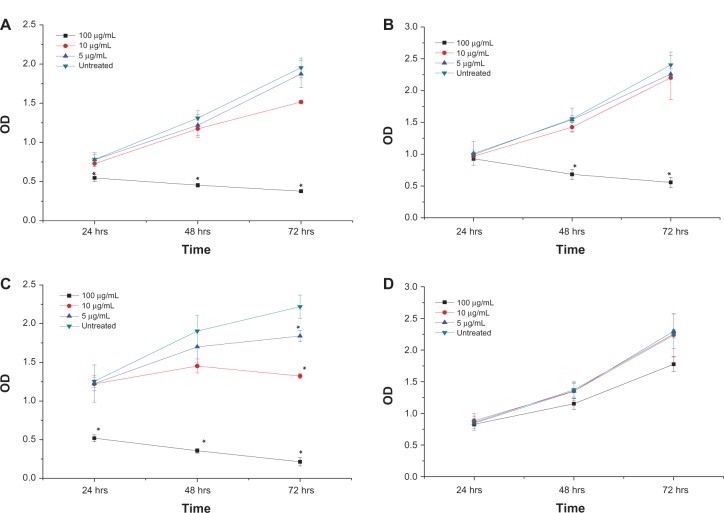
To evaluate the potential of NiZn ferrite nanoparticles in the inhibition of HepG2, HT29, and MCF7 cell proliferation, the cells were treated with various concentrations of the nanoparticles for 24, 48, and 72 hours. The effect of nanoparticles on the proliferation of the cultured cancer cells was measured by the incorporation of the thymidine analogue bromodeoxyuridine into DNA. The cell proliferation of the three cells lines decreased significantly after been treated with 100 μg/mL NiZn ferrite nanoparticles at 72 hours (Figure 7).
Figure 7.
Effects of NiZn magnetic nanoparticles on the proliferation of (A) HT29, (B) MCF7, (C) HepG2, and (D) MCF10a cells in vitro.
Notes: The nanoparticle inhibits cell proliferation in a time- and dose-dependent manner. After treatment with different concentrations of nanoparticles for 24, 48, and 72 hours, the proliferation of the cells was determined by BrdU assay. Mean ± SD (n = 3 wells/treatment); *P < 0.05 compared with the untreated cells.
Abbreviations: hrs, hours; SD, standard deviation; OD, optical density.
The antiproliferative effect of magnetic nanoparticles was evident on the HepG2 cells where 10 μg/mL NiZn ferrite nanoparticles decreased the optical density from 1.452 at 48 hours to 1.322 at 72 hours. Unlike HepG2, the proliferation of MCF7 cells incubated for 3 days was not affected by the same concentration of nanoparticles. On the other hand, the exposure of HT29 cells to 10 μg/mL NiZn ferrite nanoparticles for 24 and 72 hours resulted in a reduction in cell growth from 93% to 77% in comparison with the untreated cells (Figure 7). One hundred micrograms per milliliter exerted no significant growth inhibitory effects on MCF7 cells after 24 hours. At 1000 μg/mL, however, MCF10a proliferation appeared to be higher than that of the MCF7 cells.
Morphological examination of treated cells
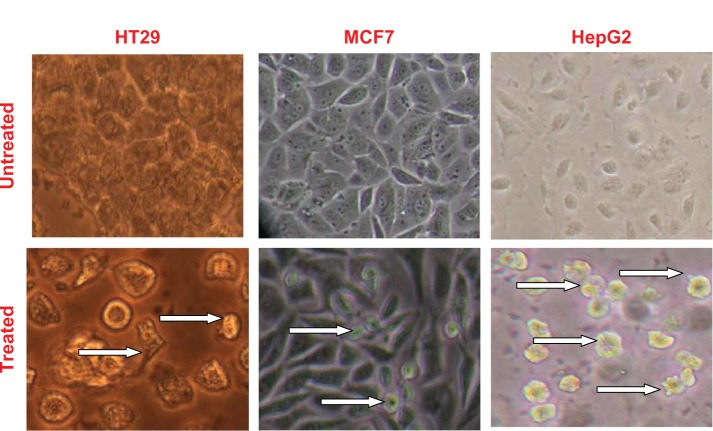
The treatment for 72 hours at the respective IC50 concentrations of NiZn ferrite nanoparticles produced substantial morphological changes in HT29, MCF7, and HepG2 cells. The cells were detached, shrunken, and dispersed with membrane blebbing and cytoplasmic shrinkage (Figure 8). However, these changes were more apparent in HepG2 cells than in the other two cancer cells lines (Figure 8).
Figure 8.
The morphological changes of HepG2, HT29, and MCF7 cells treated with NiZn magnetic nanoparticles at their respective IC50 concentrations for 72 hours.
Note: White arrows indicate apoptotic cells with typical membrane blebbing (200×).
Abbreviation: IC50, concentration of drug needed to inhibit cell growth by 50%; NiZn, nickel zinc.
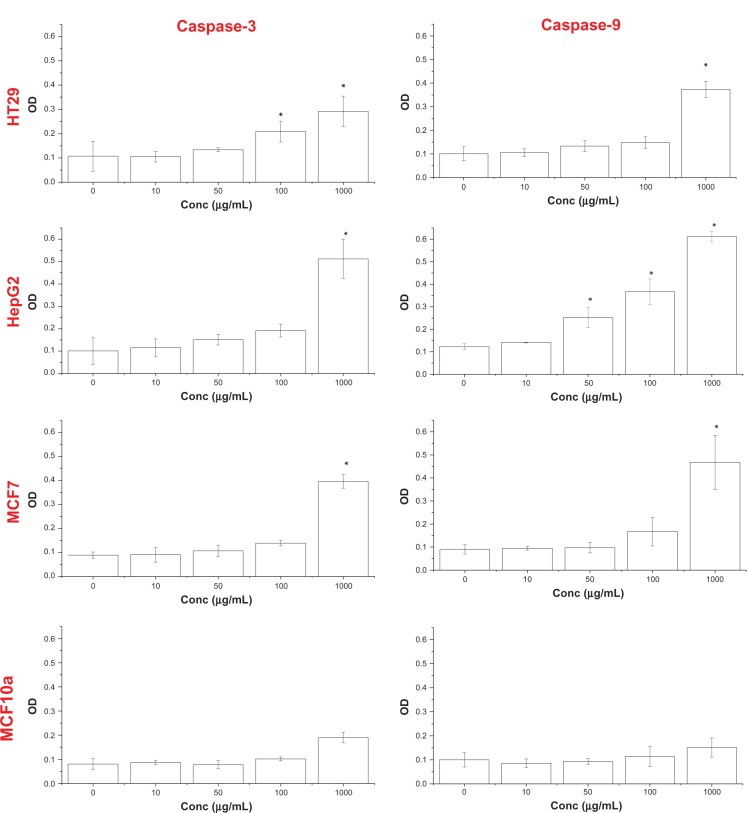
Caspase-3 and -9
Caspase-9 is an initiator for the mitochondria-mediated (intrinsic) apoptotic pathway while caspase-3 is a major enzymatic marker of apoptosis. The caspase-3 and -9 activity of NiZn ferrite nanoparticle-treated cells was analyzed by CaspACE® colorimetric caspase assay kit (Promega). Figure 9 shows that activity of both caspases increased in HT29, MCF7 and HepG2 cells significantly as compared to untreated cells. In fact, the increase in caspase-3 and -9 activities seems to parallel the increase in NiZn ferrite nanoparticle doses. The highest caspases activity was observed in HepG2 cells treated with 1,000 μg/mL of nanoparticles. After 24 hours, the caspase-3 and -9 activity increased to about fivefold higher than that in the untreated cells. In comparison with untreated cells, the caspase-3 and -9 activity increased to 156% and 168% upon treatment with 100 μg/mL nanoparticles, while it increased 3.9- and 4.7-fold in MCF7 cells after treatment with 1,000 μg/mL nanoparticles. In HT29 cells, the caspase-3 activity increased significantly (2.7-fold) after treatment with 1,000 μg/mL nanoparticles. In the normal MCF10a cells, caspase-3 activity was not significantly different from that in the untreated cells at all concentrations tested.
Figure 9.
Treatment of HepG2, HT29, MCF10a, and MCF7 cells with NiZn magnetic nanoparticles results in activation of caspase-3 and -9.
Notes: Cells were treated with 10, 50, 100, and 1,000 μg/mL for 24 hours, and then caspase-3 and -9 activities were determined using the CaspAce® system (Promega, Madison, WI, USA). Mean ± SD (n = 3 wells/treatment). *P < 0.05 compared with the untreated cells.
Abbreviations: NiZn, nickel zinc; SD, standard deviation; OD, optical density.
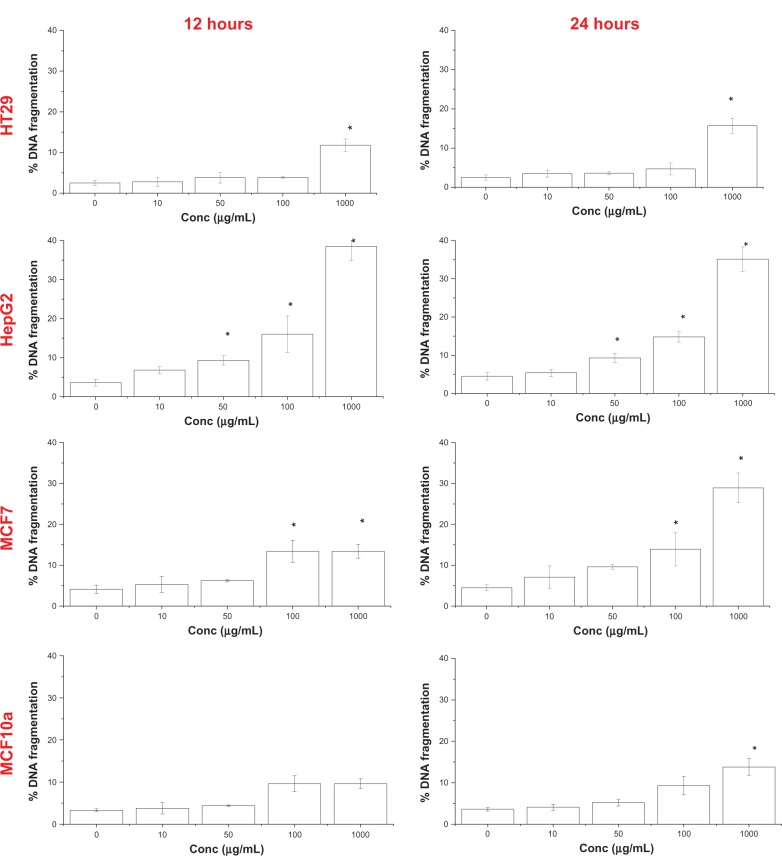
DNA fragmentation
The relative quantity of small DNA fragments in the cells treated with 10 μg/mL nanoparticles is shown in Figure 10. In HepG2 and HT29 cells, NiZn ferrite nanoparticle treatment increased fragmented DNA in a time- and concentration-dependent manner. The increase in concentration of nanoparticles from 100 to 1,000 μg/mL did not significantly affect the relative quantity of DNA fragments in MCF7 cells after 12 hours of treatment. However, exposure to 1,000 μg/mL nanoparticles produced higher DNA fragmentation in HepG2, MCF7, and HT29 cells at 38.5% ± 3.6%, 13.4% ± 1.7%, and 11.8% ± 1.5%, respectively, than in the untreated cells at 3.6% ± 0.9%, 4.1% ± 1.1%, and 2.5% ± 0.7%, respectively.
Figure 10.
Quantitation of DNA fragments induced by NiZn ferrite nanoparticles.
Notes: HepG2, HT29, MCF10a, and MCF7 cells were treated with the nanoparticles for 12 and 24 hours. The amount of DNA fragments was determined by DPA and compared to that of total DNA. Mean ± SD (n = 3 wells/treatment); *P < 0.05 compared with the untreated cells.
Abbreviations: Conc, concentration; DNA, deoxyribo-nucleic acid; DPA, diphenylamine; NiZn, nickel zinc; SD, standard deviation.
Discussion
The role of NiZn ferrite nanoparticles in biomedical applications can be attributed to their magnetic properties, particularly under cryogenic temperatures, which verify their superparamagnetic nature. The magnetic properties of these magnetic nanoparticles have been discussed.21
In vitro cytotoxicity testing can be done efficiently and economically in a previous study and is used to screen chemicals before testing in animal studies.22,23 The concentration response is an important part of cytotoxicity determinations, which should be included to provide a preclinical evaluation of the range over which toxicant-induced cytotoxicity or response occurs.24 As demonstrated in Figures 6 and 7, it is evident that HepG2, HT29, and MCF7 cells responded differently to NiZn ferrite nanoparticle exposure. The viability values indicate that HepG2 cells were more sensitive to the nanoparticles than either MCF7 or HT29 cells after 72 hours exposure while normal breast MCF10a cells exhibited the highest resistance. Using normal cell lines to compare the cytotoxicity effects of NiZn magnetic nanoparticles with cancer cell lines is advisable to confirm these results. This is an important consideration when evaluating a newly formulated drug.25 Hathaway et al26 developed a biologically targeted magnetic nanoparticle that exploits differences between MCF7, BT-474, and MDA-MB-231 breast cancer cells and normal Chinese hamster ovary cells, which permits greater specificity for cancer cells with less damage to normal cells.
Time-response studies are also useful in cytotoxicity determinations because they are indicators of association between functional change and irreversible cell damage.27,28 The 72 hours of incubation with NiZn ferrite nanoparticles had the most inhibitory effects in BrdU incorporation assays, with loss of cell viability being both dose- and time-dependent. At 72 hours, HepG2 and HT29 cells retained 60% and 77% of controlled proliferation after treatment with 10 μg/mL nanoparticles, respectively, while in MCF7 cells, controlled proliferation was reduced to 91%. The cytotoxicity of NiZn ferrite nanoparticles is similar to that of another magnetic nanoparticle ie, nickel ferrite nanoparticles, which exhibited antitumor activities in human alveolar adenocarcinoma A549 cell lines at 100 μg/mL.19
In another supporting study, nickel ferrite nanoparticles prepared by a different method, have shown similar effects on the cervical adenocarcinoma HeLa cell lines.18 At 10 μg/mL, nickel ferrite nanoparticles produced only slight antitumor activity. However, the effect was more pronounced after 72 hours with 83% cytotoxicity compared to untreated cells.18 However, these results are clearly distinct from previous findings, in which 1,000 μg/mL magnetic nanoparticles showed a slight inhibition in both human umbilical vein endothelial cells and PC3 prostate cancer cells incubated for 48 hours.29
Cells treated with NiZn ferrite nanoparticles have been shown to manifest the characteristic morphological changes associated with apoptosis or programmed cell death. In fact, a light microscope revealed the morphological characteristics of apoptosis that are attributed to the cytotoxic effect of the nanoparticles. Magnetic nanoparticle-induced apoptosis may be an integral component of the cellular mechanism relating to its therapeutic effects and cytotoxicities. The magnetic nanoparticles are rapidly distributed in epithelial tissue with strong binding to plasma proteins, principally albumin.30
Activation of endogenous nuclease enzymes is considered to be a key biochemical event in apoptosis, leading to the cleavage of DNA into nucleosomesized fragments, and it is well-known that caspase-3 is a key mediator of nuclease activation.31 The three cell lines, HT29, MCF7, and HepG2, were treated with increasing concentrations of NiZn ferrite nanoparticles to determine conditions that could induce apoptosis as measured by a standard interchromosomal DNA fragmentation assay. NiZn ferrite nanoparticles induced dose-dependent apoptosis in the treated cells with maximal effective dose of about 100 μg/mL in HepG2 cells and 1,000 μg/mL for both HT29 and MCF7 cells after 12 hours. This effect is similar to that produced by nickel ferrite nanoparticles, but in the human alveolar adenocarcinoma A549 cell line with a similar effective concentration of 100 μg/mL or less.19 Nevertheless, previous data has provided evidence that magnetic nanoparticles fulfill two basic criteria for an effective chemotherapeutic agent, ie, tumor specificity and minimal toxicity to normal cells.26
Conclusion
NiZn ferrite nanoparticles exhibited greater cytotoxicity on liver cancer HepG2 cells among the cancer cell lines examined. The normal breast MCF10a cells seemed to show the lowest sensitivity to the cytotoxic effect of the nanoparticles. NiZn ferrite nanoparticles selectively killed liver cancer cells through suppression of proliferation and induction of apoptosis. Our magnetic nanoparticles induced apoptosis in cancer cells via a caspase-9-dependent pathway. In fact, a significant increase of caspase-3 activity, which correlates with the DPA assay findings, a marker of DNA fragmentation, was observed in HT29, MCF7, and HepG2 cell lines as a result of treatment with NiZn ferrite nanoparticles.
Extrapolation of the in vitro cytotoxic effects of our NiZn ferrite nanoparticles to in vivo anticancer application would require further studies to determine its stability, half-life, and biologically significant concentrations in the induction of the apoptotic pathway in vivo. Therefore, taking this into consideration, our findings strongly suggest that NiZn ferrite nanoparticles merit further investigation as a cancer chemotherapeutic agent.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sun C, Lee JSH, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60(11):1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu H, Ho PL, Tsang KWT, Wang L, Xu B. Using biofunctional magnetic nanoparticles to capture vancomycin-resistant enterococci and other gram-positive bacteria at ultralow concentration. J Am Chem Soc. 2003;125(51):15702–15703. doi: 10.1021/ja0359310. [DOI] [PubMed] [Google Scholar]
- 3.Taylor EN, Webster TJ. The use of superparamagnetic nanoparticles for prosthetic biofilm prevention. Int J Nanomedicine. 2009;4:145–152. [PMC free article] [PubMed] [Google Scholar]
- 4.Peyre J, Humblot V, Méthivier C, Berjeauc JM, Pradier CM. Co-grafting of amino-poly-ethylene-glycol and magainin I on a TiO2 surface: Tests of antifouling and antibacterial activities. J Phys Chem B. 2012;116(47):13839–13847. doi: 10.1021/jp305597y. [DOI] [PubMed] [Google Scholar]
- 5.Lee KJ, An JH, Shin JS, Kim DH. Synthesis and characterization of bicalutamide-loaded magnetic nanoparticles as anti-tumor drug carriers. J Nanosci Nanotechnol. 2012;12(2):1611–1615. doi: 10.1166/jnn.2012.4632. [DOI] [PubMed] [Google Scholar]
- 6.Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003;63(23):8122–8125. [PubMed] [Google Scholar]
- 7.Brunke O, Odenbach S, Jurgons R, Alexiou C, Hilger I, Beckmann F. Determination of the magnetic particle distribution in tumour tissue by means of x-ray tomography. J Phys Condens Matter. 2006;18(38):S2903. [Google Scholar]
- 8.Pray L. Molecular Diagnostics: New Growth, New Markets. Waltham, MA: Cambridge Healthtech Advisors; 2005. [Google Scholar]
- 9.Leuschner C, Kumar CSSR, Hansel W, Soboyejo W, Zhou J, Hormes J. LHRH-conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastases. Breast Cancer Res Treat. 2006;99(2):163–176. doi: 10.1007/s10549-006-9199-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Q, Wang L, Cheng R, et al. Magnetic nanoparticle-based hyperthermia for head and neck cancer in mouse models. Theranostics. 2012;2(1):113–121. doi: 10.7150/thno.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas LA, Dekker L, Kallumadil M, et al. Carboxylic acid-stabilised iron oxide nanoparticles for use in magnetic hyperthermia. J Mater Chem. 2009;19(36):6529–6535. [Google Scholar]
- 12.Babincov M, Altanerov V, Altaner C, Bergemann C, Babinec P. In vitro analysis of cisplatin functionalized magnetic nanoparticles in combined cancer chemotherapy and electromagnetic hyperthermia. IEEE Trans Nanobiosci. 2008;7(1):15–19. doi: 10.1109/TNB.2008.2000145. [DOI] [PubMed] [Google Scholar]
- 13.Maeng JH, Lee DH, Jung KH, et al. Multifunctional doxorubicin-loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials. 2010;31(18):4995–5006. doi: 10.1016/j.biomaterials.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 14.Jia Y, Yuan M, Yuan H, et al. Co-encapsulation of magnetic Fe3O4 nanoparticles and doxorubicin into biodegradable PLGA nanocarriers for intratumoral drug delivery. Int J Nanomedicine. 2012;63:1697–1708. doi: 10.2147/IJN.S28629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin J. British National Formulary: September 2010. London: BMJ Group and RPS Publishing; 2010. [Google Scholar]
- 16.de Freitas ERL, Soares PRO, de Paula Santos R, et al. In vitro biological activities of anionic-Fe2O3 nanoparticles on human melanoma cells. J Nanosci Nanotechnol. 2008;8(5):2385–2391. doi: 10.1166/jnn.2008.275. [DOI] [PubMed] [Google Scholar]
- 17.Yang G, Li X, Zhao Z, Wang W. Preparation, characterization, in vivo and in vitro studies of arsenic trioxide Mg-Fe ferrite magnetic nanoparticles. Acta Pharmacol Sin. 2009;30(12):1688–1693. doi: 10.1038/aps.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomitaka A, Koshi T, Hatsugai S, Yamada T, Takemura Y. Magnetic characterization of surface-coated magnetic nanoparticles for biomedical application. J Magn Magn Mater. 2011;323(10):1398–1403. [Google Scholar]
- 19.Ahamed M, Akhtar MJ, Siddiqui MA, et al. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology. 2011;283(2–3):101–108. doi: 10.1016/j.tox.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Modak S, Ammar M, Mazaleyrat F, Das S, Chakrabarti PK. XRD, HRTEM and magnetic properties of mixed spinel nanocrystalline Ni–Zn–Cu–ferrite. J Alloys Compd. 2009;473(1–2):15–19. [Google Scholar]
- 21.Flaifel MH, Ahmad SH, Abdullah MH, Al-Asbahi BA. NiZn Ferrite filled thermoplastic natural rubber nanocomposites: effect of low temperature on their magnetic behaviour. Cryogenics. 2012;52(10):523–529. [Google Scholar]
- 22.Malich G, Markovic B, Winder C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology. 1997;124(3):179–192. doi: 10.1016/s0300-483x(97)00151-0. [DOI] [PubMed] [Google Scholar]
- 23.Shokri F, Heidari M, Gharagozloo S, Ghazi-Khansari M. In vitro inhibitory effects of antioxidants on cytotoxicity of T-2 toxin. Toxicology. 2000;146(2):171–176. doi: 10.1016/s0300-483x(00)00172-4. [DOI] [PubMed] [Google Scholar]
- 24.Miller FJ, Schlosser PM, Janszen DB. Haber’s rule: a special case in a family of curves relating concentration and duration of exposure to a fixed level of response for a given endpoint. Toxicology. 2000;149(1):21–34. doi: 10.1016/s0300-483x(00)00229-8. [DOI] [PubMed] [Google Scholar]
- 25.Al-Qubaisi M, Rozita R, Yeap SK, Omar AR, Ali AM, Alitheen NB. Selective cytotoxicity of goniothalamin against hepatoblastoma HepG2 cells. Molecules. 2011;16(4):2944–2959. doi: 10.3390/molecules16042944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hathaway HJ, Butler KS, Adolphi NL, et al. Detection of breast cancer cells using targeted magnetic nanoparticles and ultra-sensitive magnetic field sensors. Breast Cancer Res. 2011;13(5):R108. doi: 10.1186/bcr3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozman KK, Doull J. Dose and time as variables of toxicity. Toxicology. 2000;144(1):169–178. doi: 10.1016/s0300-483x(99)00204-8. [DOI] [PubMed] [Google Scholar]
- 28.Tennekes HA. The significance of the Druckrey–Küpfmüller equation for risk assessment – The toxicity of neonicotinoid insecticides to arthropods is reinforced by exposure time: Responding to a Letter to the Editor by Drs C Maus and R Nauen of Bayer CropScience AG. Toxicology. 2011;280(3):173. doi: 10.1016/j.tox.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Häfeli UO, Riffle JS, Harris-Shekhawat L, et al. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol Pharm. 2009;6(5):1417–1428. doi: 10.1021/mp900083m. [DOI] [PubMed] [Google Scholar]
- 30.Lartigue L, Wilhelm C, Servais J, et al. Nanomagnetic sensing of blood plasma protein interactions with iron oxide nanoparticles: impact on macrophage uptake. ACS Nano. 2012;6(3):2665–2678. doi: 10.1021/nn300060u. [DOI] [PubMed] [Google Scholar]
- 31.Mitamura S, Ikawa H, Mizuno N, Kaziro Y, Itoh H. Cytosolic nuclease activated by caspase-3 and inhibited by DFF-45. Biochem Biophys Res Comm. 1998;243(2):480–484. doi: 10.1006/bbrc.1998.8122. [DOI] [PubMed] [Google Scholar]