Abstract
Background
High concomitant intolerance attributed to odorous/pungent chemicals, certain buildings, electromagnetic fields (EMF), and everyday sounds calls for a questionnaire instrument that can assess symptom prevalence in various environmental intolerances. The Environmental Hypersensitivity Symptom Inventory (EHSI) was therefore developed and metrically evaluated, and normative data were established. The EHSI consists of 34 symptom items, requires limited time to respond to, and provides a detailed and broad description of the individual’s symptomology.
Methods
Data from 3406 individuals who took part in the Västerbotten Environmental Health Study were used. The participants constitute a random sample of inhabitants in the county of Västerbotten in Sweden, aged 18 to 79 years, stratified for age and gender.
Results
Exploratory factor analysis identified five significant factors: airway symptoms (9 items; Kuder-Richardson Formula 20 coefficient, KR-20, of internal consistency = 0.74), skin and eye symptoms (6 items; KR-20 = 0.60), cardiac, dizziness and nausea symptoms (4 items; KR-20 = 0.55), head-related and gastrointestinal symptoms (5 items; KR-20 = 0.55), and cognitive and affective symptoms (10 items; KR-20 = 0.80). The KR-20 was 0.85 for the entire 34-item EHSI. Symptom prevalence rates in percentage for having the specific symptoms every week over the preceding three months constitute normative data.
Conclusions
The EHSI can be recommended for assessment of symptom prevalence in various types of environmental hypersensitivity, and with the advantage of comparing prevalence rates with normality.
Keywords: Chemical intolerance, Electromagnetic fields, Hyperacusis, Idiopathic environmental intolerance, Prevalence, Sick building syndrome
Background
Health symptoms attributed to environmental agents are an extensive occupational and public health problem. Apart from toxic and allergenic substances, symptoms are commonly attributed to chemicals and biological materials (e.g., mold) that generate odor and sensory irritation (e.g., pungency), to electrical equipment that generate electromagnetic fields (EMF), and to mechanical phenomena that generate sound. Health effects of exposure to strong EMF are well documented, and such exposure is controlled by regulations and guidelines [1]. However, there is no existing evidence for health effects from low-level EMF exposure. Instead there is evidence for a nocebo effect in triggering acute health effects [2-4]. Nevertheless, health problems evoked in the presence of electrical equipment is a concern.
Clinical diagnoses for these environmental intolerances (EI) include multiple chemical sensitivity (MCS) [5], nonspecific building-related symptoms (sick building syndrome) [6], idiopathic environmental intolerance attributed to electromagnetic fields (IEI-EMF) [7], and sound sensitivity (hyperacusis) [8]. As many as 6.3% of a general Swedish population report having a physician-based diagnosis of at least one of these four intolerances, and 21.6% report an intolerance (not necessarily diagnosed) to at least one of the environmental factors odorous/pungent chemicals, certain buildings, EMF, and everyday sounds [9].
Apart from general symptoms (e.g., fatigue and headache) that are common in these EI, certain symptoms seem to be more common in certain types of intolerance. For example, airway symptoms are common in intolerance to odorous/pungent chemicals [10], eye, upper respiratory and skin symptoms among nonspecific building-related symptoms [11], skin symptoms in intolerance attributed to EMF [12], and emotional symptoms and concentration difficulties in sound sensitivity [13]. Regarding EMF, skin symptoms dominate among those who attribute their symptoms to computer screens, fluorescent lamps and television sets, whereas those who attribute their symptoms to EMF in general have a more cognitive and emotional symptom picture [14,15]. The symptom picture varies within intolerances in general, and there is overlap between intolerances.
Studies of quality of life in EI have mostly been focused on individuals with severe MCS [16-20], with exception of sound sensitivity [21]. The impact on quality of life is predominantly manifested as not having access to society, and having difficulties keeping a job and maintaining social relations. Hence, in addition to health symptoms that per se are bothersome, attempts to avoid the symptoms by avoiding the environmental exposure results in isolation for the afflicted individual. Indeed, avoidance of the environmental exposure is the most commonly reported coping strategy in MCS [22], and is common also in symptom-attribution to EMF [23] and sounds [13].
Self-reports are important for diagnosing EI due to the lack of objective markers that are generally agreed on. In this context information on the afflicted individual’s symptomology is valuable, which also may contribute to the understanding of possible underlying mechanisms. For example, a symptom picture of predominantly airway symptoms may possibly indicate C-fiber hypersensitivity as in sensory hyperreactivity [24], whereas a picture of predominantly cognitive and affective symptoms may indicate an anxiety and stress-related condition [25].
Questionnaire instruments have been developed and metrically evaluated for assessment of specific symptoms in certain types of EI. These include the Quick Environmental Exposure and Sensitivity Inventory [26] and the Idiopathic Environmental Intolerance Symptom Inventory (IEISI) [10] for intolerance to odorous/pungent chemicals, and the MM-questionnaires for nonspecific building-related symptoms [27]. However, there is no documentation of metrically evaluated instruments for specific symptoms attributed to exposure to EMF or everyday sounds. Studies show that concomitant intolerance attributed to odorous/pungent chemicals, certain buildings, EMF, and sounds is common [9,12,28]. This motivates simultaneous investigation of these intolerances, which requests a questionnaire instrument that includes relevant symptoms in all these intolerances.
An objective of the current study was to develop and psychometrically evaluate a questionnaire instrument, referred to as the Environmental Hypersensitivity Symptom Inventory (EHSI), for asessment of symtomology in persons with symptoms attributed to odorous/pungent chemicals, certain buildings, EMF, and everyday sounds. The EHSI consists of 34 symptom items, requires limited time to respond to (about 5 min), yet it provides a detailed and broad description of the individual’s symptomology. The metric evaluation included investigation of dimensionality (factor structure) and reliability (internal consistency). The dimensionality was assessed with factor analysis to accomplish appropriate symptom categories. Thus, it is useful to group the symptoms into appropriate categories to enhance the responders’ evaluation of their symptom prevalence and the administrator’s interpretation of symptom pattern in the responses.
Another objective was to establish normative data for the EHSI. In addition to normative data for the general population, reference data were provided for combinations of specific age groups (young, middle-aged and elderly adults) and gender. These data referred to having had the specific symptom on a weekly basis over the preceding three months.
There can be a large difference in symptomology between individuals with EI. This suggests that a questionnaire-based instrument aimed at providing a detailed yet broad description of the individual’s symptom picture should provide the respondent with the possibility to also report symptoms that are not specifically listed in the questionnaire. The EHSI was therefore designed to also include open-ended questions about additional symptoms pertaining to certain symptom categories as well as to symptoms pertaining to additional, unspecified categories. The objectives of this study were addressed by means of data from a population-based study, the Västerbotten Environmental Health Study.
Methods
Population and sample
The Västerbotten Environmental Health Study is an embracing name for different investigations on the same general population regarding various forms of environmental hypersensitivity in Sweden. The study population, inhabitants in the county of Västerbotten in Northern Sweden, has an age and gender distribution that is very similar to that of Sweden in general [29]. A random sample, drawn from the municipal register, of 8600 individuals aged 18 to 79 years was invited to participate. The sample was stratified for age and gender according to the following age strata: 18–29, 30–39, 40–49, 50–59, 60–69, and 70–79 years. Of the 8600 individuals, 8520 could be reached, among whom 3406 (40.0%) agreed to participate. Age and gender distributions for the responders are given in Table 1. The sample is described in Table 2 with respect to demographics, smoking, and health conditions of relevance to symptomology in EI.
Table 1.
Numbers of responders (and response percentages) across age and gender strata
| Age strata (years) | Women | Men |
|---|---|---|
| 18-29 |
307 (32.7%) |
179 (17.7%) |
| 30-39 |
266 (40.9%) |
177 (25.2%) |
| 40-49 |
288 (40.7%) |
230 (31.3%) |
| 50-59 |
367 (51.0%) |
295 (39.7%) |
| 60-69 |
405 (58.6%) |
356 (50.7%) |
| 70-79 |
265 (53.8%) |
271 (63.9%) |
| 18-79 | 1898 (45.2%) | 1508 (34.9%) |
Table 2.
Sample characteristics (n = 3406)
| Age, mean years (SD) |
51.2 (16.8) |
| Women/men, n (%) |
1898/1508 (55.7/44.3) |
| Education (highest), n (%) |
|
| Primary school |
823 (24.5) |
| High school |
1137 (33.8) |
| University |
1405 (41.8) |
| Smoker, n (%) |
298 (8.8) |
| General health status, n (%) |
|
| Very good or excellent |
1349 (40.0) |
| Good |
1152 (34.2) |
| Somewhat good or poor |
868 (25.8) |
| Diagnosis1, n (%) |
|
| Hypertension |
838 (24.6) |
| Diabetes |
186 (5.5) |
| Rheumatic disease |
147 (4.3) |
| Disease in back, joints or muscles |
492 (14.4) |
| Multiple chemical sensitivity |
107 (3.1) |
| Nonspecific building-related symptoms |
47 (1.4) |
| IEI-EMF2 |
15 (0.4) |
| Sound sensitivity |
96 (2.8) |
| Asthma due to allergy |
164 (4.8) |
| Asthma other than allergy |
129 (3.8) |
| Allergic rhinitis |
298 (8.7) |
| Atopic dermatatis |
88 (2.6) |
| Migraine |
151 (4.4) |
| Generaliserad anxiety disorder |
32 (1.0) |
| Depression | 170 (5.0) |
1Self-report of having been given a diagnosis by a physician.
2Idiopathic environmental intolerance attributed to electromagnetic fields.
The environmental hypersensitivity symptom inventory
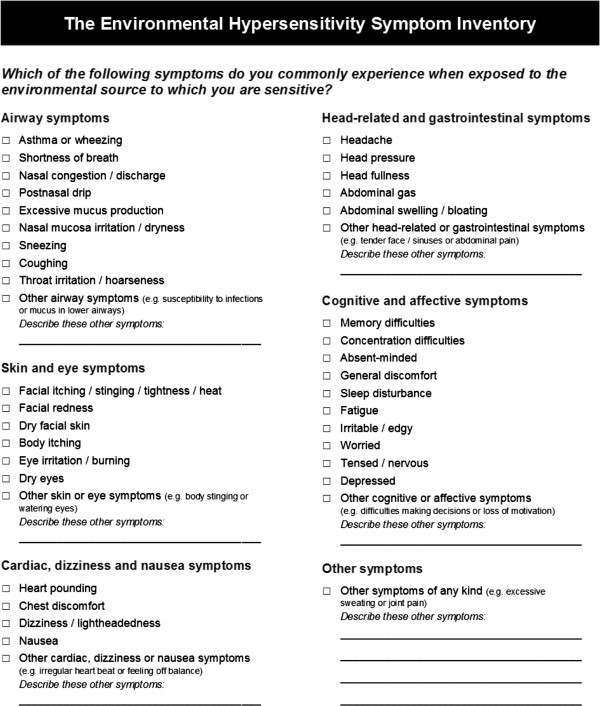
The EHSI is to a large extent based on the IEISI that was developed for assessment of symptom prevalence attributed specifically to odorous/pungent chemicals [10]. The 27 specific symptoms in the IEISI were those reported by at least 20% of a sample with moderate to severe chemical intolerance. Many of these 27 symptoms are also commonly found in nonspecific building-related symptom [6,30], IEI-EMF [12,31,32], and sound sensitivity [13]. However, certain modifications and additions to the IEISI were made to better cover the symptomology of intolerance to certain buildings, EMF, and sounds. Thus, “skin irritation/redness” was replaced with the four items “facial itching/stinging/tightness/heat”, “facial redness”, “dry facial skin”, and “body itching”; and “head fullness/pressure” was replaced with the two items “head fullness” and “head pressure”. Furthermore, “nasal mucosa irritation/dryness”, “dry eyes”, and “general discomfort” were added. In total, the EHSI consists of 34 specific symptoms.
Open-ended questions about additional symptoms pertaining to the symptom categories as well as to symptoms pertaining to additional, unspecified categories are also included in the EHSI. Since the likelihood of remembering to report a certain condition increases when that condition is provided to the respondent [33], examples of additional symptoms are given after each open-ended question in the EHSI. These examples were adopted from the IEISI. The final version of the EHSI is presented in Figure 1.
Figure 1.
The environmental hypersensitivity symptom inventory.
For the normative data, the frequency and time interval for a symptom to be considered as prevalent was having had the symptom every week over the preceding three months. This was partly based on the fact that this typically is used for the definition for nonspecific building-related symptoms [34], partly due to the three-month period being long enough to avoid memory effects and short enough to permit efficient follow-up studies after remedial measures have been taken [27].
Procedure
A questionnaire was used that included the EHSI and questions regarding demographics, smoking, and health conditions (Table 2). The responders were mailed the questionnaire, to be returned by mail with prepaid postage. Non-responders received up to two reminders. All participants responded to the questionnaire during the period March-April, 2010, before the onset of the pollen season in Västerbotten. The study was conducted in accordance with the Helsinki Declaration and approved by the Umeå Regional Ethics Board. All responders gave their informed consent to participate.
Statistical analysis
An exploratory factor analysis with Promax rotation and Kaiser normalization was conducted to study dimensionality of the 34 specific EHSI symptoms for categorization into symptom groups. An oblique factor rotation was chosen since prior studies of environmental hypersensitivity suggest strong commonalities among various types of somatic symptoms [4]. A scree test plot was made to identify the number of factors to be extracted [35]. The Kuder-Richardson Formula 20 coefficient (KR-20), comparable with the Cronbach alpha coefficient, was used for assessing internal consistency. Normative data for symptom prevalence were expressed in percentages among combinations of specific age groups [young (18–34 years), middle-aged (35–54 years), and elderly (55–79 years)] and gender, for the three age groups separately, for gender separately, and for the total sample. Since the response-rate in different age and gender strata varied, weighted prevalence rates for the entire sample were calculated as well. The weights used were calculated based on the inverse of the probability of respondents in each age and gender strata to participate [36].
Results
Dimensionality of the EHSI
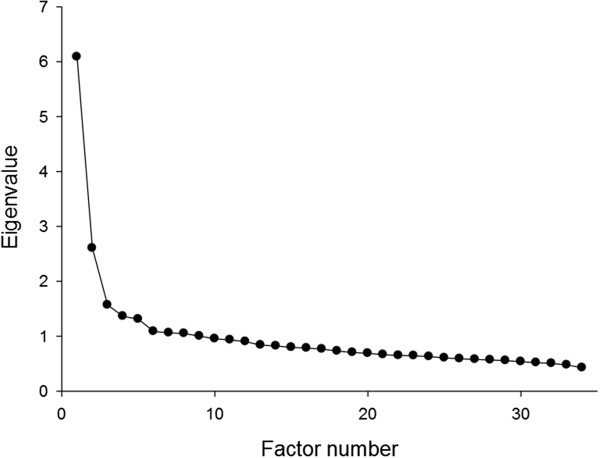
The factor analysis of the data for the 34 specific symptoms identified nine factors with an eigenvalue above 1. Their eigenvalues were 6.09 (17.90% explained variance), 2.60 (7.66%), 1.57 (4.61%), 1.36 (4.01%), 1.31 (3.86%), 1.09 (3.20%), 1.06 (3.12%), 1.05 (3.09%), and 1.01 (2.95%). However, a scree-test plot suggests only five factors to be extracted (Figure 2; the number of factors preceding the last “elbow”; [35]). The factor loadings of each EHSI item on each of the five factors are presented in Table 3.
Figure 2.
Scree test plot of eigenvalues for the 34 factors.
Table 3.
Factor loadings with the strongest loading for each symptom item given in bold
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Concentration difficulties |
.695 |
.145 |
.273 |
.216 |
.260 |
| Depressed |
.667 |
.084 |
.149 |
.235 |
.248 |
| Worried |
.649 |
.072 |
.154 |
.298 |
.239 |
| Tensed/nervous |
.644 |
.046 |
.189 |
.317 |
.210 |
| Absent-minded |
.613 |
.159 |
.210 |
.107 |
.223 |
| General discomfort |
.607 |
.080 |
.152 |
.428 |
.182 |
| Irritable/edgy |
.599 |
.090 |
.170 |
.174 |
.322 |
| Memory difficulties |
.564 |
.205 |
.263 |
.172 |
.142 |
| Fatigue |
.545 |
.142 |
.243 |
.102 |
.466 |
| Sleep disturbance |
.402 |
.163 |
.209 |
.101 |
.299 |
| Coughing |
.119 |
.683 |
.106 |
.240 |
.072 |
| Throat irritation/hoarseness |
.119 |
.665 |
.176 |
.239 |
.122 |
| Shortness of breath |
.174 |
.656 |
.124 |
.528 |
-.131 |
| Excessive mucus production |
.141 |
.587 |
.196 |
.246 |
-.057 |
| Postnasal drip |
.128 |
.564 |
.273 |
.106 |
.137 |
| Nasal congestion/discharge |
.133 |
.555 |
.194 |
.059 |
.327 |
| Sneezing |
.139 |
.555 |
.175 |
.064 |
.349 |
| Irritation/dryness of the nasal mucosa |
.145 |
.497 |
.408 |
.061 |
.236 |
| Asthma or wheezing |
.044 |
.476 |
.093 |
.397 |
-.253 |
| Facial itching/stinging/tightness/heat |
.205 |
.184 |
.691 |
.201 |
.076 |
| Facial redness |
.221 |
.136 |
.689 |
.134 |
.074 |
| Dry facial skin |
.259 |
.133 |
.599 |
.057 |
.266 |
| Dry eyes |
.090 |
.238 |
.509 |
.169 |
.213 |
| Body itching |
.207 |
.197 |
.478 |
.154 |
.147 |
| Eye irritation/burning |
.192 |
.350 |
.460 |
.173 |
.247 |
| Chest discomfort |
.238 |
.188 |
.143 |
.602 |
.106 |
| Heart pounding |
.264 |
.126 |
.206 |
.567 |
.136 |
| Nausea |
.270 |
.167 |
.107 |
.493 |
.424 |
| Dizziness/lightheadedness |
.238 |
.159 |
.254 |
.476 |
.313 |
| Abdominal gas |
.217 |
.167 |
.216 |
.040 |
.581 |
| Abdominal swelling/bloating |
.267 |
.114 |
.220 |
.195 |
.562 |
| Headache |
.273 |
.129 |
.069 |
.173 |
.535 |
| Head fullness |
.359 |
.187 |
.215 |
.307 |
.438 |
| Head pressure | .281 | .078 | .162 | .367 | .399 |
The ten items that loaded strongest on Factor 1 can be referred to as cognitive and affective symptoms; the nine items that loaded strongest on Factor 2 can be referred to as airway symptoms; the six items that loaded strongest on Factor 3 can be referred to as skin and eye symptoms; the four items that loaded strongest on Factor 4 can be referred to as cardiac, dizziness and nausea symptoms; and the five items that loaded strongest on Factor 5 can be referred to as head-related and gastrointestinal symptoms.
Reliability and normative data of the EHSI
The KR-20 coefficient was 0.74 for airway symptoms, 0.60 for skin and eye symptoms, 0.55 for cardiac, dizziness and nausea symptoms, 0.55 for head-related and gastrointestinal symptoms, 0.80 for cognitive and affective symptoms, and 0.85 for the entire 34-item EHSI. Normative data are given in Table 4 for prevalence of specific symptoms expressed in percentages of subpopulations who report having each symptom every week over the preceding three months.
Table 4.
Percentage reporting having had symptoms every week over the preceding three months, constituting normative data
| |
|
|
Middle- |
Middle- |
|
|
|
All |
|
|
|
Total |
Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Young |
Young |
aged |
aged |
Elderly |
Elderly |
All |
middle- |
All |
All |
All |
sample |
sample |
| |
women |
men |
women |
men |
women |
men |
young |
aged |
elderly |
women |
men |
unweighted |
weighted |
| (n = 441) | (n = 265) | (n = 597) | (n = 455) | (n = 860) | (n = 788) | (n = 706) | (n = 1052) | (n = 1648) | (n = 1898) | (n = 1508) | (n = 3406) | (n = 3406) | |
|
Airway symptoms |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Asthma or wheezing |
5.0 |
6.0 |
6.9 |
7.7 |
11.0 |
11.4 |
5.4 |
7.2 |
11.2 |
8.3 |
9.4 |
8.8 |
8.1 |
| Shortness of breath |
7.7 |
6.4 |
8.2 |
7.5 |
11.6 |
12.2 |
7.2 |
7.9 |
11.9 |
9.6 |
9.7 |
9.7 |
9.0 |
| Nasal congestion/discharge |
28.1 |
21.9 |
25.0 |
21.8 |
25.1 |
29.2 |
25.8 |
23.6 |
27.1 |
25.8 |
25.7 |
25.7 |
25.0 |
| Postnasal drip |
8.4 |
6.8 |
9.0 |
6.8 |
12.2 |
9.4 |
7.8 |
8.1 |
10.8 |
10.3 |
8.2 |
9.3 |
8.7 |
| Excessive mucus production |
3.4 |
3.8 |
4.2 |
5.7 |
8.1 |
8.8 |
3.5 |
4.8 |
8.4 |
5.8 |
7.0 |
6.3 |
5.7 |
| Nasal mucosa irritation/dryness |
13.8 |
8.7 |
22.8 |
12.5 |
28.8 |
17.8 |
11.9 |
18.3 |
23.5 |
23.4 |
14.6 |
19.5 |
17.3 |
| Sneezing |
27.9 |
20.4 |
24.1 |
23.7 |
30.8 |
26.8 |
25.1 |
24.0 |
28.9 |
28.0 |
24.7 |
26.6 |
25.6 |
| Coughing |
15.4 |
12.5 |
17.3 |
16.0 |
23.3 |
20.8 |
14.3 |
16.7 |
22.1 |
19.5 |
17.9 |
18.8 |
17.4 |
| Throat irritation/hoarseness |
11.6 |
9.8 |
12.7 |
10.8 |
19.8 |
17.1 |
10.9 |
11.9 |
18.5 |
15.6 |
13.9 |
14.9 |
13.8 |
|
Skin and eye symptoms |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Facial itching/stinging/tightness/heat |
5.9 |
0.4 |
6.5 |
3.3 |
7.2 |
3.8 |
3.8 |
5.1 |
5.6 |
6.7 |
3.1 |
5.1 |
4.6 |
| Facial redness |
7.0 |
3.0 |
8.2 |
4.2 |
7.2 |
4.1 |
5.5 |
6.5 |
5.7 |
7.5 |
3.9 |
5.9 |
5.5 |
| Dry facial skin |
37.2 |
15.1 |
24.3 |
13.4 |
20.0 |
8.5 |
28.9 |
19.6 |
14.5 |
25.3 |
11.1 |
19.1 |
19.1 |
| Body itching |
14.3 |
5.7 |
14.1 |
13.2 |
14.5 |
12.4 |
11.0 |
13.7 |
13.5 |
14.3 |
11.5 |
13.1 |
12.2 |
| Eye irritation/burning |
8.6 |
7.5 |
14.4 |
9.9 |
18.8 |
13.5 |
8.2 |
12.5 |
16.3 |
15.1 |
11.3 |
13.4 |
12.1 |
| Dry eyes |
11.8 |
7.2 |
14.7 |
8.8 |
21.6 |
11.3 |
10.1 |
12.2 |
16.7 |
17.2 |
9.8 |
13.9 |
12.5 |
|
Cardiac, dizziness and nausea symptoms |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Heart pounding |
8.8 |
1.9 |
8.9 |
6.4 |
12.1 |
7.0 |
6.2 |
7.8 |
9.6 |
10.3 |
5.9 |
8.4 |
7.5 |
| Chest discomfort |
6.8 |
3.4 |
5.4 |
5.3 |
7.6 |
7.4 |
5.5 |
5.3 |
7.5 |
6.7 |
6.0 |
6.4 |
6.0 |
| Dizziness/lightheadedness |
10.9 |
1.5 |
8.9 |
3.3 |
10.6 |
7.6 |
7.4 |
6.5 |
9.2 |
10.1 |
5.2 |
8.0 |
6.9 |
| Nausea |
14.3 |
2.3 |
7.2 |
5.5 |
5.8 |
2.8 |
9.8 |
6.5 |
4.4 |
8.2 |
3.5 |
6.1 |
6.1 |
|
Head-related and gastrointestinal symptoms |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Headache |
41.0 |
23.8 |
36.3 |
24.0 |
23.1 |
9.9 |
34.6 |
31.0 |
16.8 |
31.5 |
16.6 |
24.9 |
25.9 |
| Head pressure |
10.7 |
3.8 |
9.5 |
5.5 |
4.9 |
2.7 |
8.1 |
7.8 |
3.8 |
7.7 |
3.7 |
5.9 |
6.0 |
| Head fullness |
19.0 |
9.8 |
15.1 |
10.5 |
11.9 |
6.0 |
15.6 |
13.1 |
9.0 |
14.5 |
8.0 |
11.7 |
11.9 |
| Abdominal gas |
44.9 |
32.5 |
37.7 |
37.1 |
37.0 |
30.8 |
40.2 |
37.5 |
34.0 |
39.0 |
33.0 |
36.4 |
36.5 |
| Abdominal swelling/bloating |
29.7 |
7.2 |
21.9 |
11.2 |
17.1 |
8.8 |
21.2 |
17.3 |
13.1 |
21.5 |
9.2 |
16.1 |
15.8 |
|
Cognitive and affective symptoms |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Memory difficulties |
13.4 |
9.8 |
20.3 |
14.1 |
14.8 |
17.1 |
12.0 |
17.6 |
15.9 |
16.2 |
14.9 |
15.6 |
14.7 |
| Concentration difficulties |
24.9 |
16.6 |
22.3 |
16.0 |
13.7 |
8.8 |
21.8 |
19.6 |
11.3 |
19.0 |
12.3 |
16.1 |
16.7 |
| Absent-minded |
33.8 |
23.8 |
25.3 |
15.8 |
15.5 |
12.2 |
30.0 |
21.2 |
13.9 |
22.8 |
15.3 |
19.5 |
20.7 |
| General discomfort |
12.2 |
3.8 |
8.5 |
7.5 |
4.0 |
2.7 |
9.1 |
8.1 |
3.3 |
7.3 |
4.3 |
6.0 |
6.3 |
| Sleep disturbance |
26.1 |
16.6 |
32.3 |
22.6 |
33.6 |
19.5 |
22.5 |
28.1 |
26.9 |
31.5 |
20.0 |
26.4 |
24.9 |
| Fatigue |
62.8 |
41.1 |
55.6 |
39.6 |
38.6 |
25.8 |
54.7 |
48.7 |
32.5 |
49.6 |
32.6 |
42.1 |
43.2 |
| Irritable/edgy |
31.7 |
21.5 |
26.3 |
17.1 |
10.3 |
7.7 |
27.9 |
22.3 |
9.1 |
20.3 |
13.0 |
17.1 |
18.6 |
| Worried |
29.3 |
18.1 |
21.4 |
18.0 |
17.4 |
9.1 |
25.1 |
20.0 |
13.5 |
21.4 |
13.4 |
17.9 |
18.6 |
| Tensed/nervous |
23.4 |
8.3 |
16.2 |
10.8 |
7.0 |
2.7 |
17.7 |
13.9 |
4.9 |
13.7 |
6.1 |
10.3 |
11.1 |
| Depressed | 31.5 | 16.2 | 18.1 | 15.2 | 14.8 | 8.0 | 25.8 | 16.8 | 11.5 | 19.7 | 11.6 | 16.1 | 17.0 |
Young = 18–34 years, middle-aged = 35–54 years, elderly = 55–79 years.
Discussion
An objective of the present study was to develop and psychometrically evaluate a questionnaire-based instrument, the EHSI, for assessment of symptom prevalence in persons with common types of environmental hypersensitivity. The instrument was aimed at requiring limited time to respond to, yet provide a detailed and broad description of the symptomology of the individual or group under study. Although the symtomology was focused on persons who attribute their symptoms to odorous/pungent chemicals, certain buildings, EMF, and sounds, the wide range of symptoms in the EHSI is likely to cover the symtomology of several other types of environmental hypersensitivity, including asthma and allergy.
The evaluation of the EHSI suggests a factor structure of five factors: airway symptoms, skin and eye symptoms, cardiac, dizziness and nausea symptoms, head-related and gastrointestinal symptoms, and cognitive and affective symptoms. The grouping of the cardiac, dizziness and nausea symptoms can be explained by sympathetic activity such that extensive heart pounding can cause chest discomfort, nausea and dizziness. Grouping of head-related and gastrointestinal symptoms can be referred to both types of symptoms being common psychosomatic symptoms. As would be expected, the head-related and gastrointestinal symptoms were found to load relatively high on the cognitive and affective factor. The outcome from the factor analysis was similar to that reported by Miller and Mitzel [37] and by Andersson and associates [10], also using factor analysis, for those symptoms that were in common between studies.
The internal consistency of the entire EHSI and the symptom category cognitive and affective symptoms can be considered as good, the symptom category airway symptoms can be considered as acceptable, the symptom category skin and eye symptoms can be considered as questionable, and the symptom categories cardiac, dizziness and nausea symptoms, and head-related and gastrointestinal symptoms can be considered as poor. The poor consistency in the latter two symptom categories is likely to be due to these categories having few items in combination with being rather broad. A consequence is that use of a composite measure of cardiac, dizziness and nausea symptoms, and head-related and gastrointestinal symptoms from the EHSI should be supplemented with inspection of whether there is a large variability between the symptoms in this category. The validity of the EHSI was not investigated in this study. One reason for this is that the majority of its symptoms have been validated in a prior study of environmental hypersensitivity [10]. Another reason is the simplicity of assessment with the EHSI: having a specific symptom or not. Thus, the face validity [38] of the EHSI can be considered as good.
Another objective of the study was to provide normative data for various subgroups of age and gender, and for the general adult population. The population-based nature of the Västerbotten Environmental Health Study and the fact that the study population has an age and gender distribution that is very similar to that of Sweden in general [29] enhances the representativeness. However, among the randomly selected individuals only 40% volunteered, which compromises the representativeness. Research ethical regulations for conducting research in Sweden do not allow asking the selected individuals why they chose not to participate or about certain characteristics they may possess [39]. However, information on age and gender was available for those who declined participation in this study, and the largest proportion of non-responders was found among young men (Table 1). The generally low response rate increases the risk of a selection bias. Thus, the special topic of the study (environmental health) may have attracted, in particular, respondents with health problems attributed to environmental aspects [40], which may have resulted in the prevalence rates being higher than otherwise would have been the case. Comparisons with data from prior Swedish population-based studies do only partly support the notion that the current prevalence rates are too high. Whereas Eriksson and Stenberg [34] reported prevalence rates for adults aged 18–64 years that were generally lower than in the present study, Andersson and Norlén [41] reported rates based on all ages that were generally higher. The generally higher prevalence rates in women than in men (Table 4) accord with typical results on gender differences [42], and the pattern of age-related differences corresponds in general with prior Swedish population-based data for young and middle-aged adults [34].
The applicability of the EHSI is not limited to assessment of having had the specific symptoms every week over the preceding three months, or to assessment of prevalence (yes/no), for which the normative data are valid. The instrument can also be used for assessing the prevalence of symptoms as a direct result of the environmental exposure. Furthermore, the respondent can rate to what extent he/she experiences each symptom. An example of an appropriate rating scale for such a purpose is the Environmental Annoyance Scale, which is a category scale with seven semantic descriptors [Not at all (0), a little (1), partly (2), pretty much (3), rather much (4), to a large extent (5), and extremely much (6)], and with ratio-scale properties and good reliability and validity [43].
Conclusions
The 34-item EHSI for assessment of symptoms in various types of environmental hypersensitivity requires limited time to respond to, yet provides a detailed and broad description of the symptomology, including airway, skin, eye, cardiac, dizziness, nausea, head-related, gastrointestinal, cognitive and affective symptoms. Measures of internal consistency suggest that symptom prevalence can reliably be combined for a composite measure for the entire EHSI and for the symptom categories airway symptoms, skin and eye symptoms, and cognitive and affective symptoms. In contrast, caution should be taken when combining items for the symptom categories cardiac, dizziness and nausea symptoms, and head-related and gastrointestinal symptoms. Normative data for various subgroups of age and gender, and for the general adult population are available for having had the specific symptoms every week over the preceding three months.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors contributed in planning the study. SN and EP organized the data collection. SN drafted the article, and EP, ASC and BS read and approved the final version of the manuscript.
Contributor Information
Steven Nordin, Email: steven.nordin@psy.umu.se.
Eva Palmquist, Email: eva.palmquist@psy.umu.se.
Anna-Sara Claeson, Email: anna-sara.claeson@psy.umu.se.
Berndt Stenberg, Email: berndt.stenberg@vll.se.
Acknowledgements
This study was supported by grants from the European territorial cooperation program Botnia-Atlantica, Region Västerbotten (Sweden), and the Regional Council of Ostrobothnia (Finland). We gratefully acknowledge Annika Glader for supervising the TEMA project of which this work was part.
References
- International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz) Health Phys. 1998;74:494–522. [PubMed] [Google Scholar]
- Rubin GJ, Rosa Nieto-Hernandez R, Wessely S. Idiopathic environmental intolerance attributed to electromagnetic fields (formerly ‘electromagnetic hypersensitivity’): an updated systematic review of provocation studies. Bioelectromagnetics. 2010;31:1–11. doi: 10.1002/bem.20536. [DOI] [PubMed] [Google Scholar]
- Szemerszky R, Köteles F, Lihi R, Bárdos G. Polluted places or polluted minds? An experimental sham-exposure study on background psychological factors of symptom formation in ‘Idiophatic Environmental Intolerance attributed to electromagnetic fields’. Int J Hyg Environ Health. 2010;213:387–394. doi: 10.1016/j.ijheh.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Witthöft M, Rubin GJ. Are media warnings about the adverse health effects of modern life self-fulfilling? An experimental study on idiopathic environmental intolerance attributed to electromagnetic fields (IEI-EMF) J Psychosom Res. 2013;74:206–212. doi: 10.1016/j.jpsychores.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Labarge AS, McCaffrey RJ. Multiple chemical sensitivity: a review of the theoretical and research literature. Neuropsychological Review. 2010;10:183–211. doi: 10.1023/a:1026460726965. [DOI] [PubMed] [Google Scholar]
- Hodgson MJ, Addorisio MR. In: Textbook of Clinical Occupational and Environmental Medicine. 2. Rosenstock L, Cullen MR, Brodkin CA, Redlich CA, editor. Philadelphia: Elsevier Saunders; 2005. Exposures in indoor environments; pp. 1133–1142. [Google Scholar]
- Genuis SJ, Lipp CT. Electromagnetic hypersensitivity: fact or fiction? Sci Total Environ. 2012;414:103–112. doi: 10.1016/j.scitotenv.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Baguley DM. Hyperacusis. J Royal Soc Med. 2003;96:582–585. doi: 10.1258/jrsm.96.12.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin S, Söderholm A, Palmquist E, Andersson L, Claeson A-S, Nordin M. In: Byggnadsrelaterad ohälsa i Kvarkenregionen - nio delprojekt om miljökänslighet, luftkvalitet och sjuka hus ur ett tvärvetenskapligt perspektiv hälsa. Osterberg M, editor. Vasa: Novia produktion och publikation; 2012. Miljökänslighet: den osynliga folksjukdomen. Ett detektivarbete kring orsakerna till miljörelaterad överkänslighet; pp. 30–43. Series R: Report no. [Google Scholar]
- Andersson MJE, Andersson L, Bende M, Millqvist E, Nordin S. The idiopathic environmental intolerance symptom inventory: development, evaluation and application. J Occup Environ Med. 2009;51:838–847. doi: 10.1097/JOM.0b013e3181a7f021. [DOI] [PubMed] [Google Scholar]
- Edvardsson B, Stenberg B, Bergdahl J, Eriksson N, Lindén G, Widman L. Medical and social prognoses of non-specific building-related symptoms (Sick Building Syndrome): a follow-up study of patients previously referred to hospital. Int Arch Occup Environ Health. 2008;81:805–812. doi: 10.1007/s00420-007-0267-z. [DOI] [PubMed] [Google Scholar]
- Hillert L, Berglind N, Arnetz BB, Bellander T. Prevalence of self-reported hypersensitivity to electric or magnetic fields in a population-based questionnaire survey. Scand J Work Environ Health. 2002;28:33–41. doi: 10.5271/sjweh.644. [DOI] [PubMed] [Google Scholar]
- Andersson G, Lindvall N, Hursti T, Carlbring P. Hypersensitvity to sound (hyperacusis): a prevalence study conducted via the internet and post. Int J Audiol. 2002;41:545–54. doi: 10.3109/14992020209056075. [DOI] [PubMed] [Google Scholar]
- Bergdahl J, Stenberg B, Eriksson N, Lindén G, Widman L. Coping and self-image in patients with visual display terminal-related skin symptoms and perceived hypersensitivity to electricity. Int Arch Occup Environ Health. 2004;77:538–542. doi: 10.1007/s00420-004-0546-x. [DOI] [PubMed] [Google Scholar]
- Johansson A, Nordin S, Heiden M, Sandström M. Symptoms, personality traits, and stress in people with mobile phone-related symptoms and electromagnetic hypersensitivity. J Psychosom Res. 2010;68:37–45. doi: 10.1016/j.jpsychores.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Lipson JG. Multiple chemical sensitivities: stigma and social experiences. Med Anthropol. 2004;18:200–213. doi: 10.1525/maq.2004.18.2.200. [DOI] [PubMed] [Google Scholar]
- Larsson C, Mårtensson L. Experiences of problems in individuals with hypersensitivity to odours and chemicals. J Clin Nurs. 2009;18:737–744. doi: 10.1111/j.1365-2702.2008.02599.x. [DOI] [PubMed] [Google Scholar]
- Skovbjerg S, Brorson S, Rasmussen A, Johansen JD, Elberling J. Impact of self-reported multiple chemical sensitivity on everyday life: a qualitative study. Scand J Publ Health. 2009;37:621–626. doi: 10.1177/1403494809105430. [DOI] [PubMed] [Google Scholar]
- Gibson PR. Of the world but not in it: barriers to community access and education for persons with environmental sensitivities. Health Care Women Int. 2010;31:3–16. doi: 10.1080/07399330903042823. [DOI] [PubMed] [Google Scholar]
- Söderholm A, Söderberg A, Nordin S. The experience of living with sensory hyperreactivity: accessibility, financial security and social relationships. Health Care Women Int. 2011;32:686–707. doi: 10.1080/07399332.2011.585727. [DOI] [PubMed] [Google Scholar]
- Shepherd D, Welch D, Dirks KN, Mathews R. Exploring the relationship between noise sensitivity, annoyance and health-related quality of life in a sample of adults exposed to environmental noise. Int J Environ Res Publ Health. 2010;7:3579–3594. doi: 10.3390/ijerph7103580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M, Andersson L, Nordin S. Coping strategies, social support and responsibility in chemical intolerance. J Clin Nurs. 2010;19:2162–2173. doi: 10.1111/j.1365-2702.2010.03264.x. [DOI] [PubMed] [Google Scholar]
- Stenberg B, Bergdahl J, Edvardsson B, Eriksson N, Lindén G, Widman L. Medical and social prognosis for patients with perceived hypersensitivity to electricity and skin symptoms related to the use of visual display terminals. Scand J Work Environ Health. 2002;28:349–357. doi: 10.5271/sjweh.685. [DOI] [PubMed] [Google Scholar]
- Millqvist E. Mechanisms of increased airway sensitivity to occupational chemicals and odors. Curr Opin Allergy Clin Immunol. 2008;8:135–139. doi: 10.1097/ACI.0b013e3282f647ec. [DOI] [PubMed] [Google Scholar]
- Fiedler N, Kelly-McNeil K, Ohman-Strickland P, Zhang J, Ottenweller J, Kipen HM. Negative affect and chemical intolerance as risk factors for building-related symptoms: a controlled exposure study. Psychosom Med. 2008;70:254–262. doi: 10.1097/PSY.0b013e31816074f4. [DOI] [PubMed] [Google Scholar]
- Miller CS, Prihoda TJ. The environmental exposure and sensitivity inventory (EESI): a standardized approach for measuring chemical intolerances for research and clinical applications. Toxicol Ind Health. 1999;15:370–385. doi: 10.1177/074823379901500311. [DOI] [PubMed] [Google Scholar]
- Andersson K. Epidemiological approach to indoor air problems. Indoor Air. 1998;4(Suppl):32–39. [Google Scholar]
- Carlsson F, Karlsson B, Orbaek P, Österberg K, Östergren PO. Prevalance of annoyance attributed to electrical equipment and smells in a Swedish population, and relationsship with subjective health and daily function. Public Health. 2005;119:568–577. doi: 10.1016/j.puhe.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Statistics Sweden. Tables of Sweden’s population 2009: 1.3.1 Population by sex, age, marital status by county Dec. 31, 2009 according to the administrative subdivisions of January 1, 2010. 2013. http://www.scb.se/statistik/_publikationer/BE0101_2009A01_BR_05_BE0110TAB.pdf.
- World Health Organization (WHO) Indoor air quality and research. Euro Reports and Studies 103. Geneva: WHO; 1986. [Google Scholar]
- Röösli M, Moser M, Baldinini Y, Meier M, Braun-Fahrländer C. Symptoms of ill health ascribed to electromagnetic field exposure: a questionnaire survey. Int J Hyg Environ Health. 2004;207:141–150. doi: 10.1078/1438-4639-00269. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Electromagnetic fields and public health. Fact sheet No 296. Geneva: WHO Media Centre; 2005. [Google Scholar]
- Schwarz N. Self-reports: how the questions shape the answers. Am Psychol. 1999;54:93–105. [Google Scholar]
- Eriksson NM, Stenberg BGT. Baseline prevalence of symptoms related to indoor environment. Scand J Publ Health. 2006;34:387–396. doi: 10.1080/14034940500228281. [DOI] [PubMed] [Google Scholar]
- Cattell RB. The scree test for the number of factors. Multivar Behav Res. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Höfler M, Pfister H, Lieb R, Wittchen H-U. The use of weights to account for non-response and drop-out. Soc Psychiatry Psychiatr Epidemiol. 2004;40:291–299. doi: 10.1007/s00127-005-0882-5. [DOI] [PubMed] [Google Scholar]
- Miller C, Mitzel HC. Chemical sensitivity attributed to pesticide exposure versus remodeling. Arch Environ Health. 1995;50:119–129. doi: 10.1080/00039896.1995.9940889. [DOI] [PubMed] [Google Scholar]
- Anastasia A. Psychological testing. New York: Macmillan; 1988. [Google Scholar]
- Proposition 2007/08:44: Vissa etikprövningsfrågor m.m. http://www.regeringen.se/content/1/c6/09/48/06/b497e80c.pdf.
- Groves RM, Couper MP, Presser S, Singer E, Tourangeau R, Acosta GP, Nelson L. Experiments in producing nonresponse bias. Publ Opin Quart. 2006;70:720–736. [Google Scholar]
- Andersson K, Norlén U. In: Indoor air quality in practice: moisture and cold climate solutions. Flatheim G, Berg K, Edvardsen K, editor. Oslo: Norwegian Society of Chartered Engineers; 1995. Indoor climate and health effects: The indoor climate in the Swedish housing stock; pp. 23–28. [Google Scholar]
- Gijsbergs van Wijk CMT, Kolk AM. Sex differences in physical symptoms: the contribution of symptom perception theory. Soc Sci Med. 1977;45:231–246. doi: 10.1016/s0277-9536(96)00340-1. [DOI] [PubMed] [Google Scholar]
- Nordin S, Lidén E, Gidlöf-Gunnarsson A. Development and evaluation of a category ratio scale with semantic descriptors: the environmental annoyance scale. Scand J Psychol. 2009;50:93–100. doi: 10.1111/j.1467-9450.2008.00692.x. [DOI] [PubMed] [Google Scholar]