Abstract
Study Objectives:
This double-blind study evaluated the efficacy and safety of modafinil for treating excessive daytime sleepiness in Japanese patients with obstructive sleep apnea syndrome (OSAS).
Methods:
Patients with residual excessive sleepiness (Epworth Sleepiness Scale [ESS] ≥ 11) on optimal nasal continuous positive airway pressure (nCPAP) therapy (apnea-hypopnea index ≤ 10) were randomized to either 200 mg modafinil (n = 52) or placebo (n = 62) once daily for 4 weeks. Outcomes included baseline-week 4 changes in ESS total score, sleep latency on maintenance of wakefulness test (SL-MWT), nocturnal polysomnography, Pittsburgh Sleep Quality Index (PSQI), and safety.
Results:
All 114 randomized patients completed the study. Mean change in ESS total score (-6.6 vs -2.4, p < 0.001) and SL-MWT (+2.8 vs -0.4 minutes, p = 0.009) were significantly greater with modafinil than with placebo. ESS total score decreased from > 11 to < 11 at the final assessment in 69.2% of modafinil-treated patients and 30.6% of placebo-treated patients (p < 0.001). Corresponding rates at week 1 were 57.7% and 33.9% (p = 0.014). Changes in nocturnal polysomnography, PSQI, and apnea-hypopnea index from baseline to the final assessment were similar in both groups. Adverse drug reactions occurred in 36.5% and 22.6% of patients in the modafinil and placebo groups, respectively (p = 0.146).
Conclusions:
Once-daily modafinil was effective and well tolerated for managing residual daytime sleepiness in Japanese OSAS patients with residual excessive daytime sleepiness on optimal nCPAP therapy.
Clinical Trial Registration:
JapicCTI-No.090777
Citation:
Inoue Y; Takasaki Y; Yamashiro Y. Efficacy and safety of adjunctive modafinil treatment on residual excessive daytime sleepiness among nasal continuous positive airway pressure-treated Japanese patients with obstructive sleep apnea syndrome: a double-blind placebo-controlled study. J Clin Sleep Med 2013;9(8):751-757.
Keywords: Randomized clinical trial, daytime sleepiness, Epworth Sleepiness Scale, modafinil, nasal continuous positive airway pressure, maintenance of wakefulness test, obstructive sleep apnea, safety
Obstructive sleep apnea syndrome (OSAS) is a chronic condition characterized by recurrent episodes of upper airway collapse that occur during sleep. OSAS frequently causes nocturnal intermittent hypoxemia, sympathetic activation and fragmented/disrupted sleep.1 Studies of Caucasian and Asian populations have consistently estimated that the prevalence of OSAS associated with excessive daytime sleepiness ranges from 3% to 7% in adult men and from 2% to 5% in adult women.2 In Japan, Nakaya-Ashida et al.3 reported that the prevalence of moderate to severe sleep disordered breathing (respiratory disturbance index ≥ 15) was 22.3% in male workers aged 23-59 years.
Factors predisposing to OSAS include obesity, advanced age, male sex, and craniofacial abnormalities.4,5 The diagnosis of OSAS generally requires objective measurement of obstructive respiratory events and the presence of characteristic symptoms, such as excessive daytime sleepiness and unrestored nocturnal sleep that could not be better explained by other factors.6 Severe OSAS is often associated with vascular morbidities,7,8 cognitive impairment, occupational and vehicular accidents attributable to excessive daytime sleepiness, and worse quality of life than unaffected individuals.9
BRIEF SUMMARY
Current knowledge/Study Rationale: Very few studies, and none in Asian patients, have examined the effects of modafinil on subjective or objective sleep measures in patients with residual sleepiness on optimal nCPAP.
Study Impact: Treatment with 200 mg modafinil once daily improved residual daytime sleepiness in Japanese patients with OSAS on optimal nCPAP compared with placebo.
Management of OSAS requires the use of nasal continuous positive airway pressure (nCPAP) therapy, a first -line treatment, which acts as a pneumatic splint to maintain patency of the upper airway. nCPAP therapy is widely accepted to reduce excessive sleepiness and to improve daytime functioning and self-reported health status.10–12 However, despite the reported improvements of respiratory events, clinically significant excessive sleepiness persists in some patients on optimal nCPAP. In some of these patients, the residual sleepiness may reflect the presence of other sleep disorders, including narcolepsy, behaviorally induced sleep insufficiency syndrome and periodic limb movement disorders.13 In other patients, this outcome may be caused by hypoxia-induced cerebral metabolic changes.14 In a recent study in France, 6.0% (95% confidence interval [CI] 3.9-8.0) of OSAS patients who were optimally treated with nCPAP had evidence of residual excessive sleepiness.15 Considering the potential adverse outcomes that may affect the health and safety of the patients, residual sleepiness requires prompt attention.
The Standards of Practice Committee of the American Academy of Sleep Medicine recommends use of the wake-promoting agent modafinil in nCPAP-treated patients without other identifiable causes for their residual sleepiness.16 Modafinil differs from other amphetamine-like wake-promoting agents, such as methamphetamine and methylphenidate, in its chemical structure and mechanisms of action.17–20 Modafinil mainly interacts with the dopamine transporter,21,22 and affects the γ-amino butyric acid (GABA)-ergic, serotonergic, glutaminergic, noradrenergic, and histaminergic neurotransmitter systems,22–26 which may contribute to its wake-promoting activity. Double-blind placebo-controlled clinical studies on nCPAP-treated patients with residual sleepiness associated with OSAS have revealed that modafinil significantly improved objectively determined sleep latency, overall subjective severity of sleepiness, health-related quality of life, and functional status, and that it was well tolerated.27–29 To date, however, no studies have examined the effects of modafinil on residual excessive sleepiness in Japanese patients with OSAS on optimal nCPAP treatment. Furthermore, although central nervous system stimulants may theoretically disturb nocturnal sleep,30 previous studies have not documented the effects of modafinil on subjective or objective nocturnal sleep measures.
Therefore, in the present study, we evaluated the effects of modafinil on the efficacy and safety of modafinil in Japanese patients with OSAS and excessive daytime sleepiness despite optimal therapeutic use of nCPAP. We also examined the effects of modafinil on subjective and objective measures of nocturnal sleep in these patients.
METHODS
Study Design
This randomized, double-blind, placebo-controlled, parallel-group study was conducted at 37 sites specialized in sleep disorders in Japan between May 2009 and December 2009. The study included a screening visit, an observation period ≥ 15 days, and a 4-week double-blind treatment period. The protocol and the informed consent form were reviewed and approved by the internal review board at each institution. All patients provided written informed consent to participate in this study.
Patients
Patients evaluated in this study were required to be receiving effective nCPAP therapy to rule out inadequate or incorrect nCPAP use as a cause of their residual sleepiness. Patients with sleep disorders other than OSAS were excluded from the study. The main inclusion criteria for eligible patients in this study were as follows: men and women aged 20-70 years; confirmed diagnosis of OSAS; and the presence of subjective excessive sleepiness (i.e., Epworth Sleepiness Scale [ESS] total score ≥ 11)31 despite optimal use of nCPAP; having received nCPAP therapy for ≥ 3 months and being willing and able to continue its use during the study period; the use of nCPAP for ≥ 70% of nights for ≥ 4 h/night32 for 14 days before the baseline visit; and an apnea-hypopnea index (AHI) ≤ 10 determined by nocturnal polysomnography (PSG) during the observation period. Definitive diagnosis of OSAS or other sleep disorders was made using PSG data obtained before randomization based on Rechtschaffen and Kales criteria33 and American Sleep Disorders Association arousal criteria.34 The data were scored according to American Association of Sleep Medicine criteria.35 Patients who met any of the following criteria were excluded from this study: diagnosis of other sleep disorders (e.g., narcolepsy, periodic limb movement disorders, and central sleep apnea); pregnant, potentially pregnant, or lactating women; presence of arrhythmias, angina, and clinically significant cardiac, respiratory, cardiovascular diseases, psychiatric disorders (e.g., depression36), or hypertension with systolic blood pressure ≥ 160 mm Hg and diastolic blood pressure ≥ 100 mm Hg,37 as specified in the exclusion criteria of the U.S. OSAS study.29 Patients who were concomitantly administered prohibited drugs, such as central nervous system stimulants, sedative medications, antidepressants, antiepileptic drugs, acetazolamide, warfarin, monoamine oxidase inhibitors, or antimigraine drugs, within 2 weeks before the start of the study, were also excluded. Patients fulfilling these criteria were identified by the physicians and invited to participate in the study at the physician's request.
Randomization and Dosing
Patients were randomly assigned in a blocked randomization manner to receive 2 tablets of 100 mg modafinil (total dose, 200 mg/day) or placebo once daily in the morning, to be administered before or after meals. Randomization was performed using a computer-generated random number list prepared by an independent contract research organization. Clinicians contacted the organization via telephone to obtain the randomization sequence for each patient.
Efficacy Measures
Efficacy assessments were conducted at the start (i.e., baseline) and at Weeks 1 and 4 of the double-blind treatment period. The primary efficacy measure was ESS score at Week 4 of treatment. The secondary efficacy measure was mean sleep latency on the maintenance of wakefulness test (MWT).38,39 The MWT was conducted in a subset of patients (modafinil, n = 22; placebo, n = 28) at baseline and at Week 4 of the double-blind period on the days immediately after nocturnal PSG. Each MWT session lasted 20 min. Because of the methodology, the MWT was only performed at study sites with the facilities required to conduct the test. Some patients at these facilities were unable to do the MWT because of the burden associated with the test. Other secondary variables were ESS score at each visit, and the total score of Japanese version of Pittsburgh Sleep Quality Index (PSQI),40 which represents the severity of subjective sleep disturbance, and sleep parameters measured by nocturnal PSG at baseline and Week 4. PSG and MWT were conducted in an inpatient setting.
Safety
Safety was assessed by evaluating adverse drug reactions (ADRs) as well as the results of general laboratory tests (blood and urine), physiological variables (blood pressure and pulse rate), 12-lead electrocardiograms, and physical examinations. ADRs were defined as any unfavorable or unintended symptom or disease that was considered to be associated with the study drug during the study period.
Statistical Analysis
Continuous demographic variables were compared using the 2-sample t-test. Categorical variables were compared using the Fisher exact test. The efficacy population (n = 114) was defined as patients who received ≥ 1 dose of modafinil and underwent ≥ 1 postbaseline evaluation for any efficacy or safety variable during the treatment period. The changes in efficacy variables (ESS and MWT) from baseline to the final assessment (Week 4) were compared between the modafinil and placebo groups using analysis of covariance with the baseline value as a covariate. To verify the efficacy of modafinil administration, the point estimate and 2-sided 95% (CI) of the difference between the modafinil and placebo groups were calculated using the least squares mean (LS mean) method. Statistical tests were performed at a significance level of 5% using SAS System (Release 9.1.3, SAS Institute Inc., Cary, NC, USA). Changes in other secondary variables (PSQI and nocturnal PSG) from baseline were also compared between the modafinil- and placebo-treated groups. Safety data are summarized using descriptive statistics.
RESULTS
Subjects
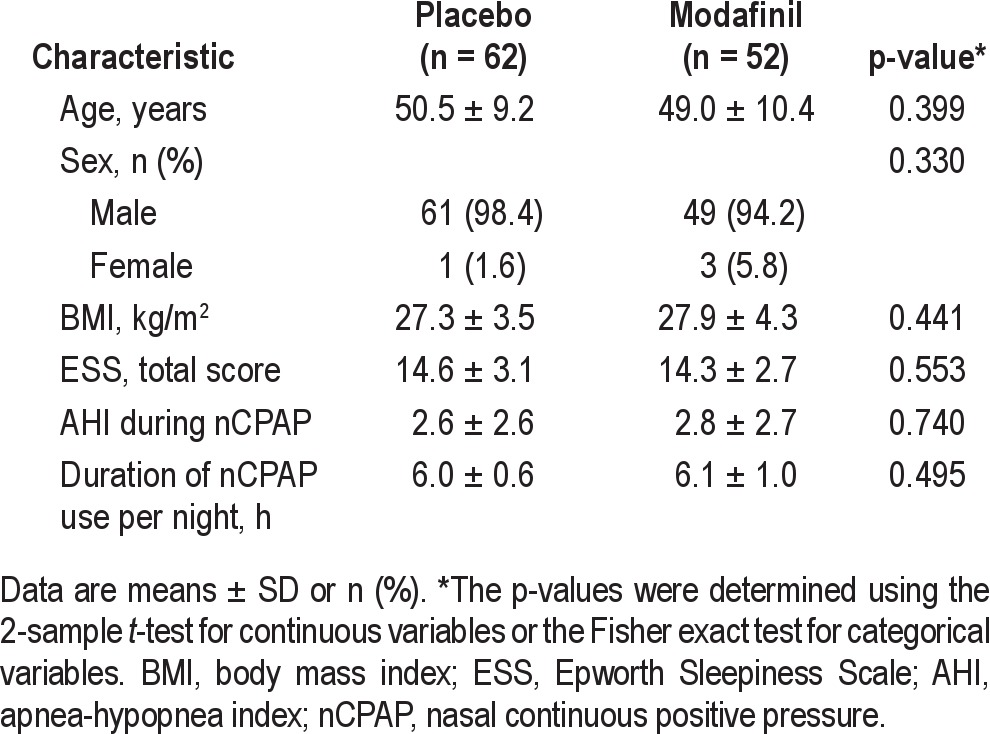
A total of 114 patients were randomized—52 patients to modafinil and 62 patients to placebo. All 114 patients completed the study (Figure 1). There were no differences between the 2 groups in terms of demographic and baseline characteristics (Table 1). Males accounted for > 94% of the patients in both groups. Before starting treatment with the study drug, the patients in both groups had moderate levels of residual sleepiness, with mean total ESS scores ≥ 14 despite effective nC-PAP therapy; mean AHI was ≤ 10 in both groups. The mean duration of nCPAP use per night was 6.1 ± 1.0 and 6.0 ± 0.6 h in the modafinil and placebo groups, respectively (Table 1). Concomitant diseases included hypertension (modafinil, n = 13 [25%]; placebo, n = 20 [32%]) and hyperlipidemia (modafinil, n = 4 [8%]; placebo, n = 14 [23%]).
Figure 1. Patient disposition.
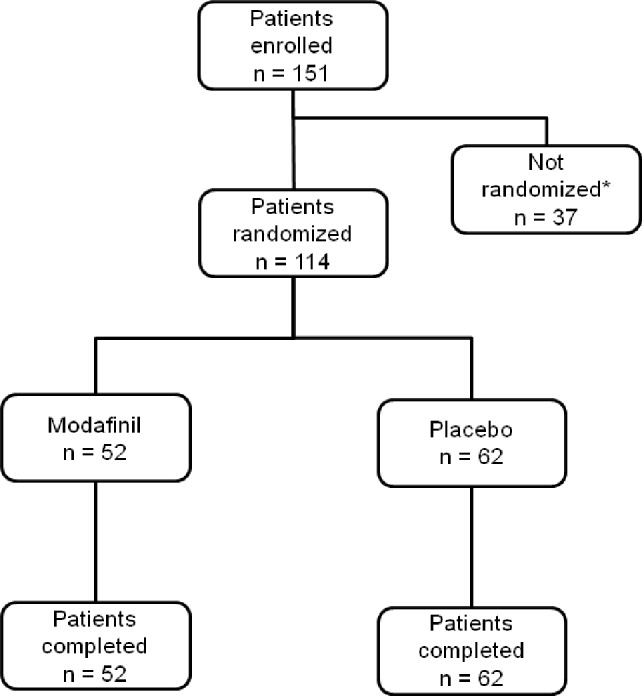
*37 patients were not randomized to the study for the following reasons: 35 patients did not meet the eligibility criteria, one patient withdrew consent, and one patient gave up on the starting dose because of changes in work schedule.
Table 1.
Patient characteristics
Subjective Sleepiness
Mean ESS total scores were determined at baseline and at the final assessment in both groups. The mean changes in ESS total score from baseline to the final assessment were -6.61 in the modafinil group and -2.44 in the placebo group (LS mean). The between-group difference of -4.17 (95% CI -5.66 to -2.69) was therefore significantly greater with modafinil than with placebo (p < 0.001). The change in mean ESS total score at 1 week after starting treatment was also significantly greater in the modafinil group than in the placebo group (p < 0.001; Figure 2).
Figure 2. Mean Epworth Sleepiness Scale total score at baseline, and after 1 and 4 weeks of treatment with modafinil or placebo.
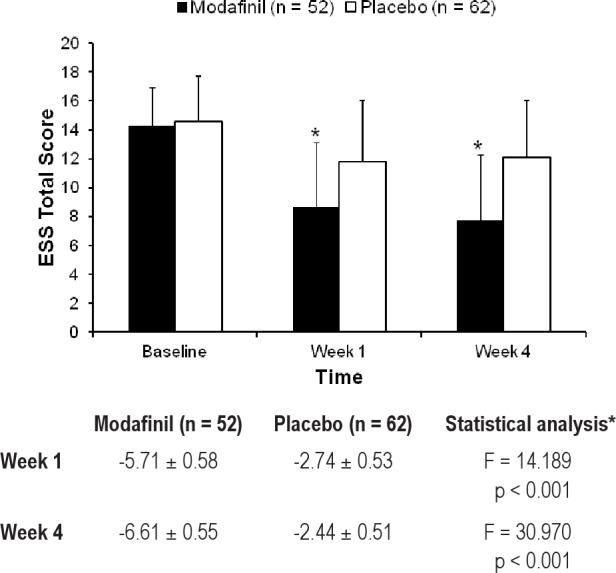
The least significant mean change from baseline ± standard error is shown in the table. *The pvalues were determined by analysis of covariance.
The patients whose ESS total scores were ≥ 11 at baseline and decreased to < 11 at the final assessment were defined as responders with normalization of ESS. Overall, 69.2% of patients (36/52) treated with modafinil and 30.6% of patients (19/62) treated with placebo were classified as responders. The Fisher exact test showed that a significantly higher percentage of patients treated with modafinil were classified as responders compared with patients treated with placebo (p < 0.001). The corresponding response rates at week 1 were 57.7% (30/52) and 33.9% (21/62) (p = 0.014).
Objective Sleepiness
Fifty patients (modafinil, n = 22; placebo, n = 28) underwent the MWT. There were no differences in patient characteristics, including baseline ESS total score, between patients who did or did not undergo the MWT (p = 0.292). Mean sleep latencies determined by MWT at baseline and at the final assessment in both groups are shown in Figure 3. The LS mean change in MWT sleep latency from baseline to the final assessment was 2.8 min in the modafinil group and -0.40 min in the placebo group. The between-group difference of 3.2 min (95% CI 0.8 to 5.6) was statistically significant, showing greater effects of modafinil versus placebo (p = 0.009).
Figure 3. Mean maintenance of wakefulness test sleep latency at baseline and after 4 weeks of treatment with modafinil or placebo.
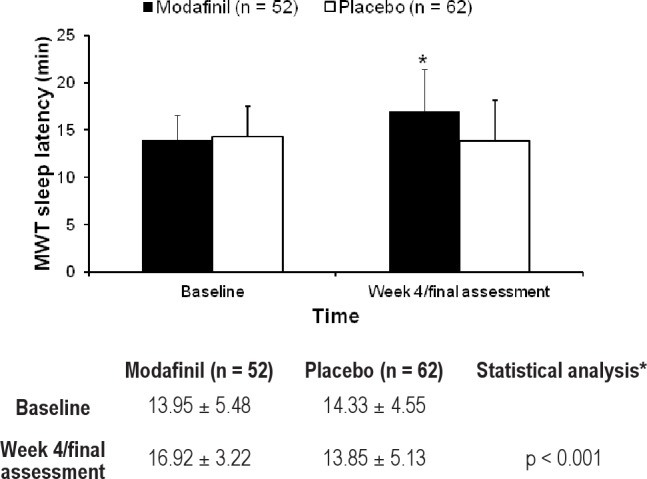
*The p-value was determined by analysis of covariance.
Objective and Subjective Measures of Nocturnal Sleep
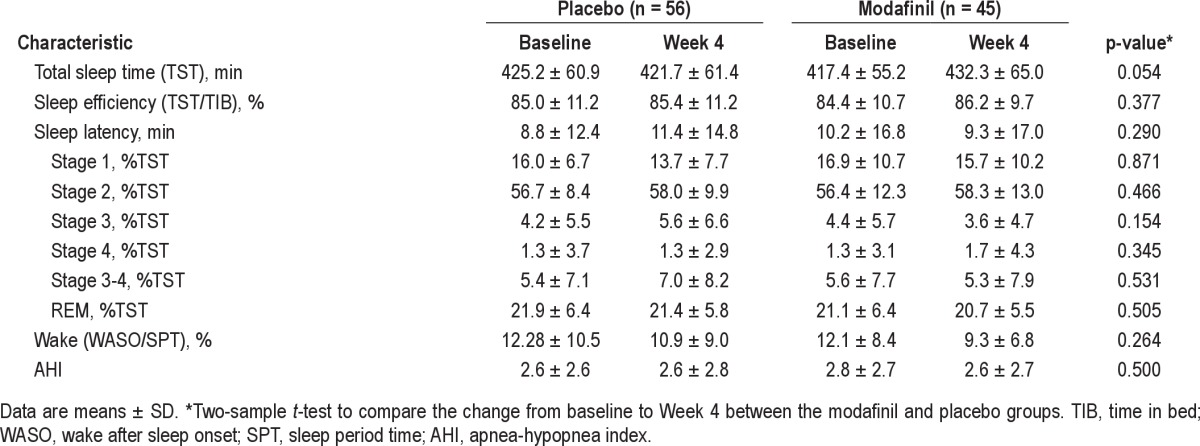
Summary statistics for sleep parameters were determined in 101 patients who underwent nocturnal PSG at baseline and at the final assessment (modafinil, n = 45; placebo, n = 56). As shown in Table 2, there were no significant differences in the changes in any nocturnal PSG parameters between the 2 groups.
Table 2.
Comparison of nocturnal PSG indices at baseline and after 4 weeks of treatment with modafinil and placebo
The total PSQI score decreased from 6.3 ± 2.7 at baseline to 4.8 ± 2.2 at the final assessment in the modafinil group, as compared with a change from 6.1 ± 2.3 to 5.4 ± 1.7 in the placebo group. The mean difference in total PSQI score between the 2 groups for the change from baseline to the final assessment was -0.7 points (95% CI: -1.5 to 0.0 points) and was not statistically significant.
Safety Outcomes
ADRs were reported by 19 patients (36.5%) in the modafinil group and 14 patients (22.6%) in the placebo group. There were no significant differences in the rate of ADRs between the 2 groups (p = 0.146; Fisher exact test). The most frequent ADRs in the modafinil group were headache (n = 6, 11.5%), insomnia (n = 2, 3.8%), and palpitation (n = 2, 3.8%). The most frequent ADRs in the placebo group were headache (n = 4, 6.5%) and upper abdominal pain (n = 2, 3.5%) (Table 3). All of these ADRs were mild or moderate in severity, and no deaths or other serious adverse events were reported. None of the patients withdrew from the study because of ADRs.
Table 3.
Adverse events occurring in two or more patients in either treatment group

Regarding the time of onset of ADRs, the frequency of ADRs was greatest within 7 days after starting treatment in both the modafinil group (15/19 patients who experienced ADRs) and the placebo group (10/14 patients).
Laboratory test abnormalities included increased γ-glutamyl transpeptidase in one patient in each group; increased alkaline phosphatase in one patient in each group; increased alanine aminotransferase and increased thyroid stimulating hormone in one patient each in the modafinil group; the presence of urinary glucose and decreased white blood cell count in one patient each treated with placebo; and multiple liver enzyme abnormalities (increased aspartate aminotransferase, alanine aminotransferase, and γ-glutamyl transpeptidase) in one patient treated with placebo. Decreased body weight (from 77.0 kg at baseline to 73.0 kg at week 4 of the treatment period) was observed in one patient treated with modafinil. Sinus tachycardia (at Week 1 of the treatment period) and sinus bradycardia (at Week 4 of the treatment period) were observed in one patient each in the modafinil group. Ventricular extrasystole (at Week 1 of the treatment period) was observed in one patient treated with placebo. No clinically relevant abnormalities were observed in the other variables, including laboratory tests, blood pressure, pulse rate, body weight, or electrocardiogram.
DISCUSSION
This study was the first Asian study to investigate the efficacy of modafinil for treating residual sleepiness in patients with nCPAP-treated OSAS using both subjective and objective measures. In this study, residual sleepiness was defined as excessive daytime sleepiness in patients who were compliant with OSAS treatment but had subjective sleepiness without any other identifiable cause of sleepiness, applying enrollment criteria identical to those in studies in the United States.27,29 The degree of residual sleepiness at baseline, as represented by the ESS total score, of the patients enrolled in this study, was also comparable to that in the US studies.27,29
The use of the MWT was limited to a subgroup of patients in this study; hence, a subjective measure of sleepiness (the ESS) was used as the primary efficacy parameter. Consequently, in this 4-week study, the improvement in ESS total score was significantly greater in the modafinil group than in the placebo group. Four weeks of treatment with modafinil normalized the ESS total score in approximately two-thirds of the patients. In terms of ESS total score, significant improvements of excessive daytime sleepiness in the modafinil group were observed at the first posttreatment evaluation, i.e., one week after starting treatment, compared with the placebo group. Overall, the mean ESS total scores were normalized (to < 11) within 1 week of modafinil administration in 57.7% of patients, increasing to 69.2% at week 4. Modafinil also improved sleep latency determined by the MWT, representing the patient's ability to maintain wakefulness.
As described above, modafinil exerts its wake-promoting activities by targeting several neurotransmitter systems, rather than a specific molecule or specific neurotransmitter system. Thus, the effects of modafinil on residual sleepiness in OSAS patients are at least partly attributable to its nonspecific pharmacological actions.
The present study evaluated adjunctive oncedaily administration of 200 mg modafinil. Another placebo-controlled study evaluated this dose and a higher dose (400 mg once daily) in a comparable patient population.29 Interestingly, when the efficacy data for the 200 mg doses in both studies were compared, the changes in ESS score from baseline to Week 4 of treatment were -6.52 ± 5.04 (n = 52) and -3.20 ± 4.25 (n = 95) in our study and in the US study,29 respectively. The respective changes in MWT sleep latency were 2.97 ± 5.25 (n = 22) and 1.20 ± 4.33 (n = 84). These data suggest that the response to 200 mg/day modafinil is greater in Japanese patients than in US patients, which may indicate slight differences in pharmacokinetic profiles among different ethnicities, as already reported among other ethnic groups.41,42 Differences in the pharmacokinetic profiles, including the absorption and distribution of modafinil, may also be attributable to the differences in body size between Japanese and US patients, as the mean BMI of patients treated with 200 mg modafinil was 27.9 ± 4.3 kg/m2 in our study versus 36.2 ± 7.6 kg/m2 in the US study.29 Alternatively, excess obesity is known to exacerbate daytime sleepiness,43,44 possibly resulting in more severe symptoms or less apparent improvements in symptoms in US patients than in Japanese patients. Additionally, differences in the timing or content of the morning meal may partly explain the differences in clinical outcomes between these studies. Nevertheless, the precise reasons for this difference between Japanese and US patients are unclear, and this study was conducted to evaluate safety and efficacy in Japanese OSA patients and not to elucidate the difference between US and Japanese patients. However, the trends in positive outcomes for patient-reported sleepiness and objectively determined sleep latency in the present study were similar to those reported in the US study.29
Of note, in this study of Japanese patients, treatment with modafinil did not significantly affect sleep parameters in terms of nocturnal PSG findings or total PSQI score. Therefore, the administration of modafinil in the morning did not seem to adversely affect the structure or quality of nocturnal sleep.
Generally, modafinil was well tolerated. The safety and tolerability findings of the current study are consistent with those of other double-blind placebo-controlled studies.27,29 Headache, insomnia, and palpitation were the most common ADRs in modafinil-treated patients. However, modafinil therapy was not associated with clinically significant changes in blood pressure or heart rate relative to placebo. Furthermore, there were no serious adverse events in either group in this study.
Some limitations of this study should be mentioned. First, most of the patients in this study were male, and there are some differences in the pharmacokinetics of modafinil between males and females.41,42,45 Second, we only included patients with OSAS on nCPAP. The efficacy of modafinil should therefore be evaluated in OSAS patients with residual sleepiness on other treatments.
In conclusion, residual daytime sleepiness was improved in Japanese patients with OSAS treated with 200 mg modafinil once daily. We found significant improvements in ESS total scores at 1 week after starting modafinil that were maintained until the end of the 4-week study. Modafinil may be an effective and well-tolerated adjunct treatment for the chronic management of residual daytime sleepiness in patients with OSAS who experience excessive daytime sleepiness despite regular nCPAP use.
DISCLOSURE STATEMENT
This study was funded by Alfresa Pharma. Dr. Inoue has consulted for Hisamitsu Pharmaceutical Co., Inc. He has also provided expert testimony for Nippon BoehringerIngelheim Co., Ltd.; Philips Respironics GK; Alfresa Pharma Corporation; Takeda Pharmaceutical Company Limited; MSD, K.K.; Pacific Medico Co., Ltd.; Otsuka Pharmaceutical Co., Ltd.; Eisai Co., Ltd.; and Mitsubishi Tanabe Pharma Corporation. He has received lecture fees or service bureau fees from Philips Respironics GK; Takeda Pharmaceutical Corporation; Otsuka Pharmaceutical Co., Ltd.; and Eisai Co., Ltd. Lastly, he has received manuscript preparation fees from Takeda Pharmaceutical Company Limited and Eisai Co., Ltd. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the members of the Modafinil OSAS Study Group in Japan for their help in this study.
ABBREVIATIONS
- ADR
adverse drug reactions
- AHI
apnea-hypopnea index
- CI
confidence interval
- ESS
Epworth Sleepiness Scale
- GABA
γ-aminobutyric acid
- LS
least squares
- nCPAP
nasal continuous positive airway pressure
- OSAS
obstructive sleep apnea syndrome
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- SL-MWT
sleep latency on maintenance of wakefulness test
REFERENCES
- 1.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–53. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakayama-Ashida Y, Takegami M, Chin K, et al. Sleep disordered breathing in the usual lifestyle setting as detected with home monitoring in a population of working men in Japan. Sleep. 2008;31:419–25. doi: 10.1093/sleep/31.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito D, Akashiba T, Yamamoto H, Kosaka N, Horie T. Craniofacial abnormalities in Japanese patients with severe obstructive sleep apnoea syndrome. Respirology. 2001;6:157–61. doi: 10.1046/j.1440-1843.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 6.McNicholas WT. Diagnosis of obstructive sleep apnea in adults. Proc Am Thorac Soc. 2008;5:154–60. doi: 10.1513/pats.200708-118MG. [DOI] [PubMed] [Google Scholar]
- 7.Nieto FJ, Young TB, Lind BK, et al. Association of sleep disordered breathing, sleep apnea and hypertension in a large community-based study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 9.Finn L, Young T, Palta M, Fryback DG. Sleep disordered breathing and self-reported general health status in the Wisconsin Sleep Cohort Study. Sleep. 1998;21:701–6. [PubMed] [Google Scholar]
- 10.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–5. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Long-term benefits in self-reported health status of nasal continuous positive airway pressure therapy for obstructive sleep apnoea. QJM. 2001;94:95–9. doi: 10.1093/qjmed/94.2.95. [DOI] [PubMed] [Google Scholar]
- 12.Hack M, Davies RJ, Mullins R, et al. Randomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnea. Thorax. 2000;55:224–31. doi: 10.1136/thorax.55.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilleminault C, Philip P. Tiredness and somnolence despite initial treatment of obstructive sleep apnea syndrome (what to do when an OSA patient stays hypersomnolent despite treatment) Sleep. 1996;29:S117–22. doi: 10.1093/sleep/19.suppl_9.s117. [DOI] [PubMed] [Google Scholar]
- 14.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- 15.Pépin J-L, Viot-Blanc V, Escourrou P, et al. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J. 2009;33:1062–7. doi: 10.1183/09031936.00016808. [DOI] [PubMed] [Google Scholar]
- 16.Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Standards of Practice Committee American Academy of Sleep Medicine. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29:1031–5. [PubMed] [Google Scholar]
- 17.Duteil J, Rambert F, Pessonnier J, Hermant JF, Gombert R, Assous E. Central α1-adrenergic stimulation in relation to the behavior stimulating effect of modafinil; studies with experimental animals. Eur J Pharmacol. 1990;180:49–58. doi: 10.1016/0014-2999(90)90591-s. [DOI] [PubMed] [Google Scholar]
- 18.Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M. Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res. 1992;591:319–26. doi: 10.1016/0006-8993(92)91713-o. [DOI] [PubMed] [Google Scholar]
- 19.Simon PH, Panissaud CH, Costentin J. The stimulant effect of modafinil on wakefulness is not associated with an increase in anxiety in mice: a comparison with dexamphetamine. Psychopharmacology. 1994;114:597–600. doi: 10.1007/BF02244990. [DOI] [PubMed] [Google Scholar]
- 20.Shelton J, Nishino S, Vaught J, Dement WC, Mignot E. Comparative effects of modafinil and amphetamine on daytime sleepiness and cataplexy of narcoleptic dogs. Sleep. 1995;18:817–26. [PubMed] [Google Scholar]
- 21.Mignot E, Nishino S, Guilleminault C, Dement WC. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17:436–7. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- 22.Madras BK, Xie Z, Lin Z, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–9. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- 23.Tanganelli S, Fuxe K, Ferraro L, Janson AM, Bianchi C. Inhibitory effects of the psychoactive drug modafinil on gamma-aminobutyric acid outflow from the cerebral cortex of the awake freely moving guineapig. Possible involvement of 5-hydroxytryptamine mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:461–5. doi: 10.1007/BF00176625. [DOI] [PubMed] [Google Scholar]
- 24.Ferraro L, Tanganelli S, O'Connor WT, Antonelli T, Rambert F, Fuxe K. The vigilance promoting drug modafinil decreases GABA release in the medial preoptic area and in the posterior hypothalamus of the awake rat: possible involvement of the serotonergic 5-HT3 receptor. Neurosci Lett. 1996;220:5–8. doi: 10.1016/s0304-3940(96)13212-2. [DOI] [PubMed] [Google Scholar]
- 25.Ferraro L, Antonelli, Tanganelli S, et al. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention: by local GABAA receptor blockade. Neuropsychopharmacology. 1999;20:346–56. doi: 10.1016/S0893-133X(98)00085-2. [DOI] [PubMed] [Google Scholar]
- 26.Ishizuka T, Sakamoto Y, Sakurai T. Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci Lett. 2003;339:143–6. doi: 10.1016/s0304-3940(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 27.Pack AI, Black JE, Schwartz JRL, Matheson JK. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164:1675–81. doi: 10.1164/ajrccm.164.9.2103032. [DOI] [PubMed] [Google Scholar]
- 28.Dinges DF, Weaver TE. Effects of modafinil on sustained attention performance and quality of life in OSA patients with residual sleepiness while being treated with nCPAP. Sleep Med. 2003;4:393–402. doi: 10.1016/s1389-9457(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 29.Black JE, Hirshkowitz M. Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep. 2005;28:464–71. doi: 10.1093/sleep/28.4.464. [DOI] [PubMed] [Google Scholar]
- 30.Nishino S, Okura M, Mignot E. Narcolepsy; Genetic predisposition and neuro-pharmacological mechanisms. Sleep Med Rev. 2000;4:57–99. doi: 10.1053/smrv.1999.0069. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 32.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 33.Rechtschaffen A, Kales A. Manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Washington, DC: US Government Printing Office; 1968. [Google Scholar]
- 34.Atlas Task Force of the American Sleep Disorders Association. EEG Arousals: scoring rules and examples. Sleep. 1992;15:174–84. [PubMed] [Google Scholar]
- 35.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 36.Koutsourelakis I, Perraki E, Bonakis A, Vagiakis E, Roussos C, Zakynthinos S. Determinants of subjective sleepiness in suspected obstructive sleep apnoea. J Sleep Res. 2008;17:437–43. doi: 10.1111/j.1365-2869.2008.00663.x. [DOI] [PubMed] [Google Scholar]
- 37.National Heart Lung and Blood Institute, National Institutes of Health. What is high blood pressure? Available at: http://www.nhlbi.nih.gov/health/health-topics/topics/hbp/
- 38.Mitler MM, Gujvarty KS, Browman CP. Maintenance of wakefulness test: a polysomnographic technique for evaluating treatment efficacy in patients with excessive somnolence. Electroencephalogr Clin Neurophysiol. 1982;53:658–61. doi: 10.1016/0013-4694(82)90142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poceta JS, Timms RM, Jeong DU, Ho SL, Erman MK, Mitler MM. Maintenance of wakefulness test in obstructive sleep apnea syndrome. Chest. 1992;101:893–7. doi: 10.1378/chest.101.4.893. [DOI] [PubMed] [Google Scholar]
- 40.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 41.Guo T, Zhao LS, Xia DY. Pharmacokinetic study of modafinil in relation to gender and ethnicity in healthy young Chinese volunteers. J Pharm Pharm Sci. 2010;13:443–9. doi: 10.18433/j3fk5r. [DOI] [PubMed] [Google Scholar]
- 42.Tao G, Longshan Z, Kehua W, et al. Population pharmacokinetics of modafinil in Chinese Han, Mongolian, Korean, Uygur, and Hui healthy subjects determined by nonlinear mixed-effects modeling. Ther Drug Monit. 2010;32:189–93. doi: 10.1097/FTD.0b013e3181cf27d3. [DOI] [PubMed] [Google Scholar]
- 43.Bixler EO, Vgontzas AN, Lin HM, et al. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–5. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 44.Vgontzas AN, Bixler EO, Tan TL, et al. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. 1998;158:1333–7. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 45.Wong YN, King SP, Simcoe D, et al. Open-label, single-dose pharmacokinetic study of modafinil tablets: influence of age and gender in normal subjects. J Clin Pharmacol. 1999;39:281–8. [PubMed] [Google Scholar]


