Abstract
Objectives:
We aimed to characterize the association between jaw muscle contractions and respiratory events in patients with obstructive sleep apnea syndrome (OSAS) and to investigate the responsiveness of the contractions to respiratory events in comparison with that of leg muscles in terms of arousal types and sleep states.
Methods:
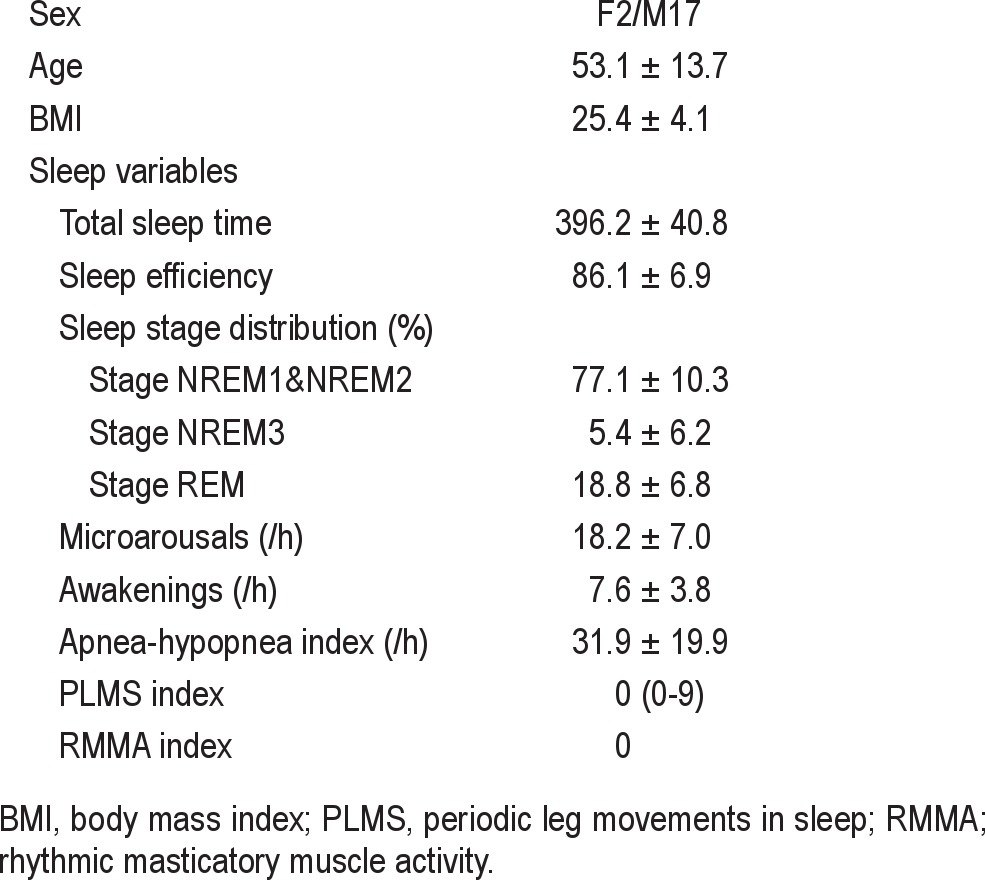
Polysomnographic (PSG) recordings were performed in 19 OSAS patients (F/M: 2/17; 53.1 ± 13.7 years; AHI: 31.8 ± 19.9/h) with no concomitant sleep bruxism or other sleep-related movement disorders. Muscle contractions of unilateral masseter (MAS) and anterior tibialis (AT) muscles were scored during sleep in association with graded arousals (microarousals and awakenings) related or unrelated to apneahypopnea events.
Results:
Arousals were scored for 68.2% and 52.3% of respiratory events during light NREM and REM sleep, respectively. Respiratory events with arousals were associated with longer event duration and/or larger transient oxygen desaturation than those without (ANOVAs: p < 0.05). Median response rates of MAS events to respiratory events were 32.1% and 18.9% during NREM and REM sleep. During two sleep states, MAS muscle was rarely activated after respiratory events without arousals, while its response rate increased significantly in association with the duration of arousals (Friedman tests: p < 0.001). A similar response pattern was found for AT muscle. Motor responsiveness of the two muscles to arousals after respiratory events did not differ from responsiveness to spontaneous arousals in two sleep stages.
Conclusion:
In patients with OSAS, the contractions of MAS and AT muscles after respiratory events can be nonspecific motor phenomena, dependent on the duration of arousals rather than the occurrence of respiratory events.
Citation:
Kato T; Katase T; Yamashita S; Sugita H; Muraki H; Mikami A; Okura M; Ohi M; Masuda Y; Taniguchi M. Responsiveness of jaw motor activation to arousals during sleep in patients with obstructive sleep apnea syndrome. J Clin Sleep Med 2013;9(8):759-765.
Keywords: masseter muscle, leg movements, arousal response, motor responsiveness, obstructive sleep apnea
Previous studies have shown that muscle contractions in the respiratory-related orofacial muscles (e.g., tongue) often occur after apnea and hypopnea events during sleep in patients with OSAS. Among the orofacial muscles, the jawclosing masseter (MAS) muscle is also activated in association with apnea and hypopnea events,1–5 and jaw closures from the jaw opening posture are often observed at the termination of the respiratory events.6,7 Since the MAS muscle is known to produce a large biting force during sleep and wakefulness,8–10 its contractions can produce a mechanical load on orodental structures. In fact, approximately 10% of OSAS patients are aware of jaw motor activity during sleep.11 This awareness is associated with jaw symptoms.11 An oral appliance is often used to manage OSAS, since it increases or maintains the upper airway space by holding the mandible and/or tongue in a forward position. OSAS patients often encounter jaw symptoms (pain/discomfort) during an initial period following the commencement of oral appliance use.12–15 In addition, longterm use of an oral appliance is associated with dentoskeletal changes,13 suggesting that oral appliances can impose a certain level of mechanical load on orodental structures. Thus the occurrence of MAS contractions may be a significant physiological factor as well as a source of additional mechanical load on orodental structures in cases where OSAS is managed with an oral appliance.
BRIEF SUMMARY
Current knowledge/Study Rationale: This study was aimed to investigate whether jaw motor events following respiratory events are specifically associated with respiratory disturbance in OSAS patients without sleep related movement disorders. We evaluate the responsiveness of jaw and leg muscles to arousals related or unrelated to respiratory events.
Study Impact: The contractions of jaw and leg muscles are nonspecific motor phenomena in response to arousals rather than respiratory events. Jaw muscle contractions can remain to occur in OSAS patients using an oral appliance. Nonspecific motor events can be a confounding factor for assessing motor events and associated clinical symptoms in patients with frequent arousals such as OSAS.
Although it has been proposed that MAS contractions play a supporting role in reinstating the compromised upper airway,16 physiological understanding of MAS contractions in patients with OSAS is limited. MAS contractions are usually associated with the termination of apneahypopnea events, while MAS contractions do not always occur after respiratory events in patients with OSAS.1–5 Previous studies have reported that MAS contractions after respiratory events occur in association with an increase in heart rate, which is a typical sign of arousal response,2,3 suggesting that the arousals associated with respiratory events are an underlying condition for MAS contractions in association with respiratory events. In patients with OSAS, varying degrees of arousal events were found to occur in relation to respiratory events.17–21 As there is a hierarchy of arousal responses,22 motor activation is more frequently observed in response to a higher level of arousals.14,23 However, the association between MAS contractions and the level of arousals after respiratory events has not been investigated in OSAS patients. In addition, in patients with OSAS, some arousals can be associated with respiratory events, while other arousals are not related to respiratory events. It is not known whether MAS is more responsive to arousals related to or unrelated to respiratory events. It also remains to be demonstrated whether the motor responsiveness of the jaw muscles is compatible with that of other muscles in the body.
The aims of this study are to characterize the association between MAS contractions and respiratory events in patients with OSAS and to investigate the responsiveness of MAS contractions to respiratory events in comparison with that of leg muscles in terms of arousal types and sleep states.
METHODS
Subjects
Fifty-four patients (F10:M44; Age: 51.9 ± 15.1 years) had been newly referred to Osaka Kaisei Hospital Sleep Medicine Center with suspected sleep apnea. They were first interviewed by sleep physicians (MO, MO, and MT) and were suspected of having OSAS based on a history of excessive daytime sleepiness, sleep disturbances, snoring, and witnessed respiratory pauses in sleep. They did not have a history of pulmonary, heart, liver or kidney disease, stroke, or other neurological disorders, and did not take sleeping pills. All patients were enrolled for a routine PSG examination. On the night of PSG recording, these patients gave written informed consent to participate in the study. They were asked to fill out a self-administered questionnaire on sleep disturbance and orofacial symptoms. After evaluating PSG data, we excluded the data of 35 patients from the final analysis for the following reasons: (1) 17 patients were aware of signs symptoms of sleep bruxism (tooth grinding during sleep and morning jaw symptoms) according to the self-administered questionnaires; (2) 4 patients did not fill out the questionnaires; (3) 4 patients with AHI index < 5 /h; (4) 9 patients had periodic leg movements in sleep (PLMS) with PLMS index > 10 /h, and (5) one patient was suspected of having an REM sleep behavior disorder. Finally, data from 19 patients (F2:M17; age: 53.1 ± 13.7 years) were used for the analysis. We also confirmed that none of these patients exhibited tooth grinding and rhythmic masticatory muscle activity (RMMA) during sleep. The study was approved by the institutional review board at the Osaka Kaisei Hospital, Osaka, Japan.
Polysomnographic Evaluations
PSG consisted of continuous recordings from 6 electroencephalographic (EEG) leads (F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1), 2 electrooculographic leads (ROC-M2, LOC-M2), 5 electromyographic leads (2 submental and bilateral anterior tibialis [AT] and unilateral MAS muscles), nasal cannula with a pressure transducer and thermal sensor for nasal airflow, strain gauges for thoracic and abdominal movements, pulse oximetry, and electrocardiography. Simultaneous audiovideo recording was made. Subjects went to bed at their usual bedtime or before 23:30, and the recording was terminated after 6:30.
Sleep stages were scored for 30-sec epochs according to the international standard criteria.24 Apnea and hypopnea were defined following the rules of the American Academy of Sleep Medicine.24 An apnea was defined as ≥ 90% reduction in airflow for ≥ 10 sec; hypopnea as ≥ 50% reduction of airflow for ≥ 10 sec associated with arousals or with ≥ 3% reduction in oxygen saturation. Transient arousals such as microarousals ([mAR] 3-15 sec) and awakenings ([AW] > 15 sec) were scored according to the criteria.20,24,25 Arousals were scored as respiratory related when they were synchronous with the termination of the respiratory event, while those without a preceding respiratory event were scored as spontaneous arousals. To confirm that patients had no concomitant sleep related movement disorders in the jaw and leg muscles, RMMA and periodic leg movements were scored according to the criteria for sleep bruxism and PLMS.24,26,27
The following sleep and respiratory variables were calculated: total sleep time (TST), total recording time (TRT), sleep efficiency (SE), sleep latency, percentage of sleep stages, and the frequency of transient events per hour of sleep (e.g., awakenings, microarousals, PLMS, RMMA, and respiratory events [apneas and hypopneas]). The duration of apneahypopnea events was measured, and the oxygen desaturation in relation to respiratory events was calculated as the difference between the oxygen saturation (SpO2) before apnea- hypopnea events and minimum SpO2 after the events. Table 1 shows the demographic and PSG data in 19 patients. Patients had little sleep Stage NREM3. Only 3 patients had respiratory events (9-26) during stage NREM3, and 6 had only one event. Since the number of respiratory events was too low to allow us to analyze the data, the data on Stage NREM3 were excluded from the following analyses.
Table 1.
Demographic and polysomnographic data in 19 patients
Motor Events
Motor activations in MAS and AT muscles were scored when the EMG level was ≥ 10% of the maximum voluntary contraction (MVC) before sleep. Minimum duration criteria were 0.25 sec for MAS events and 0.5 sec for AT events.24,27 A motor event occurring within 10 sec after the termination of a respiratory event was considered to be apneahypopnea related in case that arousal was not scored after a respiratory event. Motor response rates for the 2 muscles were calculated for the respiratory events without arousals (no arousal: NA) and with mAR and AW as well as spontaneous mAR and AW. Since 2 muscles were occasionally activated in response to a single respiratory event or spontaneous arousal, the number of muscles activated was counted as 2 (both MAS and AT muscles), 1 (either muscle), or 0 (neither muscle was activated), to assess the coincidence that motor events of the 2 muscles responded to the same arousals.
Statistical Analysis
Data are presented as mean ± SD or median (range). For pairwise comparisons, paired t tests and Wilcoxon tests were used appropriately. The duration of respiratory events and decrease in SpO2 were compared between arousal levels by repeated measure ANOVAs with post hoc paired t-tests. The response rate of motor activity to respiratory events was compared between arousal levels using Friedman tests with post hoc Wilcoxon tests for each muscle in either NREM or REM sleep. For these tests, the level of statistical significance was set at p < 0.05. Response rates to arousals were compared between respiratory related and spontaneous arousals by Wilcoxon tests for each muscle in either NREM or REM sleep. Due to multiple comparisons, the level of statistical significance was set at p < 0.0125. We used SYSTAT 11 for Windows (SYSTAT software Inc., USA) for statistical analyses.
RESULTS
Respiratory Events in NREM and REM Sleep
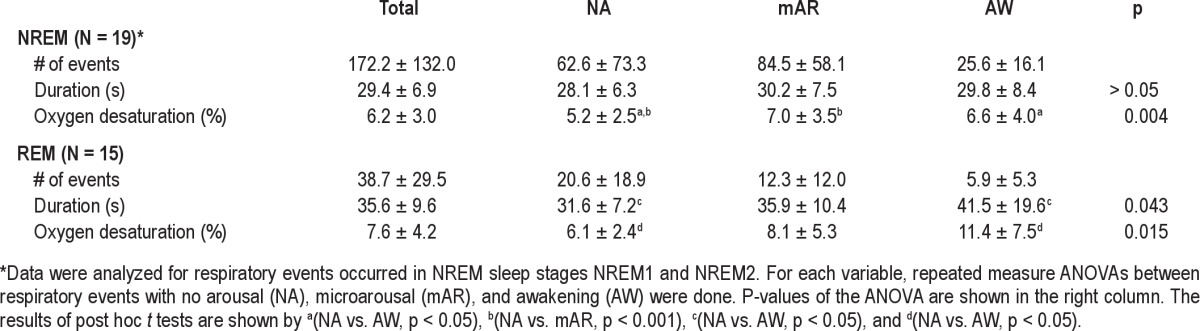
The majority of apneahypopnea events (204.2 ± 140.3 events) were scored in NREM sleep (84.4% ± 13.2% in NREM sleep and 15.6% ± 13.2% in REM sleep, N = 19). In NREM sleep, twothirds of respiratory events (68.2% ± 15.5%) were followed by transient arousals, while half of the respiratory episodes were associated with arousals during REM sleep (52.3% ± 24.1%); transient arousals were scored more frequently after respiratory events in NREM sleep than in REM sleep (paired t-test: p < 0.01).
The following variables were analyzed for NREM sleep in 19 patients and for REM sleep in 15 patients, since either or both types of transient arousals were not scored after respiratory events. In NREM sleep, duration of respiratory events did not differ among 3 types of respiratory events (e.g., nonarousal [NA] events, events with mAR and AW; Table 2). In REM sleep, the duration of respiratory events differed among the 3 types of respiratory events (ANOVA: p < 0.05). The duration of respiratory events with AW was significantly elongated compared to NA events (post hoc paired t test: p < 0.05; Table 2). The decrease in oxygen saturation was significantly different among the 3 types of respiratory events in NREM sleep (ANO-VA: p < 0.01) and in REM sleep (p < 0.05). In NREM sleep, it was significantly lower for NA events than for events with mAR (Table 2, post hoc paired t-test: p < 0.001) and those with AW (p < 0.05). In REM sleep, the decrease in oxygen saturation was significantly larger for the event with AW than for NA events (Table 2, post hoc paired t-test: p < 0.05).
Table 2.
Duration and oxygen desaturation for three types of respiratory events
Motor Activations in Relation to Arousals after Respiratory Events
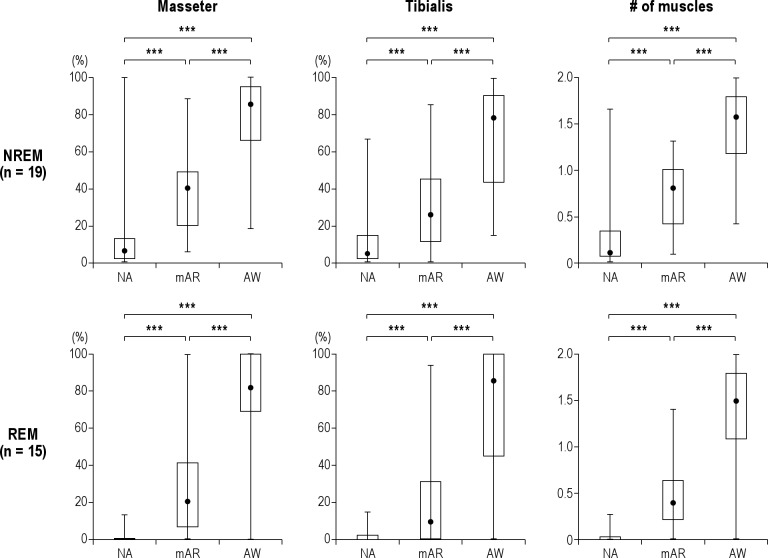
In NREM sleep, 32.1% (7.2-88.9) of respiratory events were followed by MAS contractions. The response rate of MAS contractions to respiratory events differed significantly between NA events and those with mAR and AW (Figure 1, Friedman test: p < 0.001). The contractions were found to occur increasingly from 6.1% (0-100) to 40.0% (5.1-88.6) and 85.4% (18.2-100) as the duration of post-respiratory arousals became elevated from NA to AW (post hoc Wilcoxon tests: p < 0.001 [N = 19]). AT muscle was activated after 21.7% (4.2-82.0) of respiratory events during NREM sleep. A significant difference in the responsiveness of AT muscle was found between arousal levels (Friedman test: p < 0.001): AT muscle was activated more frequently when respiratory events were followed by a higher intensity of arousals in NREM sleep (NA: 4.2% [0-66.7]; mAR: 25.5% [0-85]; AW: 78.3% [14.3-100]) (Figure 1, post hoc Wilcoxon tests: p < 0.001). The number of muscles activated following a respiratory event increased from 0.3 (0 -1.7) to 0.8 (0.1-1.3) and 1.6 (0.4-2.0) as arousal response to respiratory events became more intense in NREM sleep (Figure 1, Friedman test: p < 0.001, post hoc Wilcoxon tests: p < 0.001).
Figure 1. Responsiveness of masseter and anterior tibialis muscles to three types of respiratory events during NREM and REM.
During NREM and REM sleep, masseter (left column) and anterior tibialis (middle column) muscles were activated most frequently when awakenings (AW) occurred after respiratory events; they were activated least frequently when no EEG arousals (NA) occurred (Friedmann tests: p < 0.001). Right column shows that the number of muscles that are activated after respiratory events increased significantly during NREM and REM sleep as the arousal levels were elevated (Friedmann tests: p < 0.001). mAR, microarousal. ***p < 0.001 for post hoc Wilcoxon tests. The data are presented by median (minimum - maximum). Please see the results of statistical analysis in the text.
In REM sleep, respiratory events (18.9% [2.4-57.1]; N = 15) were less frequently associated with MAS contractions than in NREM sleep. However, a similar significant difference between the arousal levels was found during REM sleep (Figure 1, Friedman test: p < 0.001): MAS activations became more frequent in association with arousal levels (NA: 0% [0-12.7]; MA: 20.0% [0-100]; AW: 81.8% [0-100]; post hoc Wilcoxon tests: p < 0.001 [N = 15]). AT muscle contraction was scored in 12.5% (0-75.7) of respiratory events. Similar to MAS muscle, the response rate of AT muscle was significantly associated with arousal levels (Figure 1, Friedman test: p < 0.001): the response of AT muscles increased significantly from NA (0% [0-14.5]) to mAR (8.7% [0-94.1]) and AW (85.7% [0-100]) (post hoc Wilcoxon tests: p < 0.001 [N = 15]). The two muscles were activated more frequently with arousal intensity (0.0 [0-0.3], 0.4 [0-1.4], and 1.5 [0-2.0]) (Figure 1, Friedman test: p < 0.001 [N = 15]).
Motor Responsiveness between Spontaneous and Respiratory Arousals
In 19 patients, 36.5 ± 19.3 spontaneous arousals did not follow respiratory events. Of these, the majority (87.5% ± 9.0%) occurred during NREM sleep.
In NREM sleep, the response of MAS activation was significantly increased from spontaneous mAR (29.4% [4.2-92.3]) to AW (75.0% [25.0-100]) (Wilcoxon test: p < 0.001). Similarly, response rate of AT muscles were higher for AW (60.0% [9.5-100]) than for mAR (22.7% [0-87.5]) in NREM sleep (Wilcoxon test: p < 0.001). The number of muscles activated in response to a single spontaneous arousal was higher for AW (1.5 [0.5-2.0]) than for mAR (0.6 [0.1-1.3]) (Wilcoxon test: p < 0.001). The above variables did not differ between spontaneous and respiratory-related arousals for NREM sleep (Table 3).
Table 3.
Motor responsiveness to arousals in respiratory and spontaneous arousals
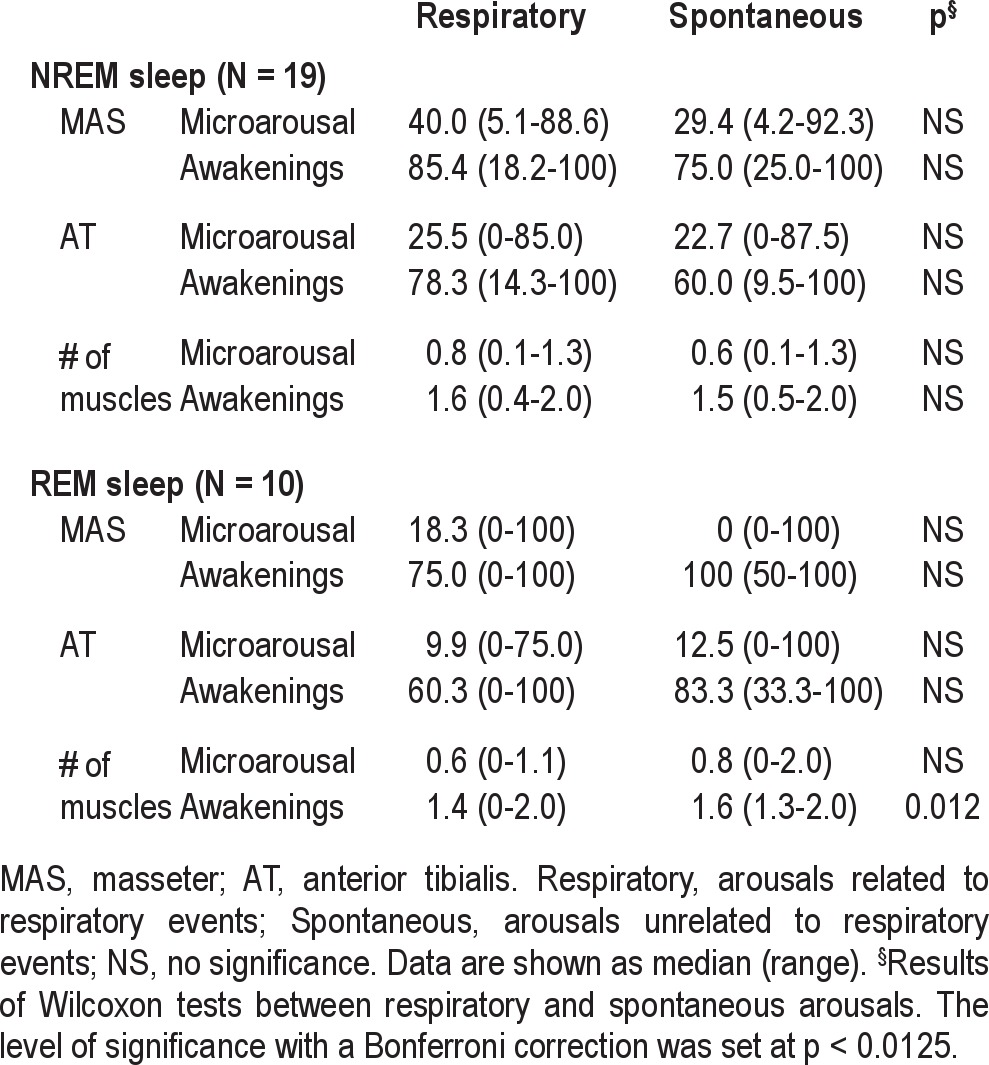
Due to less frequent arousals in REM sleep than NREM sleep, motor responses to both spontaneous mAR and AW could be assessed in 10 patients only. Similar to NREM sleep, motor responsiveness to spontaneous arousals showed a significant arousal-dependent increase in REM sleep for MAS (mAR: 0% [0- 100]; AW: 100% [50 -100], Wilcoxon test: p < 0.01), while a trend of increase was found for anterior tibialis muscles (mAR: 12.5% [0-100]; AW: 83.3% [33.3-100], Wilcoxon tests: p = 0.035; Table 3). The number of muscles activated in response to a single spontaneous arousal was higher for AW (1.6 [1.3-2.0]) than for mAR (0.8 [0-2.0]) (Wilcoxon test: p < 0.001). As to the comparison between arousal types in REM sleep, the two muscles were not more responsive to spontaneous than to respiratory-related arousals (Table 3). The number of muscles activated was higher for spontaneous AW (1.6 [1.3-2.0]) than for respiratory-related AW (1.4 [0-2.0]; p < 0.0125), while no difference was noted for the response to mAR (Table 3).
DISCUSSION
The results show that jawclosing muscles are increasingly activated in relation to the duration of arousals after respiratory events during NREM and REM sleep in patients with OSAS. The responsiveness of jaw muscles to arousals after respiratory events did not differ from that following spontaneous arousals. Moreover, the above pattern of motor response did not differ between the jaw and leg muscles. These findings suggest that, in patients with OSAS, MAS contractions after respiratory events are a nonspecific motor manifestation of the arousal response, dependent on the intensity of arousals.
Previous studies have reported that the MAS muscle was occasionally activated after respiratory events; 14.4% to 54.2% of respiratory events were followed by MAS contractions that exceeded to 20% to 40% of MVC of the MAS muscle regardless of sleep stages.3–5 In this study, 32.1% of respiratory events during NREM sleep and 18.9% during REM sleep were associated with MAS activations that exceeded to 10% MVC. Moreover, the majority of MAS activations occurred more frequently after respiratory events with arousal response than those without, as previous studies have also reported for the MAS and other craniofacial muscles.2–4,23 Although previous studies have shown that cardiac arousals without visually detectable EEG changes have been detected after respiratory events,18,21,23,28 these respiratory events were found to be rarely associated with MAS contractions. This is consistent with the findings of the recent studies in which respiratory efforts and electromyography of the tongue muscles were assessed in relation to respiratory events.23,29,30 However, when transient arousals with visually identifiable EEG changes (e.g., mAR, AW) occurred after respiratory events, the MAS was activated. This result supports a previous suggestion of an association between MAS and arousal response.31 In addition, the probability of the activation was elevated, as the duration of arousals increased from mAR to AW. These results suggest that, in patients with OSAS, the responsiveness of MAS contractions after respiratory events is dependent on the intensity of arousal responses, a finding consistent with results obtained in healthy subjects and patients with sleep disorders.3,22,31
MAS muscles were activated more frequently after respiratory events during NREM sleep than during REM sleep in the present study and in previous studies.1,4 This suggests that the threshold for MAS activation is lower during NREM sleep than during REM sleep due to the presence of active inhibition during the latter sleep state.31 In addition, arousal threshold to respiratory disturbance was higher during REM sleep than NREM sleep since a respiratory event of longer duration and larger amplitude of oxygen desaturation are needed to trigger arousals in REM sleep.17–19,21,29,32,33 In this study, submental muscle activation was needed for scoring arousals during REM sleep,25 indicating that the motor system is released from active inhibition during arousals scored. Under such conditions, MAS muscles are activated more frequently in relation to an increase in the arousal duration. This explains why the frequency of MAS contractions after respiratory events was lower during REM sleep than during NREM sleep but the responsiveness to arousals was in a similar range for the two sleep stages.
It has been reported that respiratory effort at the end of a respiratory event is a key factor for inducing arousals and activating respiratory muscles.29,32 However, arousal-dependent MAS contractions are not explained solely by the association between the degree of respiratory efforts in relation to respiratory events, since the motor responsiveness of MAS muscle to mAR and AW did not differ between arousals with and without preceding respiratory events. Since arousals during sleep are not always coupled with respiratory events in patients with OSAS,18,23,28 the lack of difference in motor responsiveness between respiratory-related and spontaneous arousals, suggests that MAS contraction after a respiratory event is dependent on the arousal response rather than respiratory events per se.
Motor responses in both jaw and leg muscles after respiratory events were similarly correlated with an intensity of arousal response. This suggests that the physiological nature of muscle contractions in patients with OSAS is not specific to jawclosing muscle. However, the two muscles are not always activated coincidently after the same respiratory event when mAR was scored. An increase in arousal duration was associated with the elevation of the probability that the two muscles would be activated. This finding is supported by the previous studies showing that, as arousal intensity increases, facilitatory influences spread over more muscles in the body.31,34
There are several limits to interpreting the results of this study. First, respiratory events, transient arousals and motor activity were assessed using visual scoring, according to the criteria used in the clinical setting and previous studies. With visual scoring, a slight increase in muscle tone was not detected in response to arousals.5,31 In addition, subtle EEG changes that can not be detected by visual scoring can be associated with MAS contractions after respiratory events without arousals in this study. Since we could not assess respiratory efforts by measuring esophageal pressure,35 we could not demonstrate how MAS contraction is linked to respiratory efforts when it occurred after respiratory events.36 The present study did not assess all factors influencing the characteristics of apnea/hypopnea events and arousal responses (e.g., age, body position, circadian rhythm, and upper airway resistance).17–19,21,32,37 Although these factors are also associated with the a severity of OSAS patients symptoms, the present study was performed using a small study sample that included mild to severe OSAS. A large inter-individual difference in arousal threshold and motor responsiveness can be associated with the severity of OSAS. We did not demonstrate whether the responsiveness of MAS contractions was modified by the severity of OSAS: we neither recorded control subjects nor assessed the effects of clinical interventions (e.g., CPAP) on the motor responsiveness.38 Obviously, all the above issues need to be assessed in the future studies.
In conclusion, MAS contraction is an orofacial manifestation of a general motor reaction to arousal occurring during sleep in patients with OSAS. Such nonspecific motor activations contribute secondarily to restoration of a compromised upper airway during respiratory events in collaboration with the activation of respiratory-related muscles in patients with OSAS.16,23,29 On the other hand, the results also show that a certain number of MAS contractions can occur in OSAS patients who have neither clinical signs of sleep bruxism nor PSG findings of RMMA. The chance of MAS contractions may be elevated in response to respiratory events and arousals when sleep structure is fragmented by transient arousals in OSAS patients. A recent study showed that an oral appliance cannot reduce some type of MAS contractions (e.g., tonic activation).39 Thus, nonspecific activations of MAS muscle would not disappear even when oral appliance therapy was successfully controlling respiratory events; they occur as an intrinsic response to arousals in OSAS patients. As well, intrinsic response to arousals can be found in the AT muscle in OSAS patients who did not exhibit PLMS. However, such nonspecific activations in jaw and leg muscles would be a confounding factor in scoring sleep bruxism and PLMS and interpreting their impacts of the commorbidity on clinical symptoms (e.g., orofacial pain, sleepiness) in patients with OSAS as well as vairous sleep disorders.24,40
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Taniguchi has received speaking fees from Sanofiaventis K.K, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Company Limited, Eisai Co., Ltd., MSD K.K., and a manuscript fee from Teijin Limited. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by the intramural research funding from Matsumoto Dental University. This work was performed at Osaka Kaisei Hospital (PSG recordings and sleep analysis) and at Matsumoto Dental University and Osaka University (EMG scoring and data analysis). We also thank physicians and technicians at Osaka Kaisei Hospital Sleep Medical Center for generously supporting this project, and Dr. Alice Petersen for editing the manuscript.
REFERENCES
- 1.Inoko Y, Shimizu K, Morita O, Kohno M. Relationship between masseter muscle activity and sleep-disordered breathing. Sleep Biol Rhythms. 2004;2:67–8. [Google Scholar]
- 2.Okeson JP, Phillips BA, Berry DT, Cook YR, Cabelka JF. Nocturnal bruxing events in subjects with sleep-disordered breathing and control subjects. J Craniomandib Disord. 1991;5:258–64. [PubMed] [Google Scholar]
- 3.Phillips BA, Okeson J, Paesani D, Gilmore R. Effect of sleep position on sleep apnea and parafunctional activity. Chest. 1986;90:424–9. doi: 10.1378/chest.90.3.424. [DOI] [PubMed] [Google Scholar]
- 4.Sjöholm TT, Lowe AA, Miyamoto K, Fleetham JA, Ryan CF. Sleep bruxism in patients with sleep-disordered breathing. Arch Oral Biol. 2000;45:889–96. doi: 10.1016/s0003-9969(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida K. A polysomnographic study on masticatory and tongue muscle activity during obstructive and central sleep apnea. J Oral Rehabil. 1998;25:603–9. doi: 10.1046/j.1365-2842.1998.00290.x. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto K, Ozbek MM, Lowe AA, et al. Mandibular posture during sleep in patients with obstructive sleep apnoea. Arch Oral Biol. 1999;44:657–64. doi: 10.1016/s0003-9969(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 7.Tsuda H, Lowe AA, Chen H, Fleetham JA, Ayas NT, Almeida FR. The relationship between mouth opening and sleep stage-related sleep disordered breathing. J Clin Sleep Med. 2011;7:181–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke NG, Townsend GC. Distribution of nocturnal bruxing patterns in man. J Oral Rehabil. 1984;11:529–34. doi: 10.1111/j.1365-2842.1984.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 9.Nishigawa K, Bando E, Nakano M. Quantitative study of bite force during sleep associated bruxism. J Oral Rehabil. 2001;28:485–91. doi: 10.1046/j.1365-2842.2001.00692.x. [DOI] [PubMed] [Google Scholar]
- 10.van der Bilt A. Assessment of mastication with implications for oral rehabilitation: a review. J Oral Rehabil. 2011;38:754–80. doi: 10.1111/j.1365-2842.2010.02197.x. [DOI] [PubMed] [Google Scholar]
- 11.Kato T, Mikami A, Sugita H, et al. Negative association between self-reported jaw symptoms and apnea-hypopnea index in patients with symptoms of obstructive sleep apnea syndrome: a pilot study. Sleep Breath. 2013;17:373–9. doi: 10.1007/s11325-012-0704-4. [DOI] [PubMed] [Google Scholar]
- 12.de Almeida FR, Lowe AA, Tsuiki S, et al. Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome. J Clin Sleep Med. 2005;1:143–52. [PubMed] [Google Scholar]
- 13.Hoffstein V. Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath. 2007;11:1–22. doi: 10.1007/s11325-006-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato T. Sleep bruxism and its relation to obstructive sleep apnea–hypopnea syndrome. Sleep Biol Rhythms. 2004;2:1–15. [Google Scholar]
- 15.Tsuda H, Almeida FR, Masumi SI, Lowe AA. Side effects of boil and bite type oral appliance therapy in sleep apnea patients. Sleep Breath. 2010;14:227–32. doi: 10.1007/s11325-009-0304-0. [DOI] [PubMed] [Google Scholar]
- 16.Hollowell DE, Suratt PM. Mandible position and activation of submental and masseter muscles during sleep. J Appl Physiol. 1991;71:2267–73. doi: 10.1152/jappl.1991.71.6.2267. [DOI] [PubMed] [Google Scholar]
- 17.Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest. 1985;87:432–6. doi: 10.1378/chest.87.4.432. [DOI] [PubMed] [Google Scholar]
- 18.Noda A, Yasuma F, Okada T, Koike Y, Nakashima N, Yokota M. Age related differences in electroencephalographic and cardiac arousal at the termination of sleep apnea/hypopnea. Intern Med. 2000;39:375–80. doi: 10.2169/internalmedicine.39.375. [DOI] [PubMed] [Google Scholar]
- 19.Oksenberg A, Khamaysi I, Silverberg DS, Tarasiuk A. Association of body position with severity of apneic events in patients with severe nonpositional obstructive sleep apnea. Chest. 2000;118:1018–24. doi: 10.1378/chest.118.4.1018. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz DJ, Moxley P. On the potential clinical relevance of the length of arousals from sleep in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2:175–80. [PubMed] [Google Scholar]
- 21.Dingli K, Fietze I, Assimakopoulos T, Quispe-Bravo S, Witt C, Douglas NJ. Arousability in sleep apnoea/hypopnoea syndrome patients. Eur Respir J. 2002;20:733–40. doi: 10.1183/09031936.02.00262002. [DOI] [PubMed] [Google Scholar]
- 22.Halasz P, Terzano M, Parrino L, Bodizs R. The nature of arousal in sleep. J Sleep Res. 2004;13:1–23. doi: 10.1111/j.1365-2869.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- 23.Jordan AS, Eckert DJ, Wellman A, Trinder JA, Malhotra A, White DP. Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am J Respir Crit Care Med. 2011;184:1183–91. doi: 10.1164/rccm.201106-0975OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 25.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 26.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 27.Kato T, Lavigne GJ. Sleep bruxism: A sleep related movement disorder. Sleep Med Clin. 2010;5:9–35. [Google Scholar]
- 28.Rees K, Spence DP, Earis JE, Calverley PM. Arousal responses from apneic events during non-rapid-eye-movement sleep. Am J Respir Crit Care Med. 1995;152:1016–21. doi: 10.1164/ajrccm.152.3.7663777. [DOI] [PubMed] [Google Scholar]
- 29.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–75. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 30.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 31.Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13:229–38. doi: 10.1111/j.1365-2869.2004.00409.x. [DOI] [PubMed] [Google Scholar]
- 32.Krieger J, Sforza E, Boudewijns A, Zamagni M, Petiau C. Respiratory effort during obstructive sleep apnea: role of age and sleep state. Chest. 1997;112:875–84. doi: 10.1378/chest.112.4.875. [DOI] [PubMed] [Google Scholar]
- 33.Muraki M, Kitaguchi S, Ichihashi H, et al. Apnoea-hypopnoea index during rapid eye movement and non-rapid eye movement sleep in obstructive sleep apnoea. J Int Med Res. 2008;36:906–13. doi: 10.1177/147323000803600506. [DOI] [PubMed] [Google Scholar]
- 34.Kato T, Masuda Y, Kanayama H, Nakamura N, Yoshida A, Morimoto T. Heteroge-neous activity level of jaw-closing and -opening muscles and its association with arousal levels during sleep in the guinea pig. Am J Physiol Regul Integr Comp Physiol. 2010;298:R34–42. doi: 10.1152/ajpregu.00205.2009. [DOI] [PubMed] [Google Scholar]
- 35.Vincken W, Guilleminault C, Silvestri L, Cosio M, Grassino A. Inspiratory muscle activity as a trigger causing the airways to open in obstructive sleep apnea. Am Rev Respir Dis. 1987;135:372–7. doi: 10.1164/arrd.1987.135.2.372. [DOI] [PubMed] [Google Scholar]
- 36.Kimoff RJ, Cheong TH, Olha AE, et al. Mechanisms of apnea termination in obstructive sleep apnea. Role of chemoreceptor and mechanoreceptor stimuli. Am J Respir Crit Care Med. 1994;149:707–14. doi: 10.1164/ajrccm.149.3.8118640. [DOI] [PubMed] [Google Scholar]
- 37.Sforza E, Krieger J, Petiau C. Arousal threshold to respiratory stimuli in OSA patients: evidence for a sleep-dependent temporal rhythm. Sleep. 1999;22:69–75. [PubMed] [Google Scholar]
- 38.Haba-Rubio J, Sforza E, Weiss T, Schroder C, Krieger J. Effect of CPAP treatment on inspiratory arousal threshold during NREM sleep in OSAS. Sleep Breath. 2005;9:12–9. doi: 10.1007/s11325-005-0002-5. [DOI] [PubMed] [Google Scholar]
- 39.Arima T, Tomonaga A, Toyota M, Inoue SI, Ohata N, Svensson P. Does restriction of mandibular movements during sleep influence jaw-muscle activity? J Oral Rehabil. 2012;39:545–51. doi: 10.1111/j.1365-2842.2012.02310.x. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien LM, Koo J, Fan L, et al. Iron stores, periodic leg movements, and sleepiness in obstructive sleep apnea. J Clin Sleep Med. 2009;5:525–31. [PMC free article] [PubMed] [Google Scholar]