Abstract
Study Objectives:
Sleep disordered breathing (SDB) is common in patients with chronic heart failure secondary to non-valvular heart disease; however, the prevalence and characteristics of SDB in patients with rheumatic valvular heart disease (RVHD) are unclear. This study was designed to determine the prevalence, characteristics, and risk factors for SDB in RVHD patients.
Methods:
A cross-sectional study was conducted in 260 RVHD patients. The following data were recorded: types of heart valve lesions, electrocardiographic, echocardiographic, arterial blood gas analysis findings, baseline medication, 6-minute walk test (6MWT) distance, and sleep parameters.
Results:
Compared to patients with single leftsided valve lesions, patients with left- and rightsided valve lesions had a higher prevalence of SDB (46.2% vs. 31.2%, p = 0.013); the increased prevalence of SDB only involved central sleep apnea (CSA) (31.1% vs. 14.1%, p = 0.001). Patients with obstructive sleep apnea (OSA) or CSA were older and had a shorter 6MWT distance, lower left ventricle ejection fraction and PaO2, a longer lung-to-finger circulation time, and a higher prevalence of atrial fibrillation (AF) and hypertension (all p < 0.05) as compared with patients without SDB. Multinomial logistic regression analysis showed that PaO2 ≤ 85 mm Hg was the only risk factor for OSA. Male gender, AF, 6MWT distance ≤ 300 m, PaO2 ≤ 85 mmHg, and PaCO2 ≤ 40 mm Hg were risk factors for CSA.
Conclusions:
Patients with RVHD had a high prevalence of SDB (predominantly CSA). RVHD patients with SDB, particularly those who had CSA, manifested more severe symptoms and greater impairment of cardiac function. Assessments of clinical manifestations of cardiac dysfunction may be important for predicting the risk factors for SDB.
Citation:
Ding N; Ni BQ; Zhang XL; Huang HP; Su M; Zhang SL; Wang H. Prevalence and risk factors of sleep disordered breathing in patients with rheumatic valvular heart disease. J Clin Sleep Med 2013;9(8):781-787.
Keywords: Sleep disordered breathing, rheumatic valvular heart disease, central sleep apnea, obstructive sleep apnea, chronic heart failure
Chronic heart failure (CHF) and sleep disordered breathing (SDB) are major public health problems in many countries. Studies have shown a high prevalence of SDB in CHF patients. Specifically, Sin1 observed that 38% of 450 CHF patients had obstructive sleep apnea (OSA), while 33% had central sleep apnea (CSA). Another study showed that SDB was present in 76% of patients with symptomatic CHF (40% CSA, 36% OSA).2 Several studies have demonstrated increased mortality in CHF patients with SDB in contrast to those without SDB.3–5 Other studies have also revealed that OSA is a cardiovascular risk factor and that CSA is a potential marker of the severity of CHF.3,6–8
Valvular heart disease is an important comorbidity factor for CHF. The prevalence of valvular heart disease is estimated at 2.5% in the US population and sharply increases beyond the age of 65 years because of the prevalence of degenerative etiologies.9 Although a decreased incidence of rheumatic valvular heart disease (RVHD) has been observed in the past 50 years, RVHD remains common in developing countries, where the prevalence is underestimated by clinical examination. The prevalence of RVHD is estimated at 2% to 3% when using systematic echocardiographic screening.9
Previous studies have mainly focused on the prevalence of SDB in patients with non-valvular diseases, such as coronary heart disease, myocardial infarction, dilated cardiomyopathy, and hypertrophic cardiomyopathy.2,10,11 Several studies have suggested the SDB and valvular heart disease are associated12–16; however, instead of RHVD, most of the studies involved patients with degenerative valvular heart disease. In addition, the relationship between the types of heart valve lesions and SDB has not been elucidated. We hypothesized that in RVHD patients, the types of SDB were related to types of heart valve lesions and that some clinical characteristics might be predictive factors for SDB. Therefore, the current study was designed to investigate the epidemic characteristics of SDB in RVHD patients.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep disordered breathing (SDB) is common in patients with chronic heart failure caused by non-valvular heart disease. However, no data are available for the prevalence and nature of SDB in patients with rheumatic valvular heart disease (RVHD).
Study Impact: This study showed that patients with RVHD have a high prevalence of SDB, in which central sleep apnea (CSA) predominates. In addition, this study demonstrates that the risk factors for CSA are significantly different from obstructive sleep apnea (OSA). CSA has more risk factors than OSA.
METHODS
Patients
Between April 2010 and October 2011, patients with RVHD admitted to the Cardiothoracic Surgery Department of the First Affiliated Hospital of Nanjing Medical University for heart valve replacement surgery were screened for the presence of various types of SDB. Among these patients, 275 underwent fullnight polysomnography (PSG). Two hundred sixty patients were enrolled, but 15 were excluded because they lacked an adequate PSG record or underwent a short PSG (< 3 h; Figure 1). None of the patients had been screened for SDB prior to the study period.
Figure 1. Flowchart of participation.
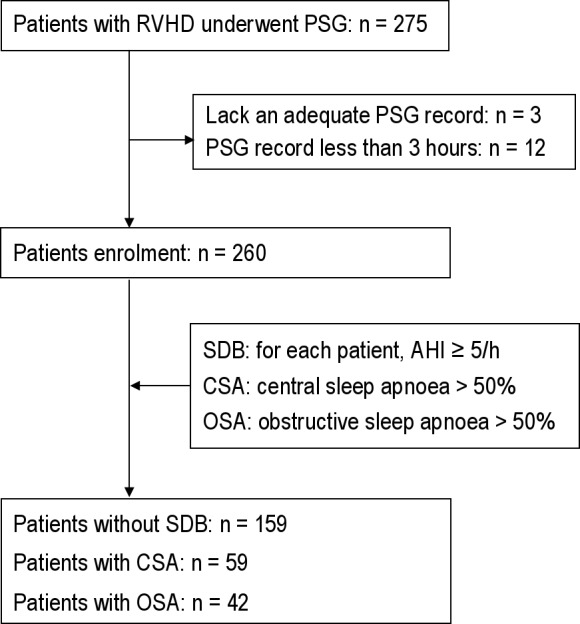
The following inclusion criteria were used: (1) age between 18 to 70 years; (2) symptomatic stable heart failure, New York Heart Association (NYHA) class ≥ II despite optimal drug therapy; (3) primary episode of rheumatic fever and current rheumatic heart disease based on 2004 WHO criteria for the diagnosis of rheumatic fever and rheumatic heart disease17; and (4) clinical features at presentation and typical rheumatic valvular lesions examined by Doppler echocardiography. Exclusion criteria were: (1) history of stroke or clinical signs of peripheral or central nervous system disorders; (2) previously known congenital heart disease, coronary heart disease, myocardial infarction, dilated cardiomyopathy, or hypertrophic cardiomyopathy; (3) chronic obstructive pulmonary disease or history of asthma; and (4) enrollment in another clinical study.
To obtain a stable clinical status for all patients, optimal drug therapy was used. The baseline medications included digoxin, diuretics, nitrates, angiotensin-converting enzyme inhibitors (ACEI), and β-blockers.
Written informed consent was obtained from all patients. This study was approved by the Clinical Study Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
Cardiac Function Assessment
NYHA classification was assessed immediately after the patients were enrolled. Atrial fibrillation (AF) was detected by 12-lead electrocardiography. Two-dimensional Doppler echocardiography was performed to assess the left ventricular ejection fraction (LVEF). On the second morning of hospitalization, an arterial blood gas was evaluated when patients discontinued oxygen therapy for at least 1 hour.
The 6-minute walk test (6MWT) was performed within 3 days after hospital admission according to the guidelines issued by the American Thoracic Society.18 For those whose lower limb joints were damaged by rheumatic fever, 6MWT was not conducted.
Bias Control
To reduce the potential bias as much as possible, the following steps were taken: (1) patients were randomly selected and according to inclusion and exclusion criteria; (2) clinical data were collected fully to avoid any possible data loss bias; (3) the same medical instrument was used for all patients for echocardiography, electrocardiography, blood gas analysis, and polysomnography; (4) all examiners and persons who analyzed the data were blinded to this study and patients enrolled in this study.
Polysomnography
An evaluation of daytime sleepiness based on the Epworth Sleepiness Scale (ESS) was performed prior to sleep study. The sleep study was performed by unattended overnight PSG (Embla S4500 System, USA). Sleep was monitored using 5 electroencephalographic channels (EEG; F4-M1, C4-M1, O2-M1, E12-M2, and E2-M2) and a submental electromyogram. Nasal airflow was measured by continuously recording the nasal pressure, snoring, pulse oximetry, and body position, as well as chest and abdominal effort. For each participant, ≥ 80% of the total recording was considered of good quality. Analyses were performed by 2 physicians who specialized in SDB but were not directly involved in this study.
The following standard definitions were used to describe and score SDB: obstructive apnea—complete cessation of airflow with continued paradoxical chest and abdominal excursion for ≥ 10 s; mixed apnea—cessation of airflow for ≥ 10 s with complete cessation of both abdominal and chest movement in at least the first half of the apnea (first 5 s), followed by paradoxical chest and abdominal excursion in the latter half (mixed apnea was classified as part of the obstructive apnea group because both had the same pathogenesis); central apnea—complete cessation of airflow as well as complete cessation of chest and abdominal excursion ≥ 10 s; and hypopnea—reduction of airflow > 50% from the baseline ≥ 10 s and associated with 4% desaturation or an increase in EEG.
SDB was further classified as either CSA or OSA based on the predominance (> 50%) of the type of sleep apnea to ensure consistency with the criteria used in other studies.2,19,20 The apnea/hypopnea index (AHI) is used as a marker of SDB severity and graded as “mild” (5-15/h), “moderate” (15-30/h), or “severe” (> 30/h) according to AASM guidelines.21 Patients with an AHI < 5/h were considered to have no SDB.
The circulation time was measured by lung-to-finger circulation time (LFCT) instead of lung-to-ear circulation time, be cause the SpO2 in our patients was assessed by a finger rather than an ear.
Statistical Analysis
Statistical analyses were performed using the SPSS statistical software (version 13.0; IBM, USA). Continuous data were expressed as mean (95% confidence interval for mean). Differences among the 3 groups (No SDB, CSA, and OSA) were compared using oneway ANOVA. Student-Newman-Keuls method was used for post hoc multiple comparisons. The χ2 test was used to analyze the frequency of each parameter. Multinomial logistic regression was used to model the association between various baseline variables and the risk of CSA and/ or OSA, for which the patients without SBD were used as the reference group. The candidate independent variables that were used to analyze the following risk factors for CSA and OSA: gender, age, history of symptomatic heart failure, body mass index (BMI), NYHA class, LVEF, and 6MWT, as well as the pH, PaO2, and PaCO2 during wakefulness. The presence of hypertension, AF, and left- and rightsided valve lesions was used to evaluate the risk factors for CSA and OSA. A pvalue < 0.05 was considered to indicate statistical significance.
RESULTS
Baseline Characteristics
The average age of the 260 patients was 51.3 years (95% CI: 50.1-52.5). Of these patients, 125 (48.1%) were males and 135 (51.9%) were females. Digoxin, diuretics, nitrates, angiotensin converting enzyme inhibitors (ACEIs), and β-blockers were used by 90.4%, 89.6%, 35.4%, 61.9%, and 59.6% of the patients, respectively. Based on the NYHA classification, 12.6%, 61.9%, and 25.4% of the patients were in NYHA classes II, III, and IV, respectively. Of the patients, 49.2% (128) had leftsided valve lesions (mitral valve lesions [23.5%], or aortic valve lesions [11.2%], or mitral valve + aortic valve lesions [14.6%]) and 50.8% (132) had left- and rightsided valve lesions (mitral valve + tricuspid valve lesions [25.0%] or mitral valve + aortic valve + tricuspid valve lesions [25.8%]). The detailed baseline characteristics of the patients are presented in Table 1.
Table 1.
Baseline clinical and sleep characteristics
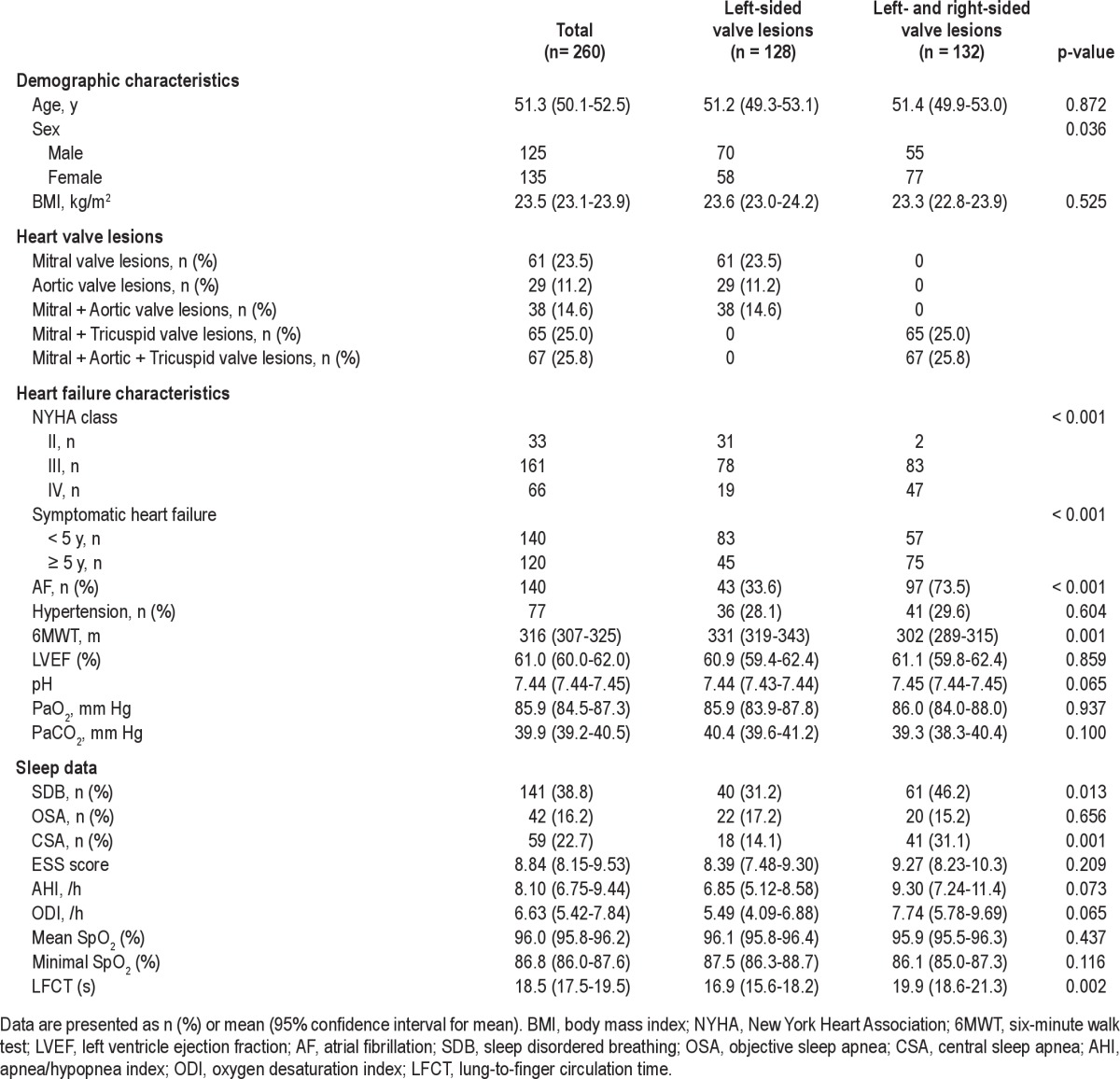
Clinical and sleep characteristics were compared between the patients with single leftsided valve lesions and left- and rightsided valve lesions. There were no differences between the 2 groups with respect to age (p = 0.872), BMI (p = 0.525), or LVEF (p = 0.859). Patients with left- and rightsided valve lesions had a remarkably shorter 6MWT distance (p = 0.001) and LFCT (p = 0.002), and a higher percentage of symptomatic heart failure ≥ 5 years (p < 0.001), CSA (p = 0.001) and AF (p < 0.001) compared to single leftsided valve lesions patients (Table 1).
The prevalence of SDB in our population was 38.8%; 16.2% had predominant OSA and 22.7% had predominant CSA (Figure 2).
Figure 2. Histograms show the prevalence of OSA and CSA in patients with RVHD.
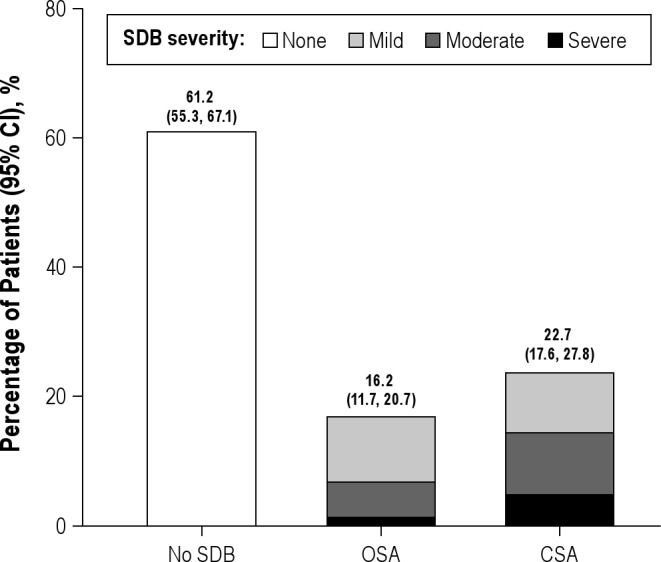
Among all patients, 61.2% did not have SDB, 16.2% had OSA, and 22.7% had CSA.
Comparisons in Patients with and without SDB
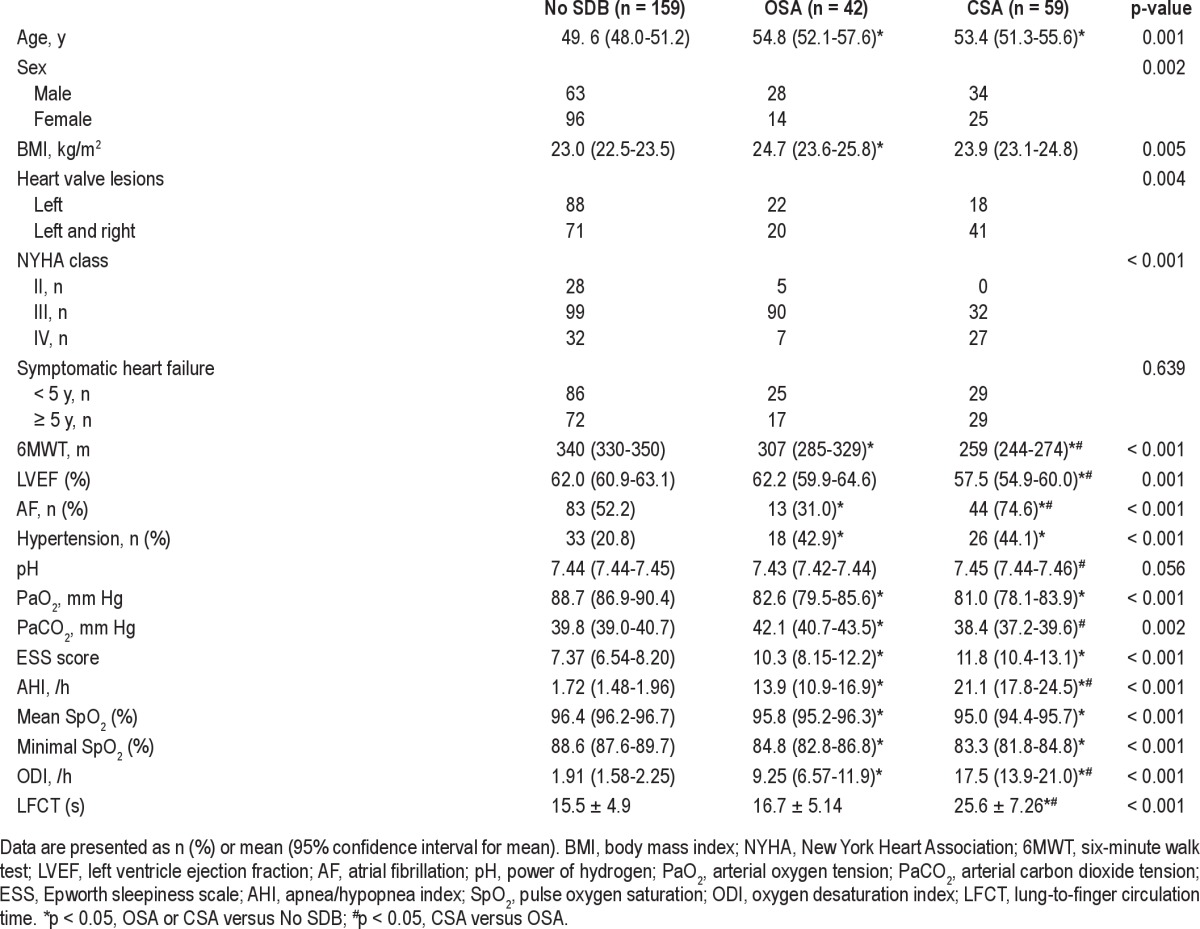
The prevalence of OSA and CSA in males was significantly higher than females (p = 0.001). Patients with OSA or CSA were older and had shorter 6MWT distances (p < 0.001) and a lower PaCO2 (p < 0.001) than patients without SDB. Compared with patients without SDB or patients with OSA only, patients with CSA exhibited a significantly higher prevalence of AF (p < 0.001), higher NYHA class (p < 0.001), and longer LFCT (p < 0.001). Decreased LVEF (p = 0.001) and worse sleep respiratory parameters (AHI, ODI, and mean and minimal SpO2) were likewise observed (Table 2).
Table 2.
Comparisons of clinical and polysomnography parameters in No SDB, OSA, and CSA patients
Risk Factors for OSA and CSA
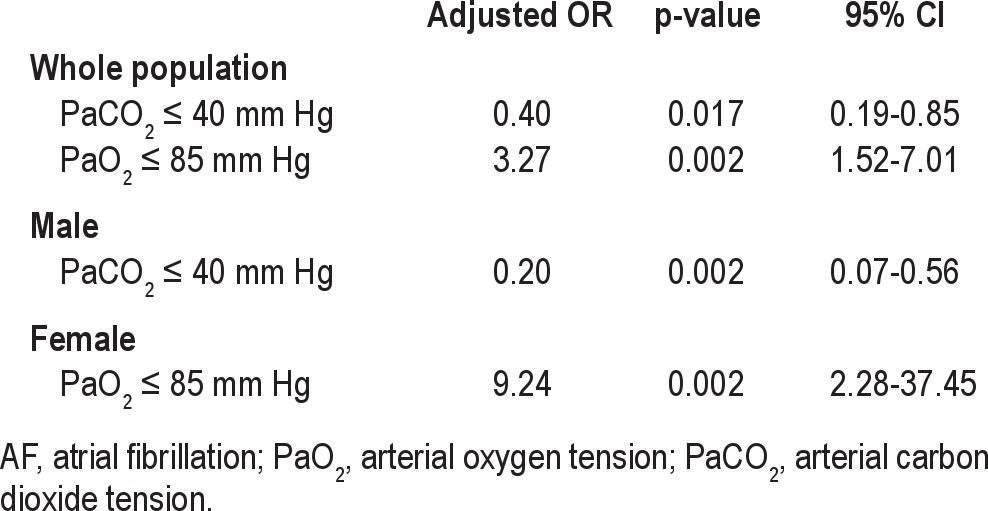
The risk factors for OSA are shown in Table 3. The decreasing PaO2 level was the dominant risk factor in the entire population and females, which was defined by a PaO2 ≤ 85 mm Hg, with adjusted odds ratios (ORs) of 3.27 (95% CI, 1.52-7.01) and 9.24 (95% CI, 2.28-37.45), respectively. PaCO2 ≤ 40 mm Hg was a protective factor for OSA in the entire population (OR = 0.40; 95% CI, 0.19-0.85) and males (OR = 0.20; 95% CI, 0.07-0.56).
Table 3.
Risk factors for OSA
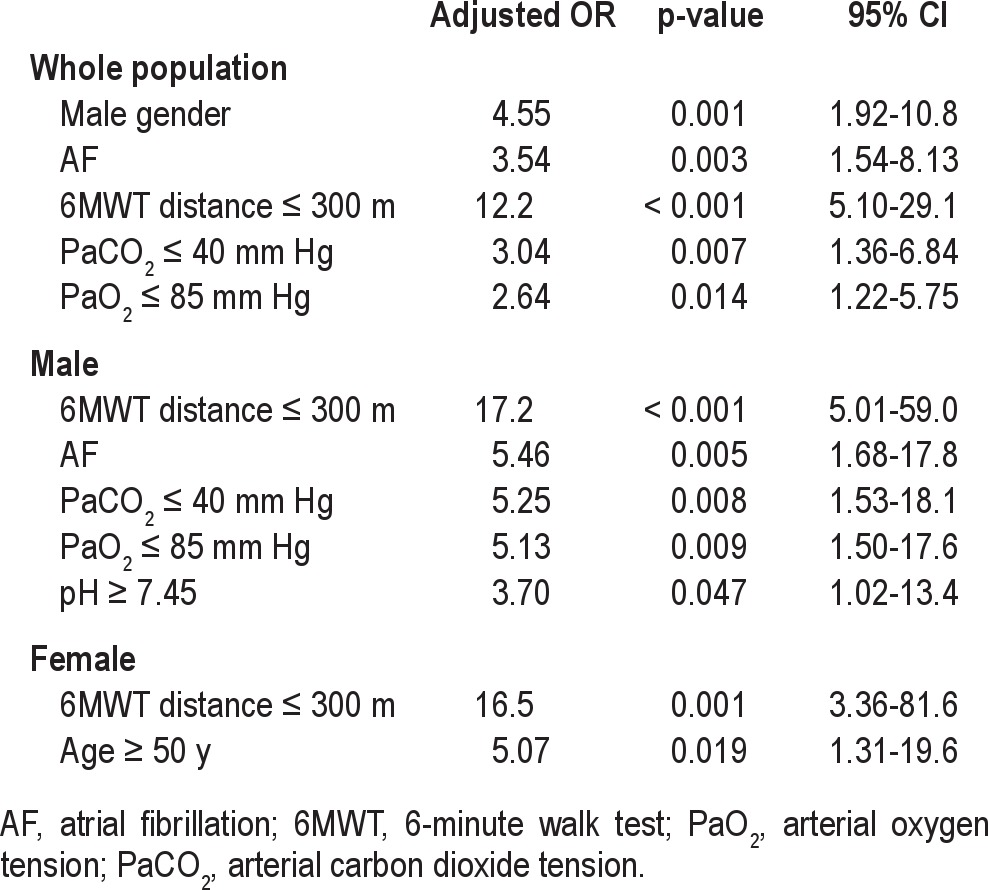
However, more risk factors were found in CSA patients than in OSA patients (both males and females). For the entire group, the risk factors for CSA were male gender, AF, 6MWT distance ≤ 300 m, PaCO2 ≤ 40 mm Hg, and PaO2 ≤ 85 mm Hg, in which the adjusted ORs were 4.55 (95% CI, 1.92-10.8), 3.536 (95% CI, 1.54-8.13), 12.2 (95% CI, 5.10-29.1), 3.04 (95% CI, 1.36-6.84), and 2.64 (95% CI, 1.22-5.75), respectively. Patients with a 6MWT distance ≤ 300 m had greater than a 15-fold increased risk for males and females. However, other risk factors for CSA differed between males and females. For males, AF, PaCO2 ≤ 40 mm Hg, PaO2 ≤ 85 mm Hg, and pH ≥ 7.45 were risk factors (OR = 5.46, 95% CI, 1.68-17.8; OR = 5.25, 95% CI, 1.53-18.1; OR = 5.13, 95% CI, 1.50-17.6; OR = 3.70, 95% CI, 1.02-13.4; respectively), whereas age (≥ 50 years) was a dependent risk factor for females (OR = 5.07, 95% CI, 1.31-19.6; Table 4). A LVEF ≤ 60% was not considered a risk factor for OSA or CSA.
Table 4.
Risk factors for CSA
DISCUSSION
In patients with CHF caused by non-valvular disease, a high prevalence of SDB has been reported1,2,10,22–24; however, data on the prevalence of SDB in patients with RVHD are not available because RVHD is not the main etiology for heart failure in developed countries. Nevertheless, RVHD remains common in developing countries and is one of the top three leading common causes of heart failure in Chinese hospitals.25
As compared with patients with single leftsided valve lesions, patients with left- and rightsided valve lesions had a remarkably higher prevalence of SDB (CSA only) and AF. The major reason might be a longer history of symptomatic heart failure and more severe degree of heart failure. In the early stages of rheumatic fever, the mitral and aortic valves are often involved. With progression of rheumatic heart disease, the tricuspid valve and right heart also become involved. Right heart failure leads to systemic congestion, delayed circulation, and PCO2 fluctuation, which might cause CSA. Because peripheral edema is more severe in right heart failure patients, nocturnal rostral fluid displaced from the legs to the neck might be a significant contributor to the pathogenesis of OSA and CSA.20 Furthermore, a long history of rheumatic disease causes the anatomic and functional alterations and electrophysiological modifications, which result in AF. CSA is predisposed to AF because of alterations in sympathetic and parasympathetic nervous system activity occurring in SDB patients, and is associated with hypoxemia, acidosis, apneas, and arousal.
A relatively lower prevalence of SDB in the study population was found. Approximately 40% of the population had OSA (16.2%) or CSA (22.7%), which was almost 50% lower than the results in previous studies.1,2,10 In addition, the mean LVEF in our study was 61.0% (62.0%, 62.2%, and 57.5% for No SDB, OSA, and CSA, respectively), which was 2-fold higher than previous studies.1,2,10 The severity and type of SDB are related to LVEF,1,2,12 which could be used as a predictor of heart systolic function. Therefore, the reduced systolic left ventricular function may lead to an increased prevalence and severity of SDB.
The patients in our study had a lower prevalence of OSA than CSA (Figure 2). Patients with OSA often have a significantly higher BMI.19 In our patients, BMI was significantly lower than previous studies,1,10,26 which may explain the lower percentage of OSA.
OSA patients had hypoxia and CO2 retention because of upper airway obstruction. However, CSA patients usually suffer from hyperventilation and hypocapnia, which occur because the background PaCO2 is closer to the apneic threshold during sleep in CSA patients than in heathy individuals. Thus, slight increases in ventilation or reductions in PaCO2 can lead to respiratory instability.27 CSA patients had a notably higher arterial blood pH and lower PaCO2 as compared with OSA patients (Table 2), which is consistent with a previous study,20,27 suggesting that the arterial blood gas differs significantly between OSA and CSA patients. Multinomial logistic regression analysis results that a PaCO2 ≤ 40 mm Hg was a risk factor for CSA, but a protective factor for OSA also agreed with these results (Tables 3 and 4, respectively).
The lung-to-ear circulation time, an estimate of the lungtocarotid chemoreceptor circulation time, is always increased in patients with CSA.28,29 Circulation delay may affect not only blood flow distribution, but also the degree of increase in minute ventilation and decrease in the PaCO2, which directly caused CSA. Our finding that the patients with CSA had a significantly longer LFCT was similar to previous studies.28,29
This study showed that the risk factors for CSA significantly differed from those for OSA, but were similar to the findings of a previous study.1 The previous study showed that risk factors for CSA were male gender, AF, age > 60 years, and hypocapnia. Two additional risk factors for CSA were demonstrated in the current study (a 6MWT distance ≤ 300 m and a PaO2 ≤ 85 mm Hg; Table 4). Moreover, the risk factors for CSA in males differed from those in females. Specifically, AF, a PaCO2 ≤ 40 mm Hg and a PaO2 ≤ 85 mm Hg were risk factors for CSA in the entire population and males, but not females; however, age (≥ 50 years) was a risk factor for CSA in females only. Sforza30 showed that hypertension was significantly associated with the OSA risk in females (OR = 1.52; p = 0.04), whereas Poletti31 reported that the risk factors for CSA included old age and a higher aminoterminal fragment of probrain natriuretic hormone levels. These results are significantly different from those in our study. Furthermore, Sin1 demonstrated that a BMI > 35 kg/m2 was the only risk factor for OSA in males and age > 60 years was the only significant risk factor for OSA in females. However, our results indicated that PaO2 ≤ 85 mm Hg was a risk factor for OSA in the entire population and females, and neither OSA nor CSA was related to BMI. These significant differences may be associated with the different etiology of CHF.
Some potential limitations are existed in the current study. First, although an AHI threshold of 10/h has been used to define the presence of SDB in several studies,1,24 we used a cutoff AHI of 5/h according to previously described recommendations,21 which may account for differences in the reported prevalence of SDB. Second, for this singlecenter study, the prevalence and risk factors of our population may not be representative of all RVHD patients. Although some important risk factors for OSA and CSA were examined in the current study, the possibility of unmeasured risk factors cannot be excluded. Third, previous studies have shown that heart valvular repair or heart transplantation improves CSA in CHF patients.15,32 Thus, further study is needed to observe the effects of the cardiac valve replacement in patients with RVHD on SDB.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by Jiangsu Provincial Department of Education [Grants CXZZ11_0726]; and the Priority Academic Program of Jiangsu Higher Education In-stitutions [Grants JX10231801]. In this study, Drs Ding and Ni contributed equally.
ABBREVIATIONS
- CHF
chronic heart failure
- SDB
sleep disordered breathing
- OSA
obstructive sleep apnea
- CSA
central sleep apnea
- RVHD
rheumatic valvular heart disease
- PSG
polysomnography
- NYHA
New York Heart Association
- ECG
electrocardiography
- LVEF
left ventricle ejection fraction
- 6MWT
6-minute walk test
- ACEI
angiotensin-converting enzyme inhibitors
- ESS
Epworth Sleepiness Scale
- EEG
electroencephalogram
- AHI
apnea/hypopnea index
- BMI
body mass index
- PaO2
arterial oxygen tension
- PaCO2
arterial carbon dioxide tension
- AF
atrial fibrillation
- SpO2
pulse oxygen saturation
- ODI
oxygen desaturation index
- LFCT
lung-to-finger circulation time
REFERENCES
- 1.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 2.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–7. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–6. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 4.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middleaged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 5.Brack T, Thuer I, Clarenbach CF, et al. Daytime Cheyne-Stokes respiration in ambulatory patients with severe congestive heart failure is associated with increased mortality. Chest. 2007;132:1463–71. doi: 10.1378/chest.07-0121. [DOI] [PubMed] [Google Scholar]
- 6.Lanfranchi PA, Somers VK, Braghiroli A, Corra U, Eleuteri E, Giannuzzi P. Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation. 2003;107:727–32. doi: 10.1161/01.cir.0000049641.11675.ee. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2010;14:131–6. doi: 10.1007/s11325-009-0298-7. [DOI] [PubMed] [Google Scholar]
- 9.Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8:162–72. doi: 10.1038/nrcardio.2010.202. [DOI] [PubMed] [Google Scholar]
- 10.Khayat RN, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. J Card Fail. 2009;15:739–46. doi: 10.1016/j.cardfail.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiza SE, Simantirakis E, Bouloukaki I, et al. Sleep disordered breathing in patients with acute coronary syndromes. J Clin Sleep Med. 2012;8:21–6. doi: 10.5664/jcsm.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomcsanyi J, Karlocai K, Papp L. Disappearance of periodic breathing after heart operations. J Thorac Cardiovasc Surg. 1994;107:317–8. [PubMed] [Google Scholar]
- 13.Yasuma F, Hayashi H, Noda S, Tsuzuki M, Tanaka M, Okada T. A case of mitral regurgitation whose nocturnal periodic breathing was improved after mitral valve replacement. Jpn Heart J. 1995;36:267–72. doi: 10.1536/ihj.36.267. [DOI] [PubMed] [Google Scholar]
- 14.Rubin AE, Gottlieb SH, Gold AR, Schwartz AR, Smith PL. Elimination of central sleep apnoea by mitral valvuloplasty: the role of feedback delay in periodic breathing. Thorax. 2004;59:174–6. doi: 10.1136/thorax.2003.007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe H, Takahashi M, Yaegashi H, et al. Valve repair improves central sleep apnea in heart failure patients with valvular heart diseases. Circ J. 2009;73:2148–53. doi: 10.1253/circj.cj-09-0307. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi M, Kasai T, Dohi T, et al. Conversion from predominant central sleep apnea to obstructive sleep apnea following valvuloplasty in a patient with mitral regurgitation. J Clin Sleep Med. 2011;7:523–5. doi: 10.5664/JCSM.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rheumatic fever and rheumatic heart disease. World Health Organ Tech Rep Ser. 2004;923:1–122. [PubMed] [Google Scholar]
- 18.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 19.Vazir A, Hastings PC, Dayer M, et al. A high prevalence of sleep disordered breathing in men with mild symptomatic chronic heart failure due to left ventricular systolic dysfunction. Eur J Heart Fail. 2007;9:243–50. doi: 10.1016/j.ejheart.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Yumino D, Redolfi S, Ruttanaumpawan P, et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010;121:1598–605. doi: 10.1161/CIRCULATIONAHA.109.902452. [DOI] [PubMed] [Google Scholar]
- 21.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 22.Zhao ZH, Sullivan C, Liu ZH, et al. Prevalence and clinical characteristics of sleep apnea in Chinese patients with heart failure. Int J Cardiol. 2007;118:122–3. doi: 10.1016/j.ijcard.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11:602–8. doi: 10.1093/eurjhf/hfp057. [DOI] [PubMed] [Google Scholar]
- 24.Ferrier K, Campbell A, Yee B, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. 2005;128:2116–22. doi: 10.1378/chest.128.4.2116. [DOI] [PubMed] [Google Scholar]
- 25.Chen HZ, Fan WH, Jin XJ, Wang Q, Zhou J, Shi ZY. Changing trends of etiologic characteristics of cardiovascular diseases among inpatients in Shanghai: a retrospective observational study from 1948 to 1999. Zhonghua Nei Ke Za Zhi. 2003;42:829–32. [PubMed] [Google Scholar]
- 26.Herrscher TE, Akre H, Overland B, Sandvik L, Westheim AS. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17:420–5. doi: 10.1016/j.cardfail.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949–54. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 28.Hall MJ, Xie A, Rutherford R, Ando S, Floras JS, Bradley TD. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med. 1996;154:376–81. doi: 10.1164/ajrccm.154.2.8756809. [DOI] [PubMed] [Google Scholar]
- 29.Ryan CM, Floras JS, Logan AG, et al. Shift in sleep apnoea type in heart failure patients in the CANPAP trial. Eur Respir J. 2010;35:592–7. doi: 10.1183/09031936.00070509. [DOI] [PubMed] [Google Scholar]
- 30.Sforza E, Chouchou F, Collet P, Pichot V, Barthelemy JC, Roche F. Sex differences in obstructive sleep apnoea in an elderly French population. Eur Respir J. 2011;37:1137–43. doi: 10.1183/09031936.00043210. [DOI] [PubMed] [Google Scholar]
- 31.Poletti R, Passino C, Giannoni A, et al. Risk factors and prognostic value of daytime Cheyne-Stokes respiration in chronic heart failure patients. Int J Cardiol. 2009;137:47–53. doi: 10.1016/j.ijcard.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Mansfield DR, Solin P, Roebuck T, Bergin P, Kaye DM, Naughton MT. The effect of successful heart transplant treatment of heart failure on central sleep apnea. Chest. 2003;124:1675–81. doi: 10.1378/chest.124.5.1675. [DOI] [PubMed] [Google Scholar]


