Abstract
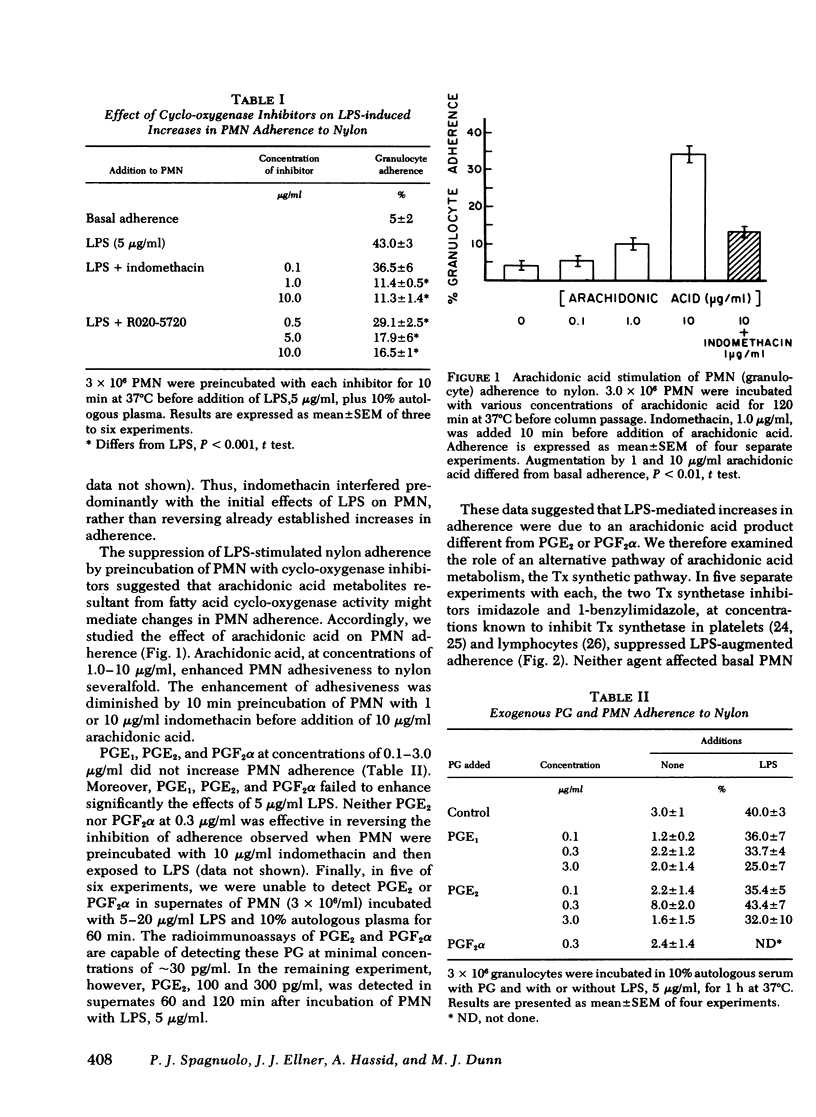
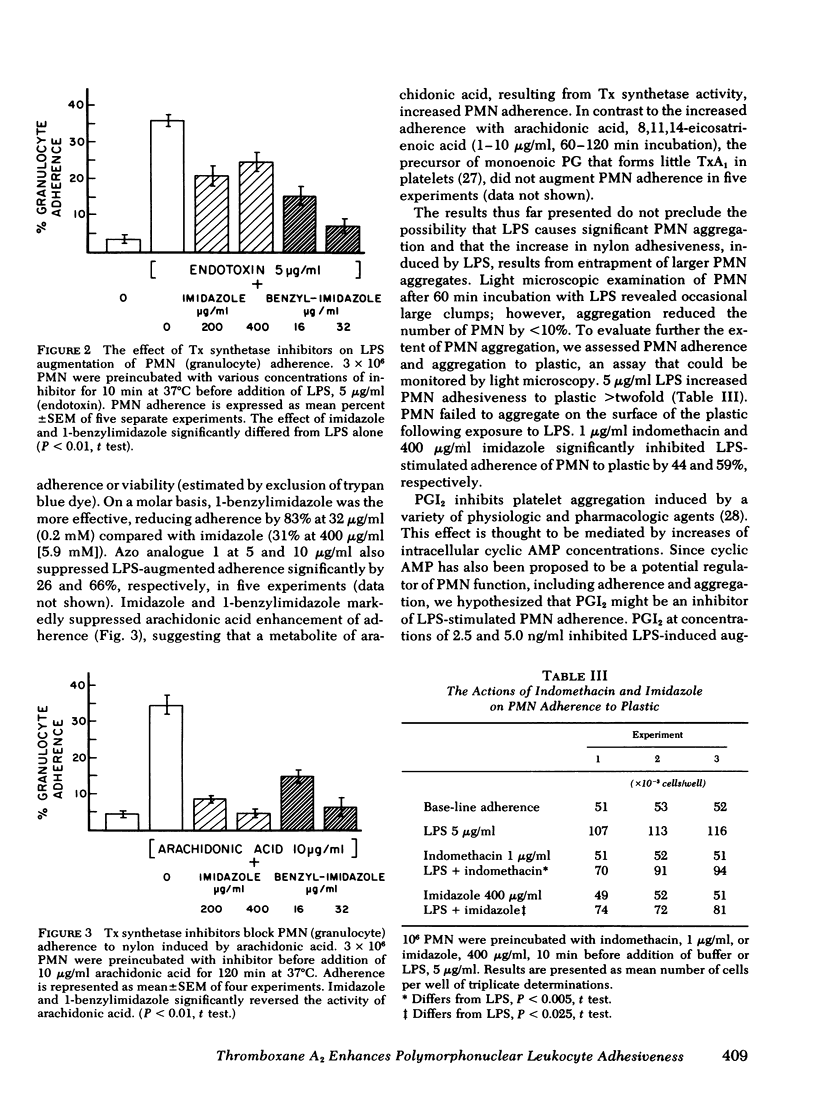
We examined the role of prostaglandins and thromboxanes as mediators of plasma-dependent increased polymorphonuclear leukocyte adhesiveness induced by Escherichia coli lipopolysaccharide. The cyclo-oxygenase inhibitors—indomethacin and d,l-6-chloro-α-methyl-carbozole-2-acetic acid (R020-5720)—reduced lipopolysaccharide-induced adherence of polymorphonuclear leukocytes by 74 and 62%, respectively. In addition, inhibitors of thromboxane synthetase—imidazole, 9,11-azoprosta-5,13-dienoic acid, and 1-benzylimidazole—suppressed the stimulation of adherence by 31, 66, and 83%, respectively. Exogenous prostaglandins E1, E2, and F2α did not increase polymorphonuclear leukocyte adherence, nor were they detected in significant quantities in supernates of polymorphonuclear leukocytes exposed to lipopolysaccharide. However, inhibitors of both cyclo-oxygenase and thromboxane synthetase reduced increases in adherence induced by arachidonic acid (10 μg/ml), suggesting that lipopolysaccharide-mediated increases in adherence were due to an arachidonic acid product other than prostaglandin E2 or F2α. 8,11,14-Eicosatrienoic acid, a precursor of monoenoic prostaglandins, did not enhance polymorphonuclear leukocyte adhesiveness.
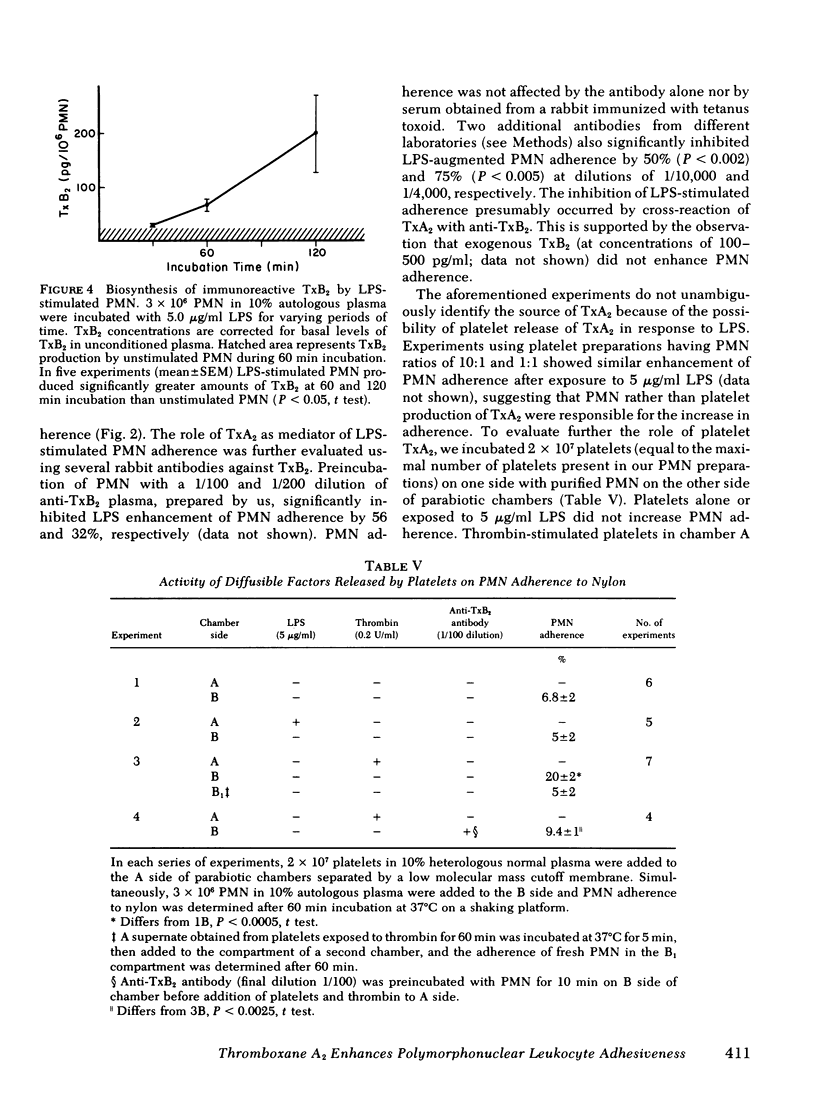
We next demonstrated lipopolysaccharide-stimulated generation, by polymorphonuclear leukocytes, of a labile, low molecular weight, dialyzable substance capable of enhancing the adherence of unstimulated leukocytes. In parallel experiments, a 10-fold increase in immunoreactive thromboxane B2 over basal levels was detected after exposure of leukocytes to lipopolysaccharide. The inhibition of lipopolysaccharide enhancement of adherence by specific rabbit antibodies to thromboxane B2 strongly supported a primary role for thromboxane A2 as the mediator of the observed increases in adherence. Lipopolysaccharide-stimulated purified platelets did not increase leukocyte adherence, whereas thrombin-stimulated platelets did increase adherence.
These studies suggest that lipopolysaccharide stimulates polymorphonuclear leukocytes to produce thromboxane A2, which enhances their adhesiveness to nylon.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON F., Jr, SMITH M. R., WOOD W. B., Jr Studies on the pathogenesis of acute inflammation. II. The action of cortisone on the inflammatory response to thermal injury. J Exp Med. 1955 Dec 1;102(6):669–676. doi: 10.1084/jem.102.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkinson N. F., Jr Prostaglandin production by human peripheral blood cells in vitro. J Lab Clin Med. 1977 Dec;90(6):1043–1053. [PubMed] [Google Scholar]
- Bryant R. E., Sutcliffe M. C. The effect of 3',5'-adenosine monophosphate on granulocyte adhesion. J Clin Invest. 1974 Nov;54(5):1241–1244. doi: 10.1172/JCI107868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting S., Higgs G. A., Moncada S., Vane J. R. Generation of thromboxane A2-like activity from prostaglandin endoperoxides by polymorphonuclear leukocyte homogenates [proceedings]. Br J Pharmacol. 1976 Oct;58(2):296P–296P. [PMC free article] [PubMed] [Google Scholar]
- Davidson E. M., Ford-Hutchinson A. W., Smith M. J., Walker J. R. The release of thromboxane B2 by rabbit peritoneal polymorphonuclear leucocytes [proceedings]. Br J Pharmacol. 1978 Jun;63(2):407P–407P. [PMC free article] [PubMed] [Google Scholar]
- Diczfalusy U., Hammarström S. A structural requirement for the conversion of prostaglandin endoperoxides to thromboxanes. FEBS Lett. 1979 Sep 15;105(2):291–295. doi: 10.1016/0014-5793(79)80632-8. [DOI] [PubMed] [Google Scholar]
- Dray F., Charbonnel B., Maclouf J. Radioimmunoassay of prostaglandins Falpha, E1 and E2 in human plasma. Eur J Clin Invest. 1975 Jul 29;5(4):311–318. doi: 10.1111/j.1365-2362.1975.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Feinstein M. B., Becker E. L., Fraser C. Thrombin, collagen and A23187 stimulated endogenous platelet arachidonate metabolism: differential inhibition by PGE1, local anesthetics and a serine-protease inhibitor. Prostaglandins. 1977;14(6):1075–1093. doi: 10.1016/0090-6980(77)90286-6. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Primary structural analysis of the polypeptide portion of human C5a anaphylatoxin. Polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J Biol Chem. 1978 Oct 10;253(19):6955–6964. [PubMed] [Google Scholar]
- Flower R. J., Cheung H. S., Cushman D. W. Quantitative determination of prostaglandins and malondialdehyde formed by the arachidonate oxygenase (prostaglandin synthetase) system of bovine seminal vesicle. Prostaglandins. 1973 Sep;4(3):325–341. doi: 10.1016/0090-6980(73)90020-8. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaut Z. N., Baruth H., Randall L. O., Ashley C., Paulsrud J. R. Stereoisomeric relationships among anti-inflammatory activity, inhibition of platelet aggregation, and inhibition of prostaglandin synthetase. Prostaglandins. 1975 Jul;10(1):59–66. doi: 10.1016/0090-6980(75)90093-3. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Woods J. M., Gorman R. R. Stimulation of human eosinophil and neutrophil polymorphonuclear leukocyte chemotaxis and random migration by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. J Clin Invest. 1977 Jan;59(1):179–183. doi: 10.1172/JCI108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Malmsten C. L., Kindahl H., Kaplan H. B., Rådmark O., Samuelsson B., Weissmann G. Thromboxane generation by human peripheral blood polymorphonuclear leukocytes. J Exp Med. 1978 Sep 1;148(3):787–792. doi: 10.1084/jem.148.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman R. R. Modulation of human platelet function by prostacyclin and thromboxane A2. Fed Proc. 1979 Jan;38(1):83–88. [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Wakabayashi T., Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc Natl Acad Sci U S A. 1974 Feb;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt D. E., White J. G., Craddock P. R., Jacob H. S. Corticosteroids inhibit complement-induced granulocyte aggregation. A possible mechanism for their efficacy in shock states. J Clin Invest. 1979 Apr;63(4):798–803. doi: 10.1172/JCI109365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs G. A., Bunting S., Moncada S., Vane J. R. Polymorphonuclear leukocytes produce thromboxane A2-like activity during phagocytosis. Prostaglandins. 1976 Nov;12(5):749–757. doi: 10.1016/0090-6980(76)90050-2. [DOI] [PubMed] [Google Scholar]
- Kaley G., Weiner R. Prostaglandin E-1: a potential mediator of the inflammatory response. Ann N Y Acad Sci. 1971 Apr 30;180:338–350. doi: 10.1111/j.1749-6632.1971.tb53203.x. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Johnson M. C., Parker C. W. Effect of inhibitors of arachidonic acid metabolism on mitogenesis in human lymphocytes: possible role of thromboxanes and products of the lipoxygenase pathway. J Immunol. 1979 Apr;122(4):1563–1571. [PubMed] [Google Scholar]
- Kitchen E. A., Boot J. R., Dawson W. Chemotactic activity of thromboxane B2, prostaglandins and their metabolites for polymorphonuclear leucocytes. Prostaglandins. 1978 Aug;16(2):239–244. doi: 10.1016/0090-6980(78)90025-4. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Macarak E. J., Kefalides N. A. Comparative adherence of granulocytes to endothelial monolayers and nylon fiber. J Clin Invest. 1978 Mar;61(3):697–702. doi: 10.1172/JCI108981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R. R., Spagnuolo P. J., Lentnek A. L. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. N Engl J Med. 1974 Sep 26;291(13):642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- McCall E., Youlten L. J. Proceedings: Prostaglandin E1 synthesis by phagocytosing rabbit polymorphonuclear leucocytes: its inhibition by indomethacin and its role in chemotaxis. J Physiol. 1973 Oct;234(2):98P–100P. [PubMed] [Google Scholar]
- McGillen J., Patterson R., Phair J. P. Adherence of polymorphonuclear leukocytes to nylon: modulation by prostacyclin (PGI2), corticosteroids, and complement activation. J Infect Dis. 1980 Mar;141(3):382–388. doi: 10.1093/infdis/141.3.382. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978 Sep;30(3):293–331. [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Raz A., Ferrendelli J. A., Minkes M. Application of imidazole as a selective inhibitor thromboxane synthetase in human platelets. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1716–1720. doi: 10.1073/pnas.74.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Effect of prostaglandins E1, E2 and F2alpha on neutrophil aggregation. Prostaglandins. 1979 Feb;17(2):201–210. doi: 10.1016/0090-6980(79)90039-x. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Neutrophil aggregation and swelling induced by chemotactic agents. J Immunol. 1977 Jul;119(1):232–239. [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. The influence of chemotactic factors on neutrophil adhesiveness. Inflammation. 1978 Mar;3(1):37–48. doi: 10.1007/BF00917320. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Becker E. L., Ward P. A. Neutrophil aggregation and degranulation. Effect of arachidonic acid. Am J Pathol. 1979 May;95(2):433–444. [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Ward P. A., Becker E. L. A possible role of arachidonic acid in human neutrophil aggregation and degranulation. Am J Pathol. 1979 Sep;96(3):799–810. [PMC free article] [PubMed] [Google Scholar]
- Rabson A. R., Anderson R., Lomnitzer R., Koornhof H. J. In vitro effects of prostaglandins on polymorphonuclear leucocyte function. S Afr Med J. 1974 Oct 16;0(0 Suppl):44–50. [PubMed] [Google Scholar]
- Snyderman R., Shin H. S., Phillips J. K., Gewurz H., Mergenhagen S. E. A neutrophil chemotatic factor derived from C'5 upon interaction of guinea pig serum with endotoxin. J Immunol. 1969 Sep;103(3):413–422. [PubMed] [Google Scholar]
- Sors H., Pradelles P., Dray F., Rigaud M., Maclouf J., Bernard P. Analytical methods for thromboxane B2 measurement and validation of radioimmunoassay by gas liquid chromatography-mass spectrometry. Prostaglandins. 1978 Aug;16(2):277–290. doi: 10.1016/0090-6980(78)90030-8. [DOI] [PubMed] [Google Scholar]
- Spagnuolo P. J., Ellner J. J. Salicylate blockade of granulocyte adherence and the inflammatory response to experimental peritonitis. Blood. 1979 May;53(5):1018–1022. [PubMed] [Google Scholar]
- Svensson J., Hamberg M., Samuelsson B. Prostaglandin endoperoxides IX. Characterization of rabbit aorta contracting substance (RCS) from guinea pig lung and human platelets. Acta Physiol Scand. 1975 Jun;94(2):222–228. doi: 10.1111/j.1748-1716.1975.tb05881.x. [DOI] [PubMed] [Google Scholar]
- Tai H. H., Yuan B. On the inhibitory potency of imidazole and its derivatives on thromboxane synthetase. Biochem Biophys Res Commun. 1978 Jan 13;80(1):236–242. doi: 10.1016/0006-291x(78)91128-2. [DOI] [PubMed] [Google Scholar]
- Till G., Kownatzki E., Seitz M., Gemsa D. Chemokinetic and chemotactic activity of various prostaglandins for neutrophil granulocytes. Clin Immunol Immunopathol. 1979 Jan;12(1):111–118. doi: 10.1016/0090-1229(79)90116-8. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Morley J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature. 1973 Nov 23;246(5430):215–217. doi: 10.1038/246215a0. [DOI] [PubMed] [Google Scholar]



