Abstract
Study Objectives:
Differentiation of narcolepsy without cataplexy from idiopathic hypersomnia relies entirely upon the multiple sleep latency test (MSLT). However, the test-retest reliability for these central nervous system hypersomnias has never been determined.
Methods:
Patients with narcolepsy without cataplexy, idiopathic hypersomnia, and physiologic hypersomnia who underwent two diagnostic multiple sleep latency tests were identified retrospectively. Correlations between the mean sleep latencies on the two studies were evaluated, and we probed for demographic and clinical features associated with reproducibility versus change in diagnosis.
Results:
Thirty-six patients (58% women, mean age 34 years) were included. Inter -test interval was 4.2 ± 3.8 years (range 2.5 months to 16.9 years). Mean sleep latencies on the first and second tests were 5.5 (± 3.7 SD) and 7.3 (± 3.9) minutes, respectively, with no significant correlation (r = 0.17, p = 0.31). A change in diagnosis occurred in 53% of patients, and was accounted for by a difference in the mean sleep latency (N = 15, 42%) or the number of sleep onset REM periods (N = 11, 31%). The only feature predictive of a diagnosis change was a history of hypnagogic or hypnopompic hallucinations.
Conclusions:
The multiple sleep latency test demonstrates poor test-retest reliability in a clinical population of patients with central nervous system hypersomnia evaluated in a tertiary referral center. Alternative diagnostic tools are needed.
Citation:
Trotti LM; Staab BA; Rye DB. Test- retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013;9(8):789-795.
Keywords: Multiple sleep latency test, idiopathic hypersomnia, narcolepsy without cataplexy, CNS hypersomnia, tes-tretest reliability
The central nervous system (CNS) hypersomnias manifest as excessive daytime sleepiness with or without prolonged nocturnal sleep in the absence of demonstrable nocturnal sleep pathology or insufficient sleep. They include the entities of narcolepsy with and without cataplexy and idiopathic hypersomnia. Narcolepsy with cataplexy is a chronic disease characterized by short latencies to both sleep and REM sleep on daytime nap opportunities during the multiple sleep latency test (MSLT) and is caused by immunogenetically mediated loss of hypocretin.1 Cataplexy is a clinical feature highly specific to hypocretin-deficient narcolepsy, although is not always evident near the onset of sleepiness. The remaining CNS hypersomnias begin at a similar age, are much less likely to be associated with hypocretin deficiency,1–4 and lack a pathognomonic sign or symptom.5,6 They have many features in common, including hallucinations and sleep paralysis,1,4 unrefreshing naps,7,8 sleep drunkenness,1,7,8 and prolonged nocturnal sleep.8–10 Therefore, differentiation between narcolepsy without cataplexy and idiopathic hypersomnia relies solely on the MSLT4 and the propensity for REM sleep to intrude into more than one daytime nap.
Perhaps because of the lack of a single defining characteristic or known pathophysiology, patients with narcolepsy without cataplexy and idiopathic hypersomnia not infrequently present to tertiary sleep centers for a second opinion regarding their diagnoses. In such instances, the clinical question frequently arises of whether or not to repeat an MSLT. Test-retest reliability of the MSLT is high in narcoleptics when two testing protocols are conducted within three weeks of each other.11 Intertest intervals are generally much longer in clinical practice, and in that case the stability of MSLT features has not been established. We therefore evaluated the test-retest reliability of the MSLT in a clinic population of patients with narcolepsy without cataplexy and idiopathic hypersomnia.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Differentiation of the syndromes of idiopathic hypersomnia and narcolepsy without cataplexy relies exclusively on the presence or absence of REM periods during the multiple sleep latency test. The test-retest reliability of the MSLT in these conditions, when applied in clinical practice, is unknown.
Study Impact: Test-retest reliability of the MSLT is poor in idiopathic hypersomnia and narcolepsy without cataplexy, because of changes in measured sleepiness and in number of REM onsets. Alternative diagnostic strategies are needed.
METHODS
Patient Selection
Patients with a clinical syndrome consistent with a CNS hypersomnia (i.e., reports of problematic, excessive daytime sleepiness persisting ≥ 3 months despite adequate or supranormal habitual sleep durations, not explained by other causes of daytime sleepiness) who had undergone 2 MSLTs were identified using separate billing, clinic, and research databases of different durations collectively spanning a period of 18 years. All patients diagnosed with narcolepsy without cataplexy or idiopathic hypersomnia were included. To minimize potential bias that could be introduced by relying upon an MSL threshold predefined as pathological in a study intended to examine the test's reliability, we also included subjects identified with “physiologic hypersomnia.” This designation required the presence of a clinical syndrome otherwise indistinguishable from idiopathic hypersomnia or narcolepsy without cataplexy but that did not meet the MSL threshold of < 8 min required for these diagnoses. Patients with unequivocal cataplexy or incomplete MSLT data (e.g., absence of data regarding mean sleep latency or sleep onset REM periods (SOREMS)) were excluded. Additionally, data were collected regarding patient demographics and clinical characteristics (Epworth Sleepiness Scale [ESS] scores, age at symptom onset, presence of sleep paralysis or sleeprelated hallucinations, habitual sleep duration, medication use at the time of study, family history of hypersomnia, and body mass index [BMI]). This study was approved by our institutional review board.
Statistical Analysis
Correlation between mean sleep latencies (MSL) on the 2 MSLTs was performed using Spearman correlation. Bland-Altman plots were constructed to allow visual inspection of the data, with the 95% limits of agreement calculated as ± 1.96*(standard deviation of the difference between paired test measurements). To determine the stability of clinical diagnosis based on MSLT, a diagnosis was assigned following ICSD-2 criteria7 as follows: sleep latency < 8 min and > 1 sleep onset REM period was diagnosed as narcolepsy without cataplexy; sleep latency < 8 min and < 2 SOREMs was diagnosed as idiopathic hypersomnia; sleep latency > 8 min (regardless of the presence or absence of SOREMs) was diagnosed as normal. Diagnoses for each individual were compared between the 2 MSLTs. To evaluate for the effects of medication on the stability of MSLT-based diagnosis, medication usage was characterized as changed or unchanged (specifically considering wakepromoting medications and selective serotonin and/ or noradrenergic reuptake inhibitors), and a Fisher exact test was performed comparing medication class changes with diagnosis change. As an exploratory analysis, demographic and clinical features were compared between those whose diagnosis changed and those whose diagnosis remained the same, using χ2 or Fisher exact test for categorical variables and Wilcoxon-Mann-Whitney test for continuous variables. Clinical characteristics across final diagnostic categories were compared using the Fisher test for categorical variables and Kruskal-Wallis test for continuous variables.
RESULTS
Patient Characteristics
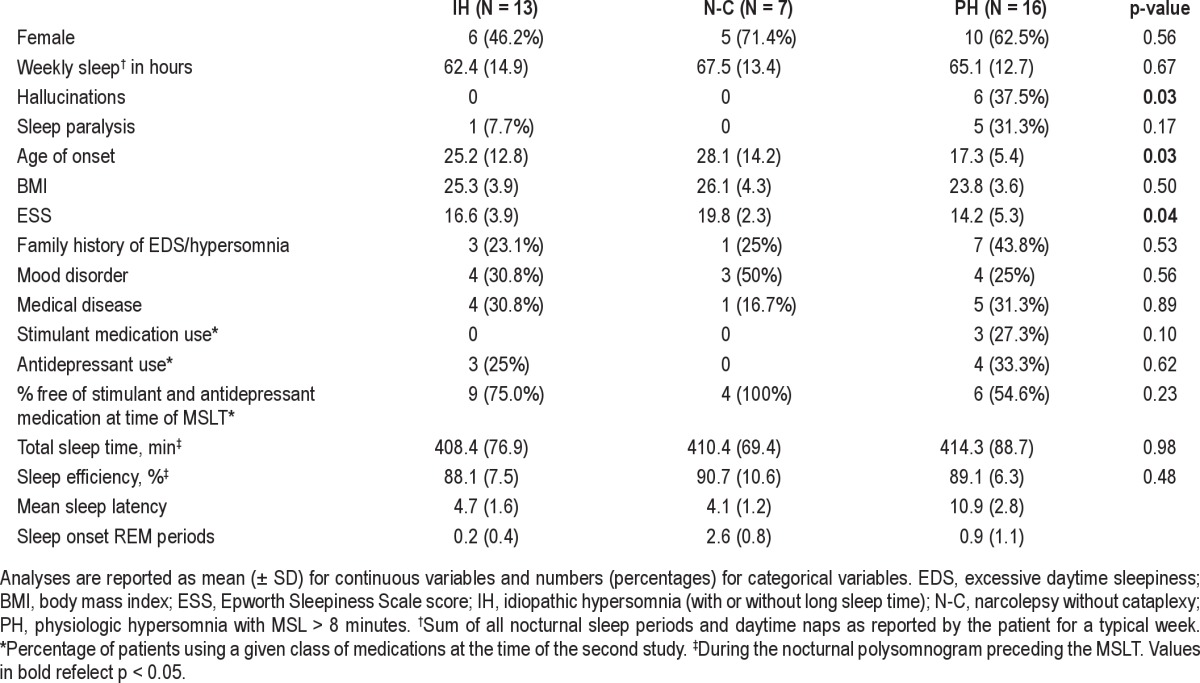
Thirty-six patients met inclusion criteria (see Table 1). The cohort was 58% female with a mean age of 34 (± 13) years at the time of first MSLT (range 15-56 years). Symptoms began at the age of 22 (± 11) years. The mean Epworth Sleepiness Score was 16 (± 5). Onethird of patients had a family history of a central nervous system hypersomnia or undiagnosed excessive daytime sleepiness. Two subjects were a mother-daughter pair. Cerebrospinal fluid measurements of hypocretin were available in 18 subjects (mean 290.2, SD 114.7). The time between studies ranged from 2.5 months to 16.9 years with an average length between studies of 4.2 ± 3.8 years. Based on the final MSLT, diagnoses included idiopathic hypersomnia (N = 13), narcolepsy without cataplexy (N = 7), and physiologic hypersomnia (N = 16); clinical features by diagnostic category are presented in Table 2 and were similar across groups except the presence of hallucinations, age of onset, and severity of sleepiness as measured by ESS. Based on the initial MSLT-based diagnosis, none of these 3 or other clinical features were significantly different across diagnostic categories, except female gender (85.7% of idiopathic hypersomnia patients, 33.3% of narcolepsy without cataplexy patients, and 57.1% of physiologic hypersomnia patients, p = 0.01) and age at first study (32.9 years for idiopathic hypersomnia, 38.3 years for narcolepsy without cataplexy, and 25.3 years for physiologic hypersomnia, p = 0.046). Diagnoses of comorbid mood disorders were common, primarily depression (N = 9), but also bipolar disorder (N = 2). Serious medical comorbidities occurred in 28% of subjects, and included HIV (N = 1), diabetes (N = 2), pulmonary hypertension (N = 1), anemia (N = 2), treated obstructive sleep apnea (N = 1), polycystic ovarian syndrome (N = 1), ulcerative colitis (N = 1), thyroid disease (N = 2), and epilepsy (N = 1). At the time of the first MSLT, 3 patients were taking a psychostimulant (dextroamphetamine, dextroamphetamine/amphetamine, or pemoline), and 3 were taking an antidepressant (fluoxetine, sertraline). For the second MSLT, 3 patients were taking a wakepromoting agent (dextroamphetamine/amphetamine, modafinil) and 7 antidepressants (citalopram, duloxetine, escitalopram, fluoxetine, sertraline, venlafaxine). Overall, change in stimulant or antidepressant medication between studies was not uncommon, occurring in 40% of patients.
Table 1.
Patient characteristics
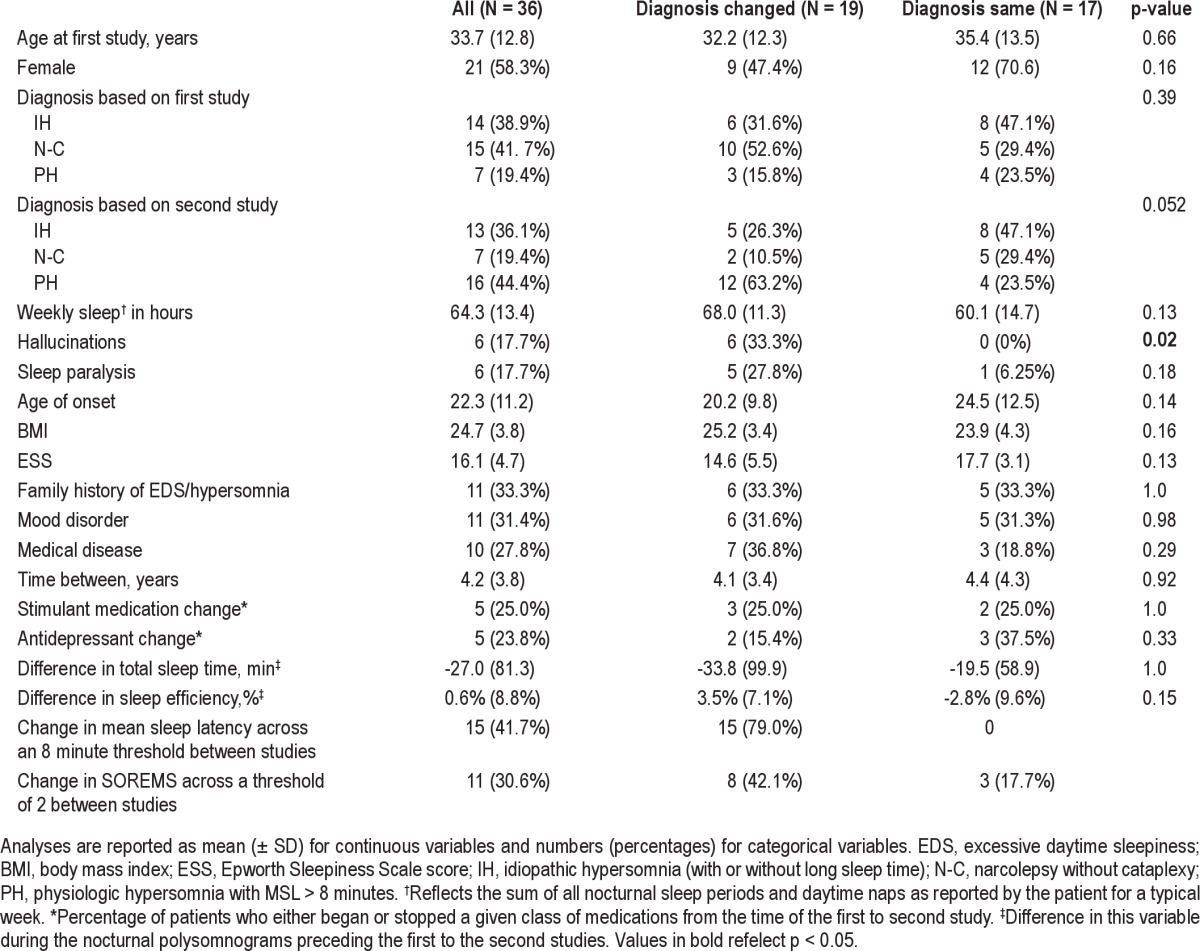
Table 2.
Clinical characteristics by final diagnostic category
MSLT Results
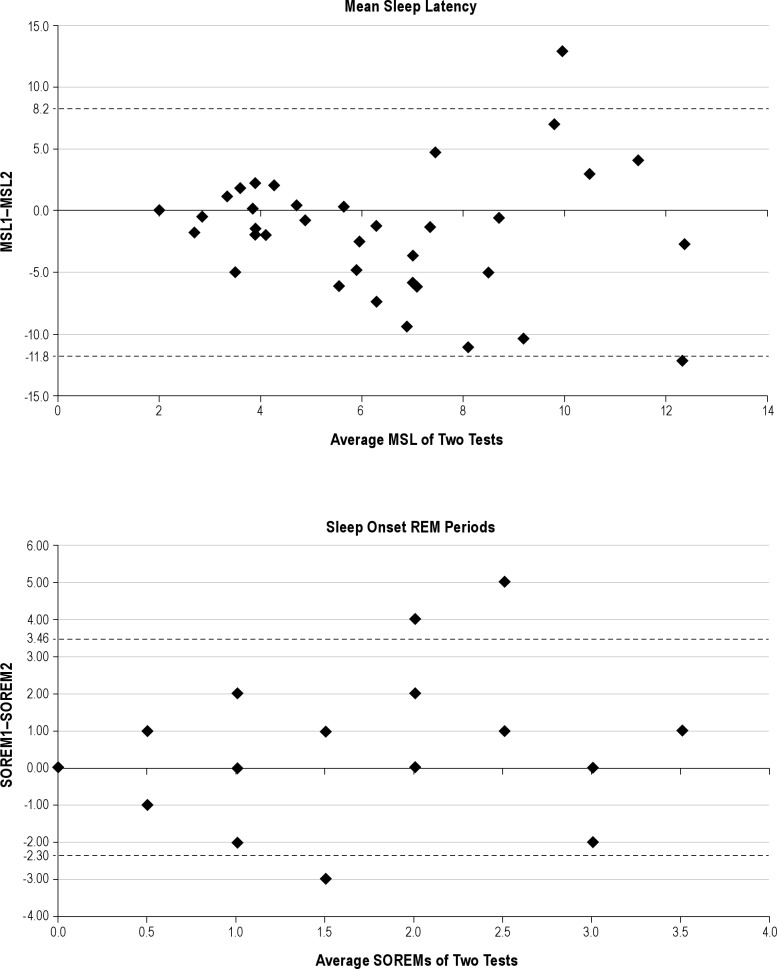
The average mean sleep latency on the first MSLT was 5.5 min (± 3.7) and on the repeat study was 7.3 (± 3.9) min, with mean sleep latencies ranging from 1 min to 18.4 min. There was no significant correlation of mean sleep latencies between studies (r = 0.17, p = 0.31). On the first study, 27.8% of subjects had no SOREMs and 50% had ≥ 2 SOREMs. On the second study, 50% of subjects had no SOREMs and 30.6% had ≥ 2. Bland-Altman plots of these variables (Figure 1) reveal very wide 95% limits of agreement for both mean sleep latencies and SOREMs, as well as a tendency for those with the highest mean values to exhibit the greatest variability. MSLT-based diagnosis frequently changed between studies (Figure 2). On repeat testing, only 17 patients (47%) retained the same diagnosis: 8 with IH, 5 with narcolepsy without cataplexy, and 4 normal (regardless of the number of SOREMs). Changes in diagnosis resulted from the mean sleep latency crossing the threshold of 8 min (N = 15, 42%) and the number of SOREMs crossing the threshold of ≥ 2 (N = 11, 31%), with both dependent variables crossing their diagnostic thresholds in 4 (11%) patients. Changes in diagnosis were not related to a change in stimulant or antidepressant medication (Table 1). Considering only those patients known to have been free of stimulant and antidepressant medications for both MSLTs, diagnosis remained the same in 45%. In exploratory analyses, a change in diagnostic category was not related to time between studies, findings on the preceding overnight polysomnography, the presence of comorbid psychiatric or medical disorders, or most symptoms of hypersomnia. The only variable that differed between those whose diagnosis changed and those whose diagnosis remained the same was the more frequent presence of hypnogogic or hypnopompic hallucinations (present in 33% of the diagnosis change group and none of the other group, p = 0.02). All 6 patients with hallucinations changed to a final diagnosis of physiologic hypersomnia, 4 from a diagnosis of narcolepsy without cataplexy and 2 from idiopathic hypersomnia.
Figure 1. Bland-Altman plots for test-retest reliability of mean sleep latency and sleep onset REMs.
Horizontal dashed lines are drawn at the 95% limits of agreement. MSL, mean sleep latency; MSL1, MSL on first test; MSL2, MSL on second test; SOREMs, sleep onset REM period (SOREM1 and SOREM2 for first and second test, respectively).
Figure 2. MSLT-based diagnosis on repeat MSLT.
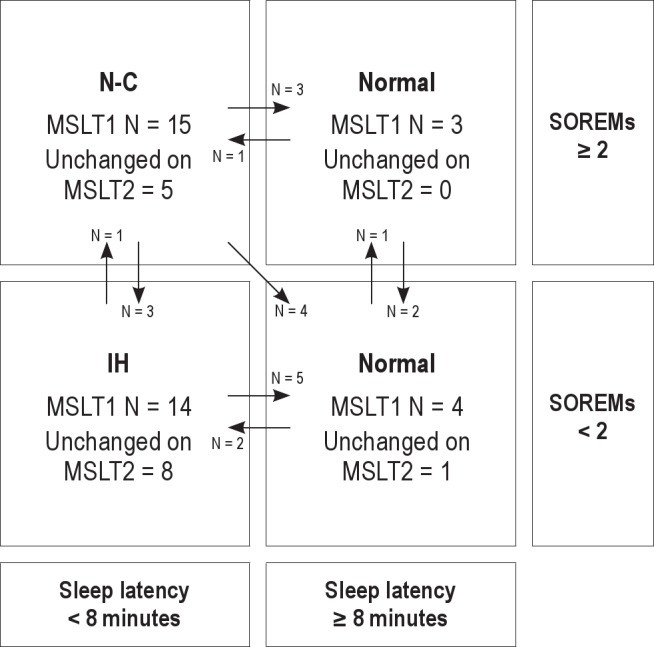
Arrows represent patients whose diagnosis changed from the first to the second MSLT. MSLT1, first MSLT; MSLT2, second MSLT; SOREMs, sleep onset REM periods. N-C, narcolepsy without cataplexy; IH, idiopathic hypersomnia.
DISCUSSION
MSLT-based diagnoses of narcolepsy without cataplexy and idiopathic hypersomnia demonstrated poor stability in clinical practice among patients evaluated in a tertiary clinic. Diagnoses changed in half of patients over an average of four years. This occurred because of changes in both sleepiness level (i.e., the MSL) and the propensity for REM sleep (i.e., SOREMs). Over 40% of patients had a change in MSL that crossed the conventional hypersomnia threshold of 8 minutes.7 In three patients, an apparently pathological level of sleepiness emerged after the initial diagnostic MSLT was interpreted as normal. In twelve, there was presumptive resolution of previously documented objective sleepiness, despite persistence of clinically significant subjective sleepiness. Such high rates of false negative and false positive MSLT results have also been highlighted in previous studies. An 8-minute MSL threshold, for example, fails to capture 22% to 39% of patients who otherwise meet clinical criteria for a CNS hypersomnia.9,12 Consistent with this, an average MSL of 8.3 minutes has been reported for patients with idiopathic hypersomnia.13 Type I error may also be problematic, as MSL < 8 was reported in 25% of one population-based sample.14 Collectively, these studies and our data call into question the appropriateness of an 8-minute threshold in capturing the complaint of sleepiness expressed by patients with nonhypocretin deficient CNS hypersomnias, and differentiating it from asymptomatic controls.
Over thirty percent of our patients crossed the threshold of two or more SOREMs between studies. Three patients initially diagnosed with narcolepsy met criteria for idiopathic hypersomnia on repeat testing, and one patient's diagnosis changed from idiopathic hypersomnia to narcolepsy without cataplexy. Excellent specificity of two or more SOREMs for narcolepsy has been suggested.15 Extension of the MSLT to diverse medical and neurological populations, however, demonstrates that although sensitive for hypocretin-deficient narcolepsy,15 the specificity of multiple SOREMs is poor. Multiple SOREMs can occur in other conditions associated with sleepiness, such as sleep apnea, Kleine-Levin syndrome, and Parkinson disease.14,16,17 Multiple SOREMs are also common in the general population, occurring in 3.9% to 13.1% of subjects.14,18 Nonetheless, SOREMs remain the sole electrodiagnostic feature that discriminates narcolepsy without cataplexy from idiopathic hypersomnia.4 The number of SOREMs is influenced by sex, sedating antidepressants, and shift work,14 but neither of the first two factors were associated with diagnosis change in our patients (shift work status was unknown). Collectively, these data and the absence of apparent therapeutic or biological significance to multiple SOREMs19 argue that the continued use of SOREMs to distinguish narcolepsy without cataplexy from idiopathic hypersomnia is not justified.
To some extent, the frequent change in MSLT results in our study may have reflected a key feature of the central hypersomnias, namely fluctuations in symptom severity. Prior work with the MSLT has shown that MSLT retest reliability is highly dependent on the population under study. In healthy subjects, the MSL has retest reliability as high as 0.97 over 4-14 months.20 In contrast, in Parkinson disease, the correlation between mean sleep latencies on consecutive days is only 0.53-0.73,21 and in insomniacs studied over eight months, it is only 0.44.22
Alternatively, there were limitations to our study that might have affected the results. Only patients with a clinical indication for repeat MSLT, i.e., persistent sleepiness, were eligible for inclusion. This may have created selection bias for more severely affected, medically refractory patients. A spontaneous remission rate of 11% has been reported in patients with idiopathic hypersomnia.13 As all patients in the present study reported persistent subjective sleepiness, our data do not address the test retest reliability of the MSLT in patients with primary hypersomnias but spontaneous remission of symptoms. Repeat testing was less often performed when the initial diagnostic MSLT was normal (< 20%). The choice to retest patients with MSLT-based diagnoses of idiopathic hypersomnia or narcolepsy without cataplexy might reflect physician or patient discomfort with these diagnoses, in which pathophysiology, treatment, socioeconomic, and prognostic implications remain ill-defined.23 In idiopathic hypersomnia in particular, evidencebased treatment standards are lacking,24 and data suggest that wake promoting agents are often incompletely effective.13,25 The average of 4 years between studies, with a maximum of 17 years, speaks to the chronicity of sleepiness experienced by these patients, but might have contributed to poor test-retest reliability. We sought to determine the reliability of testing as it is performed in clinical practice, rather than in a controlled, prospective research setting. With such a study design, as in clinical practice, factors such as treatment effects and changes in sleepwake cycle that may affect the MSLT could not be held constant. We were unable to detect any relationships between medication changes, time between MSLTs, or other available clinical features (excluding sleeprelated hallucinations) with a change in diagnosis, but our relatively small sample size does not allow us to entirely rule out this possibility. Other factors for which data were not available, such as changes in depression severity or sleep wake schedule, might have influenced the results. Alternatively, differences in the performance or interpretation of MSLTs may have affected the results (e.g., the MSLTs were generally performed at different sleep laboratories and interpreted by different individuals). The MSLT, for example, is well known to be affected by physiological levels of arousal that are distinct from sleepiness.26 The factors influencing arousal per se can be difficult to control on a single clinical MSLT, let alone a second one performed in a unique environment and under different conditions. It is less likely that scoring variability accounts for our findings as the MSLT has high interrater and intrarater reliability.14,27,28 Given these factors, our data suggests poor test-retest reliability of the MSLT in the nonhypocretin deficient CNS hypersomnias when used over time in clinical practice, although it is possible that test retest reliability would improve if tested within the confines of a tightly controlled research protocol.
Our results suggest that continued adherence to the 8-minute MSL threshold in defining hypersomnia syndromes in clinical practice is problematic. The distinction between narcolepsy without cataplexy and idiopathic hypersomnia based on MSLT testing alone also does not appear justified. It is possible that idiopathic hypersomnia and narcolepsy without cataplexy are manifestations of the same underlying pathology or exist along a spectrum with overlapping features. Family studies of narcolepsy (with and without cataplexy) support this assertion, as family members of narcoleptics have higher rates of narcolepsy, but also of idiopathic hypersomnia, excessive daytime sleepiness, and abnormal multiple sleep latency tests.29–32 Idiopathic hypersomnia is sometimes characterized as a rare disease,19 but one implication of our findings is that prevalence estimates in clinical or population cohorts are likely to be underestimates. Alternative diagnostic strategies are needed to more accurately and reliably characterize the nonhypocretin deficient CNS hypersomnias. Recognizing this need, some investigators have advocated for continuous daytime polysomnography, ad libitum sleep polysomnography, or changes to the MSLT scoring criteria.9,12,33 Similar to the MSLT, however, these alternatives are time and labor intensive. More cost-effective measures are needed, in addition to identification of a biomarker with diagnostic and therapeutic significance. While deficiencies in histamine have been proffered as one such biomarker,2 these results were not replicable with more sensitive technologies.34 Recent work suggests that somnolence in the CNS hypersomnias may derive from a gain in function in endogenous γ-aminobutyric acid (GABA) signaling mediated by a naturally occurring constituent of cerebrospinal fluid that allosterically modulates GABAA receptors.35 Ultimately, the identification of biomarkers will improve diagnostic accuracy in these conditions.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Trotti has consulted for UCB Pharma, Inc. Dr. Rye has consulted for or served on advisory boards of UCB Pharma Inc., Merck, Inc., Impax Laboratories, and Jazz Pharmaceuticals. Dr. Staab has indicated no financial conflicts of interest.
REFERENCES
- 1.Bassetti C, Gugger M, Bischof M, et al. The narcoleptic borderland: a multimodal diagnostic approach including cerebrospinal fluid levels of hypocretin-1 (orexin A) Sleep Med. 2003;4:7–12. doi: 10.1016/s1389-9457(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 2.Kanbayashi T, Kodama T, Kondo H, et al. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep. 2009;32:181–7. doi: 10.1093/sleep/32.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dauvilliers Y, Baumann CR, Carlander B, et al. CSF hypocretin-1 levels in narcolepsy, Kleine-Levin syndrome, and other hypersomnias and neurological conditions. J Neurol Neurosurg Psychiatry. 2003;74:1667–73. doi: 10.1136/jnnp.74.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heier MS, Evsiukova T, Vilming S, Gjerstad MD, Schrader H, Gautvik K. CSF hypocretin-1 levels and clinical profiles in narcolepsy and idiopathic CNS hypersomnia in Norway. Sleep. 2007;30:969–73. doi: 10.1093/sleep/30.8.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khatami R. Idiopathic hypersomnia. In: Baumann CR, Bassetti CL, Scammell TE, editors. Narcolepsy: pathophysiology, diagnosis and treatment. Springer; 2011. pp. 357–66. [Google Scholar]
- 6.Billiard M. Idiopathic hypersomnia. In: Thorpy M, Billiard M, editors. Sleepiness: causes, consequence, and treatment. NY: Cambridge University Press; 2011. pp. 126–35. [Google Scholar]
- 7.American Academy of Sleep Medicine. International classification of sleep disorders: diagnositic and coding manual (ICSD-2). 2nd ed. Westchester, Illinois: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 8.Vernet C, Arnulf I. Narcolepsy with long sleep time: a specific entity? Sleep. 2009;32:1229–35. doi: 10.1093/sleep/32.9.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32:753–9. doi: 10.1093/sleep/32.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth B. Narcolepsy and hypersomnia. New York: Karger; 1980. [Google Scholar]
- 11.Folkerts M, Rosenthal L, Roehrs T, et al. The reliability of the diagnostic features in patients with narcolepsy. Biol Psychiatry. 1996;40:208–14. doi: 10.1016/0006-3223(95)00383-5. [DOI] [PubMed] [Google Scholar]
- 12.Pizza F, Vandi S, Detto S, et al. Different sleep onset criteria at the multiple sleep latency test (MSLT): an additional marker to differentiate central nervous system (CNS) hypersomnias. J Sleep Res. 2011;20:250–6. doi: 10.1111/j.1365-2869.2009.00808.x. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KN, Pilsworth S, Sharples LD, Smith IE, Shneerson JM. Idiopathic hypersomnia: a study of 77 cases. Sleep. 2007;30:1274–81. doi: 10.1093/sleep/30.10.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the Multiple Sleep Latency Test in community adults. Brain. 2006;129:1609–23. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 15.Amira SA, Johnson TS, Logowitz NB. Diagnosis of narcolepsy using the multiple sleep latency test: analysis of current laboratory criteria. Sleep. 1985;8:325–31. doi: 10.1093/sleep/8.4.325. [DOI] [PubMed] [Google Scholar]
- 16.Chervin RD, Aldrich MS. Sleep onset REM periods during multiple sleep latency tests in patients evaluated for sleep apnea. Am J Respir Crit Care Med. 2000;161:426–31. doi: 10.1164/ajrccm.161.2.9905071. [DOI] [PubMed] [Google Scholar]
- 17.Rye DB, Bliwise DL, Dihenia B, Gurecki P. FAST TRACK: daytime sleepiness in Parkinson's disease. J Sleep Res. 2000;9:63–9. doi: 10.1046/j.1365-2869.2000.00201.x. [DOI] [PubMed] [Google Scholar]
- 18.Singh M, Drake CL, Roth T. The prevalence of multiple sleep-onset REM periods in a population-based sample. Sleep. 2006;29:890–5. doi: 10.1093/sleep/29.7.890. [DOI] [PubMed] [Google Scholar]
- 19.Mignot E. Excessive daytime sleepiness: population and etiology versus nosology. Sleep Med Rev. 2008;12:87–94. doi: 10.1016/j.smrv.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Zwyghuizen-Doorenbos A, Roehrs T, Schaefer M, Roth T. Test-retest reliability of the MSLT. Sleep. 1988;11:562–5. doi: 10.1093/sleep/11.6.562. [DOI] [PubMed] [Google Scholar]
- 21.Trotti L, Rye D, Bliwise D. Comment on Shpirer et al. (“Excessive daytime sleepiness in patients with Parkinson's disease: a polysomnographic study”) Mov Disord. 2007;22:1520. doi: 10.1002/mds.21487. [DOI] [PubMed] [Google Scholar]
- 22.Roehrs TA, Randall S, Harris E, Maan R, Roth T. MSLT in primary insomnia: stability and relation to nocturnal sleep. Sleep. 2011;34:1647–52. doi: 10.5665/sleep.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayon V, Leger D, Philip P. Socio-professional handicap and accidental risk in patients with hypersomnias of central origin. Sleep Med Rev. 2009;13:421–6. doi: 10.1016/j.smrv.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–11. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavault S, Dauvilliers Y, Drouot X, et al. Benefit and risk of modafinil in idiopathic hypersomnia vs. narcolepsy with cataplexy. Sleep Med. 2011;12:550–6. doi: 10.1016/j.sleep.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Bonnet MH. ACNS clinical controversy: MSLT and MWT have limited clinical utility. J Clin Neurophysiol. 2006;23:50–8. doi: 10.1097/01.wnp.0000190415.83841.17. [DOI] [PubMed] [Google Scholar]
- 27.Drake CL, Rice MF, Roehrs TA, Rosenthal L, Guido P, Roth T. Scoring reliability of the multiple sleep latency test in a clinical population. Sleep. 2000;23:911–3. [PubMed] [Google Scholar]
- 28.Chen L, Ho CK, Lam VK, et al. Interrater and intrarater reliability in multiple sleep latency test. J Clin Neurophysiol. 2008;25:218–21. doi: 10.1097/WNP.0b013e31817f36a6. [DOI] [PubMed] [Google Scholar]
- 29.Guilleminault C, Mignot E, Grumet FC. Familial patterns of narcolepsy. Lancet. 1989;2:1376–9. doi: 10.1016/s0140-6736(89)91977-6. [DOI] [PubMed] [Google Scholar]
- 30.Hublin C, Partinen M, Koskimies S. Familial narcolepsy in Finland. Acta Neurol Scand. 1991;83:388–93. doi: 10.1111/j.1600-0404.1991.tb03969.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Fong SY, Lam CW, et al. The familial risk and HLA susceptibility among narcolepsy patients in Hong Kong Chinese. Sleep. 2007;30:851–8. doi: 10.1093/sleep/30.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wing YK, Chen L, Lam SP, et al. Familial aggregation of narcolepsy. Sleep Med. 2011;12:947–51. doi: 10.1016/j.sleep.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Pizza F, Moghadam KK, Vandi S, et al. Daytime continuous polysomnography predicts MSLT results in hypersomnias of central origin. J Sleep Res. 2013;22:32–40. doi: 10.1111/j.1365-2869.2012.01032.x. [DOI] [PubMed] [Google Scholar]
- 34.Dauvilliers Y, Delallee N, Jaussent I, et al. Normal cerebrospinal fluid histamine and tele-methylhistamine levels in hypersomnia conditions. Sleep. 2012;35:1359–66. doi: 10.5665/sleep.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rye DB, Bliwise DL, Parker K, et al. Modulation of vigilance in the primary hypersomnias by endogenous enhancement of GABAA receptors. Sci Transl Med. 2012;4:161ra51. doi: 10.1126/scitranslmed.3004685. [DOI] [PubMed] [Google Scholar]