Abstract
We have studied the interaction between virulent egg yolk-grown Legionella pneumophila Philadelphia 1 and human blood monocytes in vitro. The leukocytes were cultured in antibiotic-free tissue culture medium supplemented with 15% autologous human serum.
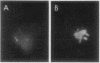
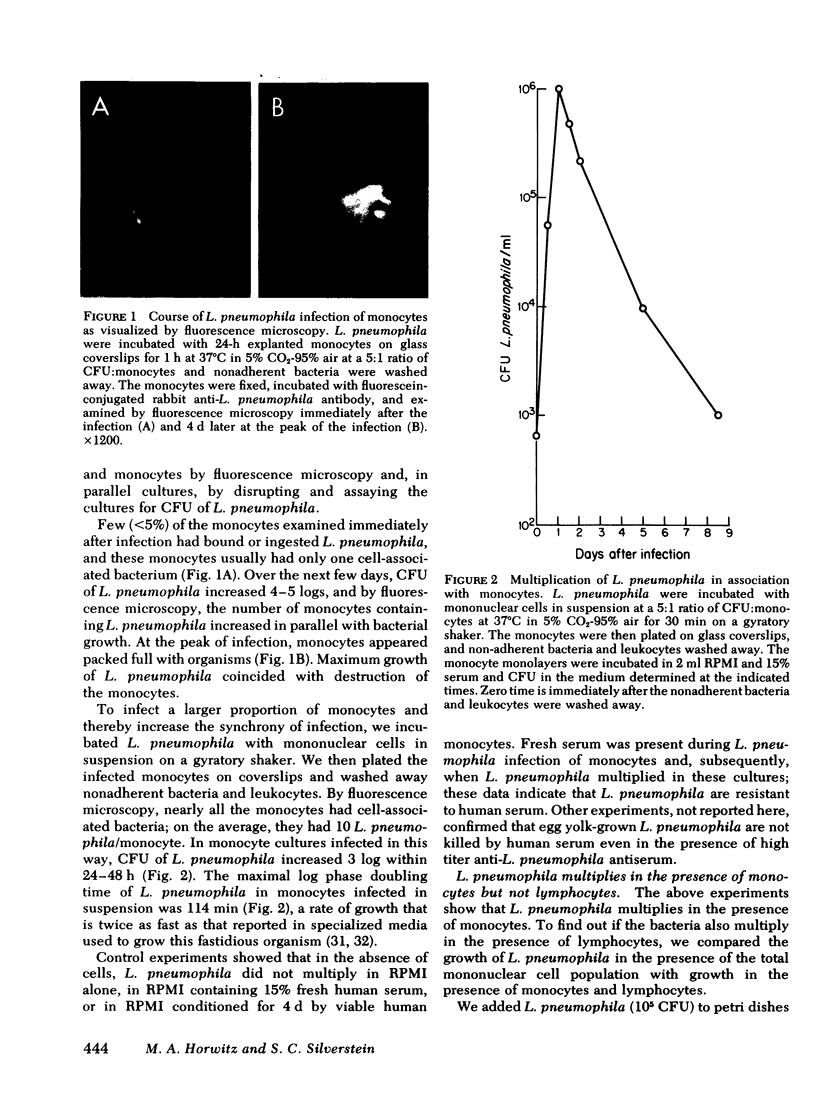
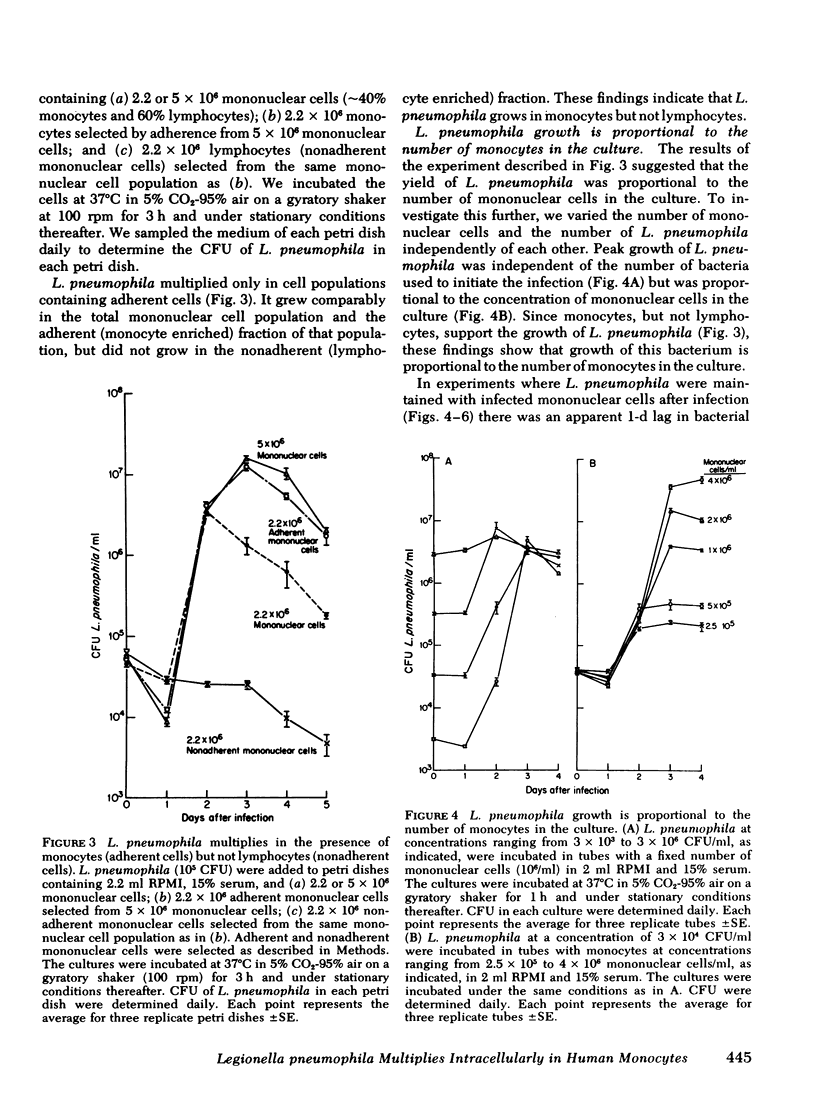
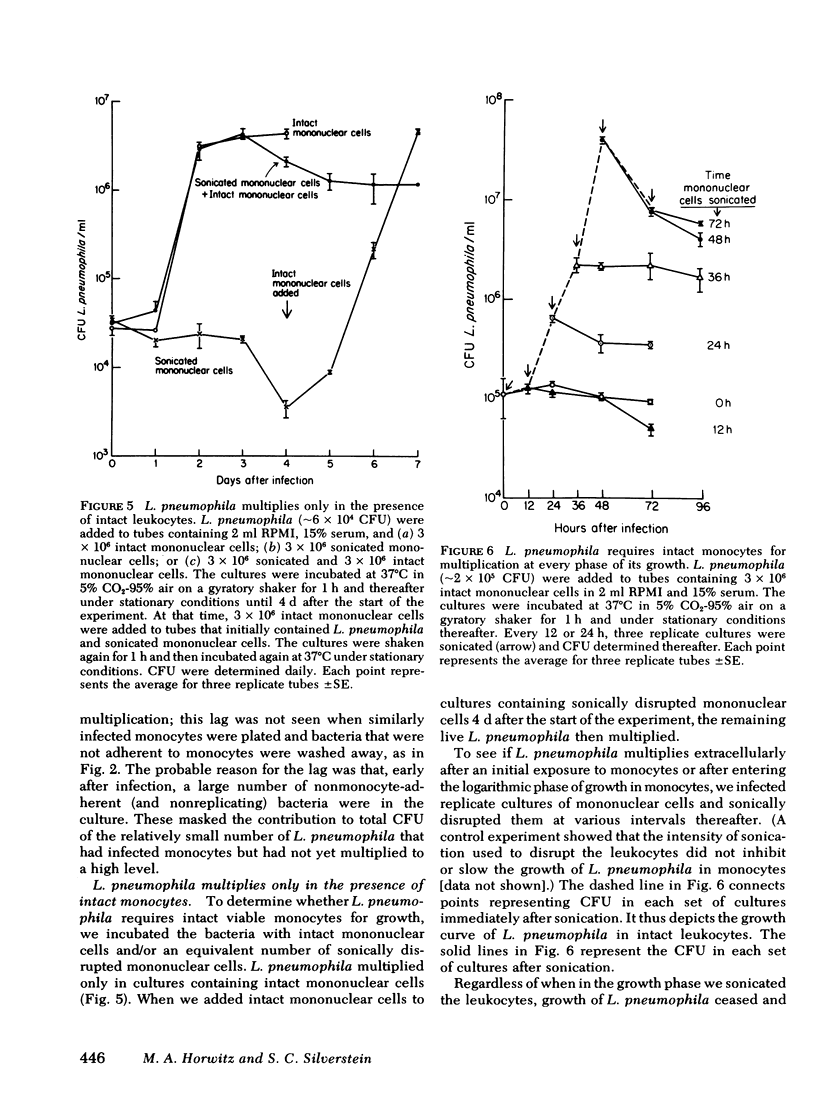
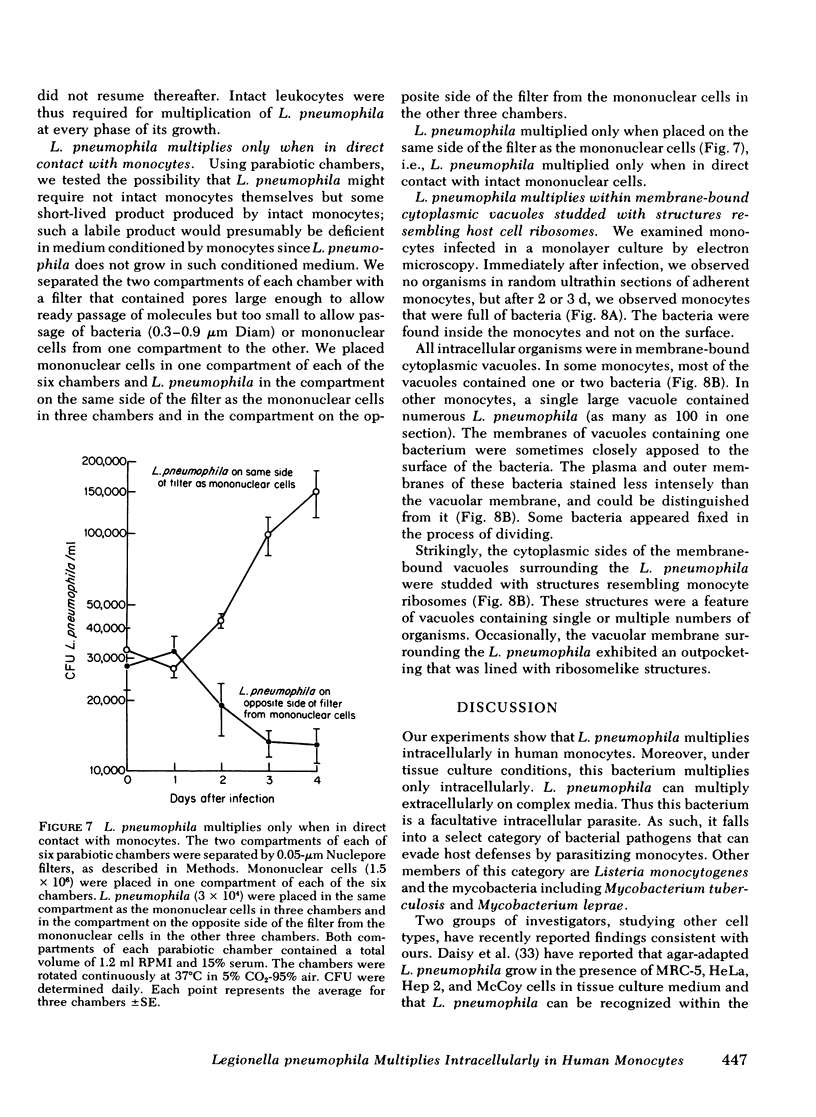
L. pneumophila multiplied several logs, as measured by colony-forming units, when incubated with monocytes or mononuclear cells; the mid-log phase doubling time was 2 h. The level to which L. pneumophila multiplied was proportional to the number of mononuclear cells in the culture. L. pneumophila multiplied only in the adherent fraction of the mononuclear cell population indicating that monocytes but not lymphocytes support growth of the bacteria. Peak growth of L. pneumophila was correlated with destruction of the monocyte monolayer. By fluorescence microscopy using fluorescein conjugated rabbit anti-L. pneumophila antiserum, the number of monocytes containing L. pneumophila increased in parallel with bacterial growth in the culture. At the peak of infection, monocytes were packed full with organisms. By electron microscopy, L. pneumophila in such monocytes were found in membrane-bound cytoplasmic vacuoles studded with structures resembling host cell ribosomes.
Several lines of evidence indicate that L. pneumophila grows within monocytes. (a) In the absence of leukocytes, L. pneumophila did not grow in tissue culture medium with or without serum even if the medium was conditioned by monocytes. (b) L. pneumophila did not grow in sonicated mononuclear cells. Lysis of these cells at various times during logarithmic growth of L. pneumophila was followed by cessation of bacterial multiplication. Growth resumed when intact mononuclear cells were added back to the culture. (3) In parabiotic chambers separated by 0.1-μm Nuclepore filters, L. pneumophila multiplied only when placed on the same side of the filter as mononuclear cells.
These findings indicate that L. pneumophila falls into a select category of bacterial pathogens that evade host defenses by parasitizing monocytes. It remains to be determined whether cell-mediated immunity plays a dominant role in host defense against L. pneumophila as it does against other intracellular pathogens.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Vickerman K. Fusion of host cell secondary lysosomes with the parasitophorous vacuoles of Leishmania mexicana-infected macrophages. J Protozool. 1975 Nov;22(4):502–508. doi: 10.1111/j.1550-7408.1975.tb05219.x. [DOI] [PubMed] [Google Scholar]
- Anderson S. E., Bautista S., Remington J. S. Induction of resistance to Toxoplasma gondii in human macrophages by soluble lymphocyte products. J Immunol. 1976 Aug;117(2):381–387. [PubMed] [Google Scholar]
- Anderson S. E., Jr, Remington J. S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974 May 1;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett C. L. Sporadic cases of Legionnaires' disease in Great Britain. Ann Intern Med. 1979 Apr;90(4):592–595. doi: 10.7326/0003-4819-90-4-592. [DOI] [PubMed] [Google Scholar]
- Bock B. V., Kirby B. D., Edelstein P. H., George W. L., Snyder K. M., Owens M. L., Hatayama C. M., Haley C. E., Lewis R. P., Meyer R. D. Legionnaires' disease in renal-transplant recipients. Lancet. 1978 Feb 25;1(8061):410–413. doi: 10.1016/s0140-6736(78)91202-3. [DOI] [PubMed] [Google Scholar]
- Borges J. S., Johnson W. D., Jr Inhibition of multiplication of Toxoplasma gondii by human monocytes exposed to T-lymphocyte products. J Exp Med. 1975 Feb 1;141(2):483–496. doi: 10.1084/jem.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cahall D. L., Youmans G. P. Conditions for production, and some characteristics, of mycobacterial growth inhibitory factor produced by spleen cells from mice immunized with viable cells of the attenuated H37Ra strain of Mycobacterium tuberculosis. Infect Immun. 1975 Oct;12(4):833–840. doi: 10.1128/iai.12.4.833-840.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler F. W., Hicklin M. D., Blackmon J. A. Demonstration of the agent of Legionnaires' disease in tissue. N Engl J Med. 1977 Dec 1;297(22):1218–1220. doi: 10.1056/NEJM197712012972206. [DOI] [PubMed] [Google Scholar]
- Chandler F. W., McDade J. E., Hicklin M. D., Blackmon J. A., Thomason B. M., Ewing E. P., Jr Pathologic findings in guinea pigs inoculated intraperitoneally with the Legionnaires' disease bacterium. Ann Intern Med. 1979 Apr;90(4):671–675. doi: 10.7326/0003-4819-90-4-671. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Broome C. V., Paris A. L., Martin W. T., Allen J. R. Fatal nosocomial Legionnaires' disease: clinical and epidemiologic characteristics. Ann Intern Med. 1979 Apr;90(4):611–613. doi: 10.7326/0003-4819-90-4-611. [DOI] [PubMed] [Google Scholar]
- Cole P. Activation of mouse peritoneal cells to kill Listeria monocytogenes by T-lymphocyte products. Infect Immun. 1975 Jul;12(1):36–41. doi: 10.1128/iai.12.1.36-41.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff T. C. Epidemiology of Legionnaires' disease. Ann Intern Med. 1979 Apr;90(4):499–502. doi: 10.7326/0003-4819-90-4-499. [DOI] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. W., McDade J. E. Legionellosis. Sci Am. 1979 Oct;241(4):82–99. doi: 10.1038/scientificamerican1079-82. [DOI] [PubMed] [Google Scholar]
- Fraser D. W., Tsai T. R., Orenstein W., Parkin W. E., Beecham H. J., Sharrar R. G., Harris J., Mallison G. F., Martin S. M., McDade J. E. Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 1977 Dec 1;297(22):1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- Glavin F. L., Winn W. C., Jr, Craighead J. E. Ultrastructure of lung in Legionnaires' disease. Observations of three biopsies done during the Vermont epidemic. Ann Intern Med. 1979 Apr;90(4):555–559. doi: 10.7326/0003-4819-90-4-555. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Phagocyte lysosomes: interactions with infectious agents, phagosomes, and experimental perturbations in function. Annu Rev Microbiol. 1977;31:507–533. doi: 10.1146/annurev.mi.31.100177.002451. [DOI] [PubMed] [Google Scholar]
- Gregory D. W., Schaffner W., Alford R. H., Kaiser A. B., McGee Z. A. Sporadic cases of Legionnaires' disease: the expanding clinical spectrum. Ann Intern Med. 1979 Apr;90(4):518–521. doi: 10.7326/0003-4819-90-4-518. [DOI] [PubMed] [Google Scholar]
- Haley C. E., Cohen M. L., Halter J., Meyer R. D. Nosocomial Legionnaires' disease: a continuing common-source epidemic at Wadsworth Medical Center. Ann Intern Med. 1979 Apr;90(4):583–586. doi: 10.7326/0003-4819-90-4-583. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A. Strain virulence and the lysosomal response in macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1974 Oct;10(4):742–746. doi: 10.1128/iai.10.4.742-746.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Len L., Hirsch J. G. Assessment in vitro of immunity against Toxoplasma gondii. J Exp Med. 1975 Feb 1;141(2):466–482. doi: 10.1084/jem.141.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T., Youmans G. P. The in vitro inhibition of growth of intracellular Listeria monocytogenes by lymphocyte products. Cell Immunol. 1973 Dec;9(3):353–362. doi: 10.1016/0008-8749(73)90050-6. [DOI] [PubMed] [Google Scholar]
- Kishimoto R. A., Kastello M. D., White J. D., Shirey F. G., McGann V. G., Larson E. W., Hedlund K. W. In vitro interaction between normal cynolmolgus monkey alveolar macrophages and Legionnaires disease bacteria. Infect Immun. 1979 Aug;25(2):761–763. doi: 10.1128/iai.25.2.761-763.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauel J., Buchmüller Y., Behin R. Studies on the mechanisms of macrophage activation. I. Destruction of intracellular Leishmania enriettii in macrophages activated by cocultivation with stimulated lymphocytes. J Exp Med. 1978 Aug 1;148(2):393–407. doi: 10.1084/jem.148.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade J. E., Brenner D. J., Bozeman F. M. Legionnaires' disease bacterium isolated in 1947. Ann Intern Med. 1979 Apr;90(4):659–661. doi: 10.7326/0003-4819-90-4-659. [DOI] [PubMed] [Google Scholar]
- McDade J. E., Shepard C. C., Fraser D. W., Tsai T. R., Redus M. A., Dowdle W. R. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977 Dec 1;297(22):1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- McDade J. E., Shepard C. C. Virulent to avirulent conversion of Legionnaires' disease bacterium (Legionella pneumophila)--its effect on isolation techniques. J Infect Dis. 1979 Jun;139(6):707–711. doi: 10.1093/infdis/139.6.707. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Gordon S., Cohn Z. Trypanosoma cruzi: modification of macrophage function during infection. J Exp Med. 1977 Jul 1;146(1):157–171. doi: 10.1084/jem.146.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M. L. Legionnaires' disease in a renal-transplant program. N Engl J Med. 1978 May 11;298(19):1089–1089. doi: 10.1056/NEJM197805112981915. [DOI] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner E. D., Helms C. M., Hierholzer W. J., Jr, Hall N., Wong Y. W., Viner J. P., Johnson W., Hausler W. J., Jr Legionnaires' disease in pneumonia patients in Iowa. A retrospective seroepidemiologic study, 1972-1977. Ann Intern Med. 1979 Apr;90(4):603–606. doi: 10.7326/0003-4819-90-4-603. [DOI] [PubMed] [Google Scholar]
- Sanford J. P. Legionnaires' disease--the first thousand days. N Engl J Med. 1979 Mar 22;300(12):654–656. doi: 10.1056/NEJM197903223001205. [DOI] [PubMed] [Google Scholar]
- Sanford J. P. Legionnaires' disease: one person's perspective. Ann Intern Med. 1979 Apr;90(4):699–703. doi: 10.7326/0003-4819-90-4-699. [DOI] [PubMed] [Google Scholar]
- Saravolatz L. D., Burch K. H., Fisher E., Madhavan T., Kiani D., Neblett T., Quinn E. L. The compromised host and Legionnaires' disease. Ann Intern Med. 1979 Apr;90(4):533–537. doi: 10.7326/0003-4819-90-4-533. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Pelster B., Suzuki N., Piekarski G., Brandis H. Immunity to Toxoplasma gondii induced in vitro in non-immune mouse macrophages with specifically immune lymphocytes. J Immunol. 1975 Oct;115(4):1151–1158. [PubMed] [Google Scholar]
- Shirahata T., Shimizu K., Noda S., Suzuki N. Studies on production of biologically active substance which inhibits the intracellular multiplication of Toxoplasma within mouse macrophages. Z Parasitenkd. 1977 Aug 25;53(1):31–40. doi: 10.1007/BF00383112. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Storch G., Baine W. B., Fraser D. W., Broome C. V., Clegg H. W., 2nd, Cohen M. L., Goings S. A., Politi B. D., Terranova W. A., Tsai T. F. Sporadic community-acquired Legionnaires' disease in the United States. A case-control study. Ann Intern Med. 1979 Apr;90(4):596–600. doi: 10.7326/0003-4819-90-4-596. [DOI] [PubMed] [Google Scholar]
- Wang W. L., Blaser M. J., Cravens J., Johnson M. A. Growth, survival, and resistance of the Legionnaires' disease bacterium. Ann Intern Med. 1979 Apr;90(4):614–618. doi: 10.7326/0003-4819-90-4-614. [DOI] [PubMed] [Google Scholar]
- Warren W. J., Miller R. D. Growth of Legionnaires disease bacterium (Legionella pneumophila) in chemically defined medium. J Clin Microbiol. 1979 Jul;10(1):50–55. doi: 10.1128/jcm.10.1.50-55.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]