Abstract
Dendritic cell-based anticancer immunotherapy is feasible, safe and results in the induction of tumor-specific immune responses, at least in a fraction of vaccinated patients. The concomitant activation of cytotoxic and helper T cells, by loading DCs with peptides or electroporating them with the corresponding mRNAs, may further enhance vaccine-induced antitumor responses.
Keywords: cancer immunotherapy, dendritic cell vaccination, helper T cells, metastatic melanoma
Dendritic cells (DCs) are central players in the induction of adaptive immune responses. Upon infection or inflammation, DCs take up antigens and migrate to lymphoid organs where they activate naïve antigen-specific T and B cells. This unique capacity of DCs is exploited worldwide for DC-based anticancer immunotherapy, a therapeutic approach aimed at eliciting specific antitumor immune responses.1 Thus far, the vaccination of cancer patients with DCs has proven to be feasible, safe and able to promote potent immune responses, provided that DCs had been appropriately matured. Nevertheless, since objective clinical responses are only observed in a minority of patients treated with DCs, further improvements are warranted before their use will be accepted in the standard clinical practice.2
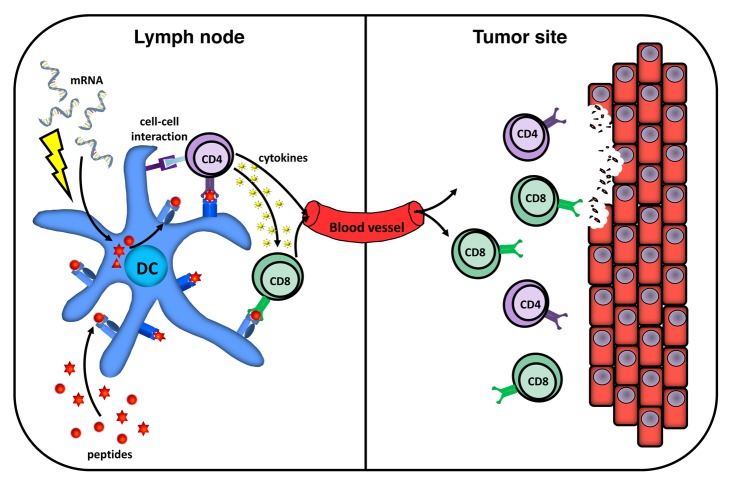
DCs present endogenous antigens complexed with MHC Class I molecules to CD8+ cytotoxic T lymphocytes (CTLs), whereas exogenous antigens are either presented in association to MHC Class II molecules to CD4+ helper T cells or cross-presented on MHC Class I molecules to CD8+ T cells. In most clinical DC-based vaccination studies performed so far, patients have been vaccinated with mature monocyte-derived DCs loaded with synthetic MHC Class I-binding peptides to induce tumor-specific CTLs. However, convincing evidence indicates that both CTLs and helper T cells are important for the induction of strong and sustained antitumor T-cell responses (Fig. 1).3 Helper T cells not only provide growth and differentiation signals to CTL precursors, but also stimulate them by reciprocally activating antigen-presenting cells, enhance their recruitment to lymph nodes and neoplastic lesions, and even exert direct antitumor effector functions. It may therefore be important to establish protocols for the simultaneous activation of both CTLs and helper T cells against tumor-associated antigens (TAAs), rather than that of CTLs alone.
Figure 1. Induction of tumor antigen-specific CD4+ and CD8+ T cells by dendritic cell-based vaccines. To achieve antigen presentation to both CD4+ helper and CD8+ cytotoxic T cells, dendritic cells (DCs) can either be loaded with HLA-binding peptides or electroporated with mRNA encoding full-length tumor-associated antigens (TAAs). Upon intranodal administration, DCs migrate to the T-cell areas of lymph nodes where they present TAA-derived peptides to and activate antigen-specific CD4+ and CD8+ T lymphocytes. CD4+ T cells provide help to the CD8+ counterparts via cell-to-cell interactions and by secreting several cytokines. Both activated antigen-specific CD4+ and CD8+ T cells are involved in tumor eradication.
In metastatic melanoma patients, we investigated the immunologic and clinical responses to the intranodal administration of monocyte-derived DCs pulsed either with MHC class I-restricted TAA-derived peptides alone or with both MHC Class I and II-binding TAA-derived epitopes simultanoeusly.4 In both groups of patients, we detected TAA-specific CD8+ T cells by means of delayed type hypersensitivity (DTH) skin tests. Nevertheless, only upon vaccination with MHC Class I/II-loaded DCs, patients developed highly functional TAA-specific CD8+ T-cells that maintained their interferon γ (IFNγ)-secreting potential even in the presence of an immunosuppressive milieu such as that generated by an interleukin (IL)-10-producing melanoma cell line. Interestingly, in this group of patients we also detected circulating TAA-specific CD4+ T cells, coinciding with their CD8+ counterparts. These findings strongly support the hypothesis that the co-activation of TAA-specific helper T cells stimulates the proliferation and effector functions of CD8+ T cells.5 When we compared the clinical outcome of metastatic melanoma patients vaccinated with DCs with carefully matched control patients treated with standard dacarbazine-based chemotherapy, only patients who received MHC Class I/II-loaded DCs exhibited a significant increase in progression-free and overall survival, suggesting that the co-activation of CD4+ T cells contributes to improved clinical responses upon DC vaccination.
Unfortunately, MHC-binding peptides are restricted to a given HLA type and may dissociate from HLA molecules due to turnover or low affinity. In addition, TAA-elicited immune responses are limited to defined epitopes, although a phenomenon of “epitope spreading” has been described upon vaccination. Electroporating DCs with a synthetic mRNA coding for specific TAAs circumvents the disadvantages of peptide pulsing and results in the endogenous synthesis of full-length TAAs as well as, depending on the presence of endosome-targeting sequences, antigen presentation on both MHC Class I and II molecules. We have demonstrated that mature monocyte-derived DCs highly and sustainably express TAAs upon mRNA electroporation6 and that TAA expression can be detected within lymph nodes 24 h after vaccination.7 In DTH tissue biopsies from patients receiving these cells a wide spectrum of tumor-specific T cells was detected.8 Importantly, in the peripheral blood of these patients the presence of vaccine-induced CD4+ T cells did not correlate with the presence of TAA-specific CD8+ T cells. This might suggest that DCs electroporated with TAA-encoding mRNA are less potent in inducing immune responses than their peptide-loaded counterparts. More likely, however, the total repertoire of TAA-specific T cells was underestimated in this study, owing to limitations in immunomonitoring with HLA-A2- and HLA-DR4-binding peptides. Indeed, our data suggest that T cells with a broader specificity than HLA-A2 and HLA-DR4 epitopes were induced.8
Also patients receiving DCs electroporated with TAA-encoding mRNA exhibited a trend toward improved overall survival as compared with matched controls. Moreover, the results confirm and extend our previous findings indicating that the presence of functional tumor-specific T cells in DTH tissue biopsies correlates with clinical outcome following DC-based vaccination.9,10 Unfortunately, the groups of patients involved in this study were too small and variable to statistically compare the immunological and clinical outcomes upon vaccination with peptide-loaded or mRNA-electroporated DCs. Nevertheless, our findings from two independent studies indicate that DC-based vaccines can induce tumor-specific CTL and helper T-cell responses that correlate with improved overall survival, provide a rationale for larger prospective studies in the future.
Both immunological and clinical responses, as measured in the context of carefully designed clinical trials, support the therapeutic potential of DC-based vaccination. Although clinical evidence is still limited, the responses that have been observed are often long-lasting. These findings call for studies aimed at further optimizing DC-based vaccines to induce a favorable clinical outcome in an ever increasing number of patients. Several variables are already being evaluated, including the maturation of DCs, the route of administration, and the use of natural circulating DC subsets.2 Additional aspects that are being explored include (1) extending the antigen repertoire based on the profiling of MHC-bound peptides on tumor cells of individual patients, (2) targeting DCs in vivo and (3) developing combinatorial approaches to overcome local and systemic immunosuppression and to limit immune escape mechanisms. In conclusion, the potential of DC-based immunotherapy has not yet been fully exploited and DC-based anticancer vaccines may constitute a valuable alternative to conventional chemotherapy.
Glossary
Abbreviations:
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- DTH
delayed type hypersensitivity
- TAA
tumor-associated antigen
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24440
References
- 1.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesterhuis WJ, Aarntzen EHJG, De Vries IJ, Schuurhuis DH, Figdor CG, Adema GJ, et al. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol. 2008;66:118–34. doi: 10.1016/j.critrevonc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–8. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res. 2013;73:19–29. doi: 10.1158/0008-5472.CAN-12-1127. [DOI] [PubMed] [Google Scholar]
- 5.Ossendorp F, Mengedé E, Camps M, Filius R, Melief CJM. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuurhuis DH, Lesterhuis WJ, Kramer M, Looman MG, van Hout-Kuijer M, Schreibelt G, et al. Polyinosinic polycytidylic acid prevents efficient antigen expression after mRNA electroporation of clinical grade dendritic cells. Cancer Immunol Immunother. 2009;58:1109–15. doi: 10.1007/s00262-008-0626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuurhuis DH, Verdijk P, Schreibelt G, Aarntzen EH, Scharenborg N, de Boer A, et al. In situ expression of tumor antigens by messenger RNA-electroporated dendritic cells in lymph nodes of melanoma patients. Cancer Res. 2009;69:2927–34. doi: 10.1158/0008-5472.CAN-08-3920. [DOI] [PubMed] [Google Scholar]
- 8.Aarntzen EH, Schreibelt G, Bol K, Lesterhuis WJ, Croockewit AJ, de Wilt JH, et al. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clin Cancer Res. 2012;18:5460–70. doi: 10.1158/1078-0432.CCR-11-3368. [DOI] [PubMed] [Google Scholar]
- 9.Aarntzen EH, Bol K, Schreibelt G, Jacobs JF, Lesterhuis WJ, Van Rossum MM, et al. Skin-test infiltrating lymphocytes early predict clinical outcome of dendritic cell-based vaccination in metastatic melanoma. Cancer Res. 2012;72:6102–10. doi: 10.1158/0008-5472.CAN-12-2479. [DOI] [PubMed] [Google Scholar]
- 10.de Vries IJM, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJ, et al. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol. 2005;23:5779–87. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]