Abstract
Tumor-associated macrophages polarize toward an M2 phenotype and express scavenger receptor A (SRA), hence promoting tumor progression. We demonstrated that SRA can be therapeutically targeted in vivo with the small peptide inhibitor 4F to prevent metastatic spread. Beyond our study, 4F is emerging as a promising anticancer agent.
Keywords: scavenger receptor, tumor-associated macrophage, ligand, apo A-I mimetic peptide, co-culture
Tumor-associated macrophages (TAMs) constitute attractive therapeutic targets because they are a constant part of the tumor microenvironment in many distinct types of malignancy.1 We and others have previously shown that tumor cells can promote the switch of macrophages toward an anti-inflammatory M2 phenotype that is characterized by the expression of scavenger receptor A (SRA).2,3 We established a co-culture model to probe the crosstalk between macrophages and cancer cells for pro-oncogenic cues from either interaction partner. Interestingly, the expression of SRA on TAMs appeared to be necessary for tumor cell invasion in co-culture experiments as well as in vivo, since Msr1−/− mice (lacking SRA upon gene knockout) failed to develop metastases.4
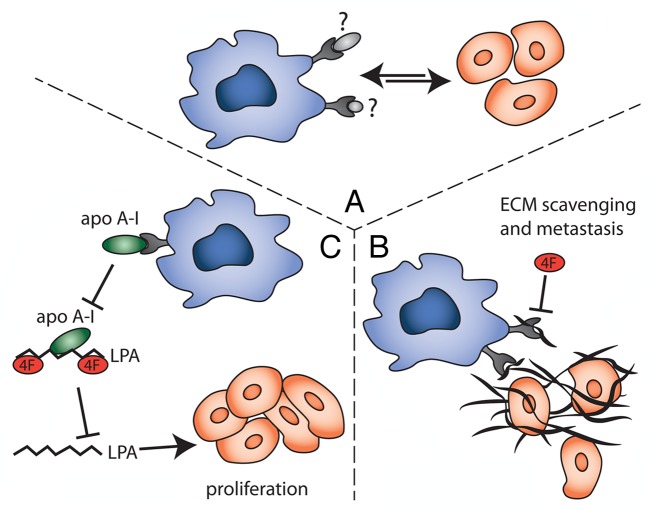
Two hypotheses could be put forward to explain the involvement of SRA in tumorigenesis (Fig. 1): TAMs might use SRA to downregulate inhibitory signals in the tumor microenvironment or to trigger activatory pathways, resulting in release of tumor-promoting mediators. In either case, the identification of an SRA ligand in co-culture experiments was expected to provide mechanistic clues. We therefore screened culture supernatants for SRA ligands and identified both proteins and lipids that are selectively produced by co-cultures but not when TAMs and cancer cells are grown separately.
Figure 1. Therapeutic effects of the scavenger receptor A inhibitor 4F. (A) The co-culture of tumor-associated macrophages (TAMs) and cancer cells results in the production of unidentified scavenger receptor A (SRA) ligands, which promote the TAM/cancer cell crosstalk and metastasis. (B) SRA on TAMs may scavenge extracellular matrix (ECM) components, thereby releasing cancer cells from their primary site and enabling metastasis. 4F might interfere with this interaction and hence, similar to the loss of SRA, prevent tumor cell egress. (C) Lysophosphatidic acid (LPA) stimulates the proliferation and migration of cancer cells, and is scavenged from the circulation by apolipoprotein A1 (APOA1) and/or APOA1 mimetic peptides (such as 4F). By interacting with APOAI, SRA might promote the local release of LPA.
Several protein ligands that we identified are components of the extracellular matrix, a promising finding since scavenger receptors (SRs) are known to interact with modified collagens and extracellular proteoglycans. The repeated incubation of co-culture supernatants with wild-type (WT), but not Msr1−/−, macrophage monolayers depleted the ligand, supporting the hypothesis of SRA-mediated scavenging. Another SR has previously been implicated in clearing the way for migrating tumor cells: stabilin 2 (STAB2, also known as FELL). STAB2 efficiently removes hyaluronic acid from the circulation, and the loss of STAB2 has been shown to suppress metastatic spread in a mouse tumor model.5 If a similar extracellular matrix clearance mechanism proved to underlie the pro-metastatic functions of SRA, it will be worthwhile to investigate inhibitors that would simultaneously target multiple SRs.
The de-lipidation of co-culture supernatants also reduced SRA ligand activity. SRA is able to interact with a range of modified lipids and lipoproteins, and pro-inflammatory lipids not only stimulate tumor growth but also constitute predictive biomarkers, at least in some settings. In the future, it will hence be interesting to characterize the SRA-binding lipids that are produced by TAM/cancer cell co-cultures. This will provide further insights into how bioactive lipids drive the TAM-tumor crosstalk.
We used known ligands to interfere with SRA function, and were able to block tumor cell invasiveness in vitro. This prompted us to test the therapeutic benefits of a small peptidic inhibitor of SRA, 4F, in vivo. This D-amino acid peptide, which is known to block SRA-mediated macrophage adhesion, prevented the progression and metastatic spread of two murine cancer cell lines, ovarian ID8 and pancreatic Panc02 cells, similarly to the complete absence of SRA. Since no additive effects were observed when 4F was administered to Msr1−/− mice, we believe that 4F and SRA operate in the same signaling pathway.
Initially designed to improve cholesterol homeostasis and to prevent atherogenesis through its antioxidant and anti-inflammatory properties, the apolipoprotein A-I (APOA1) mimetic peptide 4F is emerging as a multi-faceted anticancer therapeutic. A study by Su et al. first described the direct antineoplastic effects of 4F against mouse and human ovarian cancer cell lines in vitro. The same authors succeeded in reducing the growth of ID8 ovarian cancer cells in mice, hence improving the survival of tumor-bearing animals.6 The proposed mechanism of action hinges on the ability of 4F to reduce the circulating levels of lysophosphatidic acid (LPA), similar to overexpressed human APOA1. Pro-inflammatory and pro-angiogenic lysophospholipids such as LPA have been repeatedly associated with tumor progression and poor prognosis, and are normally cleared from the serum by APOA1, which is downregulated in ovarian, gastric and pancreatic cancer patients. In a subsequent study the same group demonstrated that the LPA-lowering, antitumor properties of 4F are shared by other apolipoprotein mimetics and can be observed in murine models of both induced and spontaneous colon cancer.7
A follow-up paper further unravelled the mechanism of action of 4F and proposed that 4F-dependent oxidative changes in cancer cells may be responsible for its therapeutic effects.8 4F-treated ID8 cells upregulated the manganese-containing superoxide dismutase (MnSOD), resulting in lower levels of oxidative stress and oxidative damage to macromolecules in the tumor microenvironment. In vivo, ID8 cells depleted of MnSOD became unresponsive to 4F, proving that one 4F exerts antineoplastic effects, at least in part, by modulating the oxidative status of cancer cells.
With regards to our study, it will be interesting to investigate whether APOA1, a known SRA ligand, is indeed cleared from the tumor microenvironment by SRA, and whether this drives the local accumulation of LPA. As noted above, the SRA ligand activity generated by TAM/cancer cell co-cultures decreased upon repeated passaging on macrophage monolayers, which is suggestive of scavenging activity, and we also detected lipid ligands in co-culture supernatants. Whether these two observations are mechanistically linked remains to be determined.
Oxidative stress leads to the modification of multiple macromolecules, including proteins (carbonylation) and lipids (peroxidation), thereby generating potential ligands for SRs. The ability of 4F to exert antioxidant effects in cancer cells may reduce the availability of both SRA-specific and less specific SR ligands in the tumor microenvironment, another plausible connection to explore.
From a therapeutic standpoint, the administration of 4F seems flexible: Su et al. reported that 4F was active regardless of oral or subcutaneous delivery, and although injection resulted in higher plasma levels than ingestion, both routes were efficient in lowering circulating LPA in mice. Bioavailability and safety tests in humans showed that oral, subcutaneous and intravenous 4F is safe and well-tolerated.9,10 Nevertheless, so far the anti-inflammatory effects on circulating serum lipids observed in 4F-treated mice could not be replicated in humans.9 An increasing number of studies reports antineoplastic effects for 4F, raising the urgent need to fully understand how 4F affects tumor progression in order to best deploy its therapeutic benefits.
Glossary
Abbreviations:
- SR
scavenger receptor
- TAM
tumor-associated macrophage
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24461
References
- 1.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 2.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Plüddemann A, et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–32. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 4.Neyen C, Plüddemann A, Mukhopadhyay S, Maniati E, Bossard M, Gordon S, et al. Macrophage scavenger receptor a promotes tumor progression in murine models of ovarian and pancreatic cancer. J Immunol. 2013;190:3798–805. doi: 10.4049/jimmunol.1203194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirose Y, Saijou E, Sugano Y, Takeshita F, Nishimura S, Nonaka H, et al. Inhibition of Stabilin-2 elevates circulating hyaluronic acid levels and prevents tumor metastasis. Proc Natl Acad Sci U S A. 2012;109:4263–8. doi: 10.1073/pnas.1117560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su F, Kozak KR, Imaizumi S, Gao F, Amneus MW, Grijalva V, et al. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:19997–20002. doi: 10.1073/pnas.1009010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su F, Grijalva V, Navab K, Ganapathy E, Meriwether D, Imaizumi S, et al. HDL mimetics inhibit tumor development in both induced and spontaneous mouse models of colon cancer. Mol Cancer Ther. 2012;11:1311–9. doi: 10.1158/1535-7163.MCT-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganapathy E, Su F, Meriwether D, Devarajan A, Grijalva V, Gao F, et al. D-4F, an apoA-I mimetic peptide, inhibits proliferation and tumorigenicity of epithelial ovarian cancer cells by upregulating the antioxidant enzyme MnSOD. Int J Cancer. 2012;130:1071–81. doi: 10.1002/ijc.26079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson CE, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, et al. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J Lipid Res. 2011;52:361–73. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–52. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]