Abstract
Immunoligands for stimulatory natural killer (NK)-cell receptors can be targeted to the surface of malignant cells by fusing them to antibody fragments. Mimicking an “induced-self” phenotype, such recombinant immunoligands signal danger, trigger NK-cell cytotoxicity and synergistically enhance antibody-dependent cellular cytotoxicity. These findings may be translated into novel immunotherapeutic approaches against cancer.
Keywords: ADCC, NK cells, NKG2D, NKp30, antibody
Activating natural killer (NK) cells against tumors represents an attractive treatment option for anticancer immunotherapy. NK cells play a key role in anticancer immunosurveillance, as they are able to recognize malignant cells, exert spontaneous cytotoxicity against them and produce a variety of immunomodulatory cytokines regulating both innate and adaptive immune responses.1 The effector functions of NK cells are governed by a complex interplay between sets of stimulatory and inhibitory cell surface receptors. Various activating receptors such as natural killer group 2 member D (NKG2D), NKp30, NKp44 and NKp46 are believed to scan host cells for the stress-induced expression of self molecules that convey a signal of danger (Fig. 1A). For example, NKG2D recognizes multiple cellular ligands including MHC Class-I related chain A (MICA), MICB and UL16-binding proteins (ULBPs) 1–6.2 All these molecules are rarely expressed on the surface of healthy tissues but become upregulated in response to stressful conditions including heat shock, pathogen infection and malignant transformation. The importance of NKG2D for the antineoplastic functions of NK cells has been illustrated in elegant animal models. However, during tumor progression, malignant cells undergo immunoediting processes and may lose the surface expression of such alert signals to escape the immune system.1 For example, NKG2D ligands have been shown to be shedded from the surface of neoplastic cells by proteolytic cleavage or to be downregulated in response to cancer cell-derived transforming growth factor β (TGFβ).2
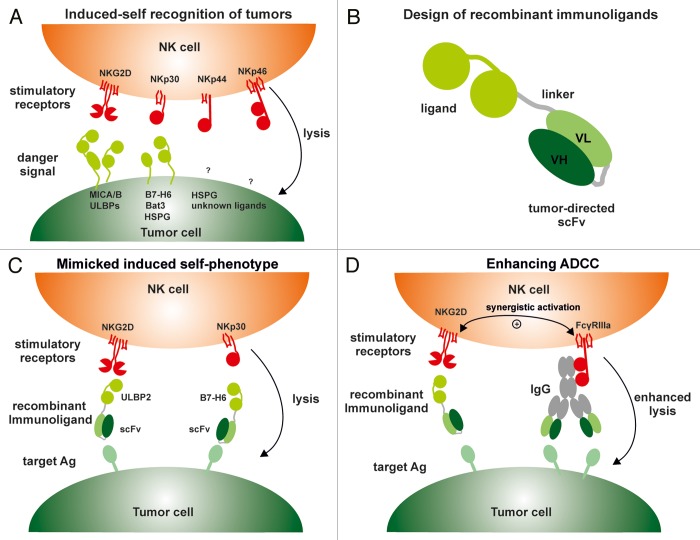
Figure 1. Enhancing natural killer cell cytotoxicity by recombinant immunoligands. (A) Natural killer (NK) cells express a variety of activating receptors such as NKG2D, NKp30, NKp44 or NKp46, binding to stress-induced self molecules that are often expressed on the cell surface upon oncogenic events. The ligands for NKG2D include MHC Class I-related chain A (MICA), MICB as well as UL16-binding proteins (ULBPs) 1–6. NKp30 recognizes B7-H6 and HLA-B-associated transcript 3 (BAT3). NKp44 and NKp46 appear to bind to heparan sulfate proteoglycans (HSPGs), but most likely additional—still elusive—surface proteins expressed by malignant cells function as bona fide ligands. (B) Tumor-directed recombinant ligands are bispecific fusion proteins consisting of a single-chain fragment variable (scFv) specific for a tumor-associated antigen (Ag) fused to the C-terminus of a ligand (e.g., ULBP2) for an activating NK cell receptor (e.g., NKG2D). (C) Binding to a tumor-associated Ag via their scFv moiety, recombinant immunoligands mimick an “induced-self” phenotype, thereby facilitating the recognition of cancer cells by NK cells and inducing NK-cell cytotoxic functions. (D) Recombinant immunoligands engaging NKG2D synergistically enhance antibody-dependent cellular cytotoxicity (ADCC) responses elicited by therapeutic antibodies. VH, heavy chain variable region; VL, light chain variable region.
The importance of stimulatory NK-cell receptors and the corresponding ligands in anticancer immunosurveillance generated the question as to whether this system could be harnessed to manipulate NK cell-based immune responses in the course of anticancer immunotherapy. Interestingly, chemical compounds such as all-trans-retinoic acid were found to stimulate the expression of NKG2D ligands on cancer cells, hence sensitizing them to NK cell-mediated killing.2 In another approach to increase the density of danger signals on the surface of cancer cells, NKG2D ligands were coupled to antibody fragments targeting different antigens associated with solid (e.g., HER2, carcinoembryonic antigen, prostate-specific membrane antigen) or hematological (e.g., CD33, CD138) tumors, either by genetic fusion or chemical conjugation.3-6 Binding to malignant cells thanks to their antibody moiety, such immunoconstructs are able to attach danger ligands to the cell surface and hence mimic an “induced-self” phenotype, which is required for the elicitation of NK cell-driven immune responses (Fig. 1B and C).
To recruit NK cells against malignant B cells, we fused the NKG2D-specific ligands MICA and ULBP2 to a CD20-specific single-chain fragment variable (scFv) derived from the antibody clone 7D8.7 The resulting immunoligands, which we designated ULBP2:7D8 and MICA:7D8, supported the recognition of malignant B cells by the immune system. Thus, lymphoma cells coated with the immunoligands efficiently activated NK-cell cytotoxicity. MICA:7D8 and ULBP2:7D8 were active at nanomolar concentrations and induced a NK cell-mediated cytotoxic response in a strictly CD20-dependent manner. Remarkably, when combined with intact antibodies, MICA:7D8 and ULBP2:7D8 synergistically enhanced antibody-dependent cellular cytotoxicity (ADCC) by NK cells (Fig. 1D), a clinically relevant mechanism of action of many therapeutic antibodies. For example, both MICA:7D8 and ULBP2:7D8 boosted ADCC as triggered by daratumumab. Daratumumab specifically recognizes CD38, a cell surface antigen of blood cells including plasma cells that is co-expressed with CD20 by certain lymphomas and chronic lymphocytic leukemias.7 Whereas the Fcγ receptor IIIa associates with FcεRI γ and CD3 ζ chains, containing immunoreceptor tyrosine-based activation motifs (ITAMs), NKG2D signals in an ITAM-independent fashion, in particular via the adaptor molecule DNAX-activating protein of 10 kDa, bearing an YXXM motif within its cytoplasmic domain and relying on different downstream signal transducers.8 The concomitant activation of these two different signaling pathways may account for the synergistic effects stemming from the use of monoclonal antibodies together with recombinant immunoligands. Thus, combining therapeutic antibodies with recombinant immunoligands that engage NKG2D may represent a novel strategy to improve antibody-based anticancer immunotherapy by enhancing NK cell-mediated ADCC. In addition, this combination strategy allows for the simultaneous targeting of two different tumor-associated antigens. If these target antigens are not co-expressed by healthy tissues, such an approach may potentially offer an elegant way to achieve the preferential killing of malignant cells. However, in contrast to MICA:7D8 and ULBP2:7D8, the CD20-specific antibody 7D8, whose variable regions were used for the construction of the immunoligands, failed to clearly enhance NK-cell cytotoxicity elicited by daratumumab. This suggests that obtaining optimal ADCC responses by combining two tumor-specific monoclonal antibodies may be difficult, probably because they compete for the binding to FcγRIIIa.
We also applied the immunoligand approach to NKp30 and its recently identified ligand B7-H6.9,10 Thus, a recombinant immunoligand containing a CD20-derived scFv and B7-H6, which we designated B7-H6:7D8, efficiently triggered the NK cell-mediated lysis of a broad range of primary leukemia and lymphoma cells. Similar to their NKG2D-targeting counterparts, the immunoligands containing B7-H6 synergistically enhanced ADCC responses elicited by monoclonal antibodies,10 although to lower extents (unpublished data). Moreover, synergistic effects were achieved when B7-H6:7D8 was combined with ULBP2:7D8. This resulted in enhanced NK-cell activation, cytokine production and cytotoxicity, indicating a cooperation between NKp30 and NKG2D in the regulation of NK-cell functions.
In conclusion, stimulatory receptors such as NKG2D and NKp30 deserve further evaluation as activating triggers for antibody-based anticancer therapeutics. With their abilities to promote NK-cell cytotoxicity and to boost ADCC responses elicited by conventional antibodies, recombinant immunoligands directed against tumor-associated antigens and engaging NKG2D or NKp30 may represent attractive agents to potentiate NK-cell responses against cancer.
Glossary
Abbreviations:
- ADCC
antibody-dependent cellular cytotoxicity
- NK
natural killer
- MIC
MHC Class I-related chain, NKG2D, natural killer group 2 member D
- ITAM
immunoreceptor tyrosine-based activation motif
- scFv
single chain fragment variable
- ULBP
UL16-binding protein
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24481
References
- 1.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol. 2009;9:568–80. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–58. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 3.von Strandmann EP, Hansen HP, Reiners KS, Schnell R, Borchmann P, Merkert S, et al. A novel bispecific protein (ULBP2-BB4) targeting the NKG2D receptor on natural killer (NK) cells and CD138 activates NK cells and has potent antitumor activity against human multiple myeloma in vitro and in vivo. Blood. 2006;107:1955–62. doi: 10.1182/blood-2005-05-2177. [DOI] [PubMed] [Google Scholar]
- 4.Jachimowicz RD, Fracasso G, Yazaki PJ, Power BE, Borchmann P, Engert A, et al. Induction of in vitro and in vivo NK cell cytotoxicity using high-avidity immunoligands targeting prostate-specific membrane antigen in prostate carcinoma. Mol Cancer Ther. 2011;10:1036–45. doi: 10.1158/1535-7163.MCT-10-1093. [DOI] [PubMed] [Google Scholar]
- 5.Germain C, Larbouret C, Cesson V, Donda A, Held W, Mach JP, et al. MHC class I-related chain A conjugated to antitumor antibodies can sensitize tumor cells to specific lysis by natural killer cells. Clin Cancer Res. 2005;11:7516–22. doi: 10.1158/1078-0432.CCR-05-0872. [DOI] [PubMed] [Google Scholar]
- 6.Stamova S, Cartellieri M, Feldmann A, Bippes CC, Bartsch H, Wehner R, et al. Simultaneous engagement of the activatory receptors NKG2D and CD3 for retargeting of effector cells to CD33-positive malignant cells. Leukemia. 2011;25:1053–6. doi: 10.1038/leu.2011.42. [DOI] [PubMed] [Google Scholar]
- 7.Kellner C, Hallack D, Glorius P, Staudinger M, Mohseni Nodehi S, de Weers M, et al. Fusion proteins between ligands for NKG2D and CD20-directed single-chain variable fragments sensitize lymphoma cells for natural killer cell-mediated lysis and enhance antibody-dependent cellular cytotoxicity. Leukemia. 2012;26:830–4. doi: 10.1038/leu.2011.288. [DOI] [PubMed] [Google Scholar]
- 8.Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517–9. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 9.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kellner C, Maurer T, Hallack D, Repp R, van de Winkel JG, Parren PW, et al. Mimicking an induced self phenotype by coating lymphomas with the NKp30 ligand B7-H6 promotes NK cell cytotoxicity. J Immunol. 2012;189:5037–46. doi: 10.4049/jimmunol.1201321. [DOI] [PubMed] [Google Scholar]