Abstract
Understanding the immune response to the death of malignant cells is critical for the development of therapeutic strategies designed to stimulate the immune system against cancer. We have developed an inducible caspase-3-mediated death switch model to explore the effects of apoptosis on the host immune system, demonstrating that the synchronous apoptotic demise of established tumors can be immunogenic and elicit anticancer T-cell responses.
Keywords: apoptosis, caspase 3, cancer, death switch, HMGB1, immunogenic cell death
Until recently, established anticancer therapies such as radiotherapy and chemotherapy were thought to mediate direct antineoplastic effects through the induction of apoptotic cell death. Previously established dogma proposes that in order to avoid autoimmune reactions during tissue homeostasis and development cells undergoing apoptosis either go unperceived by the immune system or actively promote tolerance. In this setting, apoptotic corpses are rapidly cleared by phagocytes and intracellular contents capable of functioning as endogenous “danger signals” are not released.1,2 Thus, the contribution of systemic immunity to the elimination of malignant cells and clinical remissions observed upon cytoreductive therapy has largely been ignored or rejected. However, accumulating evidence suggest that cancer cells succumbing to specific chemotherapeutic drugs including anthracyclines (e.g., adriamycin) or irradiation undergo a form of apoptosis that is immunogenic and hence elicit tumor-specific immune responses.3 The discrimination between immunogenic and non-immunogenic forms of apoptosis is based on the ectopic cell-surface expression of calreticulin,4 the liberation of the toll-like receptor (TLR) 4 ligand high mobility group box 1 (HMGB1)5 and release of ATP.6 Together, these factors promote the uptake, processing and presentation of antigens from apoptotic tumor cells by dendritic cells (DCs) and hence stimulate the priming of CD8+ T cells. The contribution of immunogenic cell death to the clinical outcome of cancer patients remains unclear. However, the response to chemoradiotherapy appears to be impaired in patients carrying loss-of-function alleles of TLR4 and P2RX7 (rendering the HMGB1 and ATP signaling pathways deficient),5,6 suggesting that—at least in some clinical settings—the induction of immunogenic apoptosis improve therapeutic outcomes.
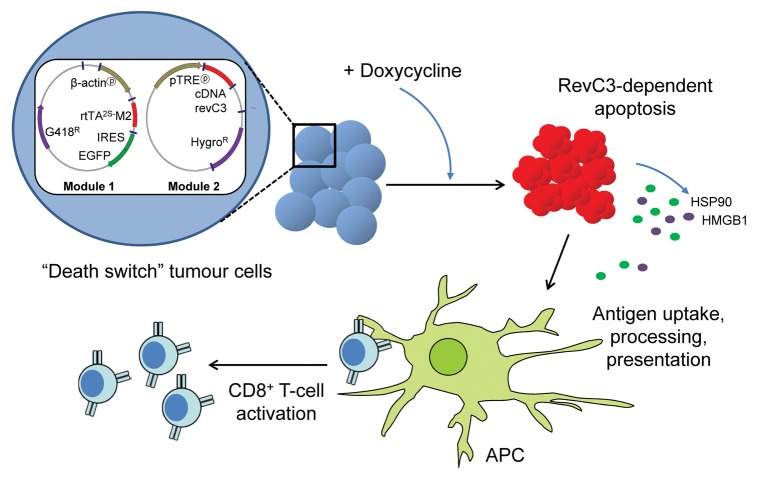
In order to obtain further insights into the immunogenic potential of malignant cells dying by apoptosis in vivo and to explore the impact of a massive, rapid and synchronous apoptotic response on the host immune system, we developed a syngeneic murine tumor model in which caspase-dependent apoptosis can be conditionally triggered in vivo.7 To this aim, variants of ovalbumin-expressing B16 melanoma cells were produced using a two-step strategy involving the production of clones expressing a doxycycline-regulated reverse transcriptional transactivator (rtTA)8 that were subsequently transfected with a vector encoding reverse caspase 3 (revC3)9 under the control of a tetracycline-responsive element. In this setting, the addition of doxycycline induces the expression of revC3, leading to a synchronous apoptotis response that we termed the “death switch.”
Cell death as triggered by the conditional expression of revC3 was rigorously characterized to confirm that cells died of apoptosis. Indeed, in response to doxycycline cells manifested the exposure of phosphatidylserine on the cell surface, apoptotic DNA fragmentation, chromatin condensation, membrane blebbing, caspase activation as well as the cleavage of poly(ADP-ribose) polymerase (PARP). Furthermore, we confirmed that such an apoptotic response was significantly inhibited by the pharmacological inhibition of caspase activity. Tumor cells expressing revC3 displayed signs of apoptosis starting from 6–12 h after the induction of the death switch, and more than 80% of cells were apoptotic within 24 h of doxycycline addition. Importantly, cells succumbing to the death switch released molecular determinants that can potentially function as damage-associated molecular patterns (DAMPs), including HMGB1 and the heat-shock protein of 90 KDa (HSP90). Kinetic studies suggested that DAMPs were released during the early stages of progression toward secondary necrosis, rather than from late secondary necrotic cells. Furthermore, tumor cells dying upon the induction of the death switch were readily engulfed by DCs in vitro.
The considerable advantage of the death switch model is that apoptosis can be conditionally activated in established tumors growing in vivo, allowing for the assessment of its immunogenic potential in the presence of an intact tumor microenvironment. A single administration of doxycycline by oral gavage was sufficient to induce apoptosis and tumor regression. In addition, the sustained administration (7 d) of doxycycline resulted in 40% of animals rejecting tumors in a stable fashion (>80 d). Interestingly, and unexpectedly, our data suggested that such a long-term tumor clearance was associated with the activation of an antitumor immune response. Evidence for this came from studies in immunodeficient mice as well as in animals that has been specifically depleted of CD8+ T cells. In this setting, tumor growth rapidly recovered following the cessation of doxycycline. In contrast, immunocompetent animals that survived for long periods after the induction of the death switch were able to reject subsequent challenges with tumor cells of the same type, indicating that an immunological memory had been established. Taken together, these data suggest that apoptosis is not always perceived as immunologically silent, and in fact can be immunogenic, resulting in the effective priming of tumor-specific cytotoxic T cell-mediated adaptive immunity (Fig. 1).
Figure 1. Relationship between doxycycline-induced reverse caspase-3-dependent apoptotic cell death and tumor-specific CD8+ T-cell responses. Tumor cells transfected with the reverse caspase-3 (revC3)-mediated “death switch” system undergo synchronous apoptosis in response to doxycycline, a process that is accompanied by the release of intracellular components such as high mobility group box 1 (HMGB1) and heat-shock protein of 90 KDa (HSP90). These molecular determinants can function as endogenous immune adjuvants, promoting the processing and presentation of tumor-associated antigens by antigen-presenting cells (APCs) and hence leading to the priming of a tumor-specific CD8+ T-cell response.
Evidence suggesting that systemic immunity contributes to the control of many cancer types after successful therapeutic intervention is growing. The importance of inducing an anticancer immune response in the course of therapy is highlighted by the development of immune based prognostic biomarkers and immune scores that predict clinical responses.10 Understanding how to optimize the immune response that cancer cells succumbing to conventional chemotherapeutics elicit is therefore imperative. Model systems such as our death switch, which facilitates the conditional induction of cell death in a tightly regulated fashion, will be of benefit for addressing this issue and will favor the development of strategies to improve clinical responses to apoptotic cancer cells. One strategy that warrants further exploration is combining radiotherapy or conventional chemotherapy with targeted anticancer agents in an attempt to potentiate immune responses either directly or upon the inhibition of immune checkpoints. Such combinatorial approaches provide exciting opportunities to improve therapeutic responses and hence prolong the survival of cancer patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest have been disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24501
References
- 1.Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–50. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–8. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 3.Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–9. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 4.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 5.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 6.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 7.Melis MH, Simpson KL, Dovedi SJ, Welman A, Macfarlane M, Dive C, et al. Sustained tumour eradication after induced caspase-3 activation and synchronous tumour apoptosis requires an intact host immune response. Cell Death Differ. 2013;20:765–73. doi: 10.1038/cdd.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welman A, Barraclough J, Dive C. Generation of cells expressing improved doxycycline-regulated reverse transcriptional transactivator rtTA2S-M2. Nat Protoc. 2006;1:803–11. doi: 10.1038/nprot.2006.117. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasula SM, Ahmad M, MacFarlane M, Luo Z, Huang Z, Fernandes-Alnemri T, et al. Generation of constitutively active recombinant caspases-3 and -6 by rearrangement of their subunits. J Biol Chem. 1998;273:10107–11. doi: 10.1074/jbc.273.17.10107. [DOI] [PubMed] [Google Scholar]
- 10.Senovilla L, Vacchelli E, Galon J, Adjemian S, Eggermont A, Fridman WH, et al. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology. 2012;1:1323–43. doi: 10.4161/onci.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]