Abstract
The transcription factor forkhead box P3 (FOXP3) has been identified as a marker of CD4+CD25+ regulatory T cells and is a key determinant of their immunosuppressive functions. FOXP3 has indeed been shown to limit antitumor immune responses during tumor progression. In addition, by expressing FOXP3, tumor cells may evade effector T-cell responses.
Keywords: colorectal cancer, FOXP3, prognosis
Strategies to Evade Effective Antitumor Responses
Tumor progression results from a disrupted regulation of cell growth as well as from the failure of the host to mount an efficient antitumor immune response. Most human colorectal cancer (CRC) patients do not develop satisfactory antitumor immune responses, pointing to the existence of strategies whereby CRCs evade immune effectors.1 CD4+CD25+ regulatory T cells (Tregs) have been shown to be critical for the maintenance of immunologic tolerance. Moreover, the transcription factor forkhead box P3 (FOXP3) has been identified not only as a key player in the immunosuppressive functions of Tregs but also as a definitive marker of CD4+CD25+ Tregs.2 Some subclasses of Tregs exert immunosuppressive functions by producing cytokines such as interleukin (IL)-10 and transforming growth factor β (TGFβ). In vivo, Tregs have been shown to specifically block cytotoxic T-lymphocyte (CTL) functions through TGFβ signaling, standing out as a crucial facet of the complex role of TGFβ in cancer biology. Tregs can also suppress the activation and/or proliferation of T cells without producing cytokines, via a mechanism that requires cell-to-cell contacts.3 FOXP3 confers Tregs with an immunosuppressive phenotype by enhancing their metabolic status and by improving cell survival. FOXP3 is able to repress the expression of specific cytokines by interacting with phosphodiesterase 3B, cGMP-inhibited (PDE3B) and the transcription factor NF-κB, a key driver of inflammation.4 In vitro, naturally occurring CD4+CD25+ Tregs as well as FOXP3-transduced CD4+ T cells inhibit the proliferation of naive T cells.2 In vivo, a high density of tumor-infiltrating FOXP3+ Tregs has been associated with poor disease outcome in patients affected by various solid tumors, including pancreatic cancer5 and hepatocellular carcinoma.6 Taken together, these observations plead in favor of a substantial role of Tregs in oncogenesis and tumor progression, which may be related to their robust immunosuppressive functions.
Another effective strategy for malignant cells to evade antitumor responses consists in the direct secretion of immunosuppressive factors such as IL-10 and TGFβ, a process that may be related to FOXP3 expression by cancer cells.7 Whether the expression of these cytokines truly provides tumors with the ability to evade immune responses remains to be elucidated. In vitro, FOXP3 expression by tumor cells has been correlated with the inhibition of T-cell proliferation, indicating that cancer cells may share growth-suppressive effects with Tregs and that mimicking Tregs functions may represent a novel mechanism of immune evasion.
Correlation between FOXP3 Expression and Tumor Progression
In various solid tumors, including ovarian and pancreatic cancer, a high density of tumor-infiltrating FOXP3+ Tregs has been linked with poor disease outcome.5 Conversely, no significant association between the absolute number of tumor-infiltrating FOXP3+ T cells and prognosis was established in several studies involving CRC patients. In addition, some studies suggest that a high frequency of tumor-infiltrating FOXP3+ Tregs may de facto be associated with a favorable, rather than a dismal, prognosis.8 Recent clinical data have provided the first evidence in support of FOXP3 expression by multiple distinct cancer cells.9 However, the biological significance of FOXP3 expression by CRC cells remains unknown.
We have recently evaluated FOXP3 expression by tumor-infiltrating Tregs and CRC cells in patients affected by different stages of the disease (UICC I–IV) and investigated its long-term prognostic significance.10 The analysis of CD4, CD25, FOXP3, IL-10 and TGFβ expression profiles suggest that CD4 and CD25 are expressed to significantly higher levels in early (UICC I/II) lesions than in advanced (UICC III/IV) tumors, while FOXP3, IL-10 and TGFβ appeared to be significantly downregulated in early, as opposed to advanced, neoplasms.10 Additional analyses demonstrated an increased amount of CD4+, CD25+, FOXP3+, IL-10+ and TGFβ+ expressing cells in early tumors as compared with advanced lesions as well as non-malignant tissues. FOXP3+ cancer cells were detected in 60 out of 65 CRC patients analyzed in this respect. We have observed that the expression of FOXP3 by cancer cells was significantly correlated with that of IL-10 and TGFβ.10
We demonstrated for the first time a significantly increased expression of FOXP3 by CRC cells. Additionally, we provided evidence for an association between increased FOXP3 expression levels by cancer cells and poor prognosis. By expressing FOXP3, malignant cells would be capable to downregulate antitumor effector T-cell responses. The overall survival of patients exhibiting low or high levels of tumor-infiltrating FOXP3+ Tregs in comparison to that of patients displaying low or high FOXP3 levels on malignant cells suggested that FOXP3 expression by cancer cells may constitute a prognostic factor. Indeed, CRC patients exhibiting high FOXP3 expression levels on cancer cells had a worse prognosis than patients with low FOXP3 levels. Conversely, no significant correlation was observed between the expression pattern of FOXP3 on tumor-infiltrating Tregs and prognosis.10
In conclusion, the expression of FOXP3 by cancer cells, resulting in the secretion of immunosuppressive cytokines such as IL-10 and TGFβ into the tumor microenvironment, may allow them to evade effector T-cell responses and hence progress (Fig. 1). In addition, FOXP3 expression by cancer cells appears as an independent prognostic factor for CRC patients.
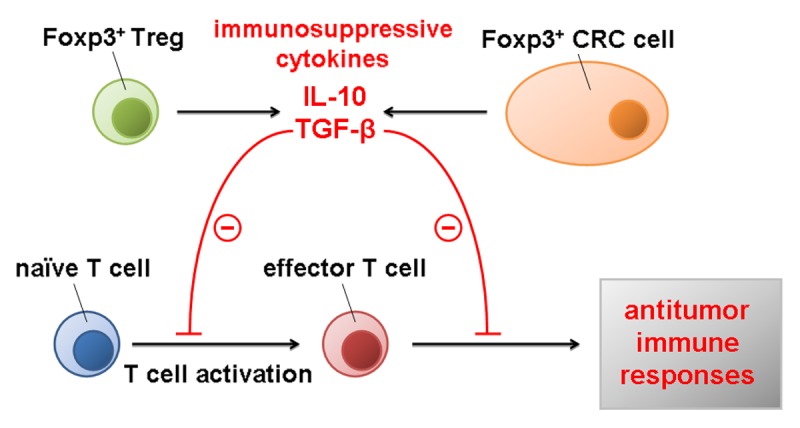
Figure 1. Impact of FOXP3 on oncogenesis and tumor progression. Immunosuppressive cytokines such as interleukin-10 (IL-10) and transforming growth factor β (TGFβ) released by either FOXP3+ regulatory T cells (Tregs) or FOXP3+ cancer cells inhibit the activation of naive T cells, hence limiting antitumor immune responses and favoring oncogenesis and tumor progression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24521
References
- 1.Strand S, Galle PR. Immune evasion by tumours: involvement of the CD95 (APO-1/Fas) system and its clinical implications. Mol Med Today. 1998;4:63–8. doi: 10.1016/S1357-4310(97)01191-X. [DOI] [PubMed] [Google Scholar]
- 2.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/S0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci U S A. 2005;102:5138–43. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–34. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 6.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–93. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 7.Karanikas V, Speletas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, et al. Foxp3 expression in human cancer cells. J Transl Med. 2008;6:19. doi: 10.1186/1479-5876-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–92. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 9.Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grüssel S, Sipos B, et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67:8344–50. doi: 10.1158/0008-5472.CAN-06-3304. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Grimmig T, Grimm M, Lazariotou M, Meier E, Rosenwald A, et al. Expression of Foxp3 in colorectal cancer but not in Treg cells correlates with disease progression in patients with colorectal cancer. PLoS One. 2013;8:e53630. doi: 10.1371/journal.pone.0053630. [DOI] [PMC free article] [PubMed] [Google Scholar]


