Abstract
Exosomes derived from dendritic cells (dexosomes) induce potent antitumor immune responses in mice. We have shown that the efficacy of dexosome-elicited antitumor immunity relies on the presence of both T- and B-cell dexosome-associated epitopes. Hence, the inclusion of B-cell epitopes in anticancer vaccines is crucial for the success of this immunotherapeutic intervention.
Keywords: exosomes, dexosomes, melanoma, CD8+ T cells, B cells, marginal zone B cells, CD4+ T cells
Dendritic-cell (DC)-derived exosomes (dexosomes) have been suggested to constitute potent anticancer immunotherapeutic agents, mainly due to their robust immunostimulatory functions, their ability to reduce tumor burden in animal models, and their unresponsiveness to the immunosuppressive tumor milieu.1,2 Two Phase I clinical trials have been conducted to investigate the antineoplastic potential of immature autologous DC-derived dexosomes loaded with MHC Class I- and II-restricted peptides in patients with melanoma and non-small cell lung cancer.3,4 Dexosomes were generally well tolerated but exerted limited therapeutic activity. We have recently investigated if the immunogenicity of dexosomes would depend—at least in part—on the quality of its antigen cargo.5 Although potent antigen-specific CD8+ T-cell activation was induced in vitro by both peptide- and whole protein-loaded dexosomes, only the latter, containing both T- and B-cell epitopes, induced specific cytotoxic T-lymphocyte (CTL) responses in vivo. The activation of CD8+ T cells in vivo was shown to be totally dependent on CD4+ T cells as well as on B cells, in particular marginal zone B cells (MZBs).
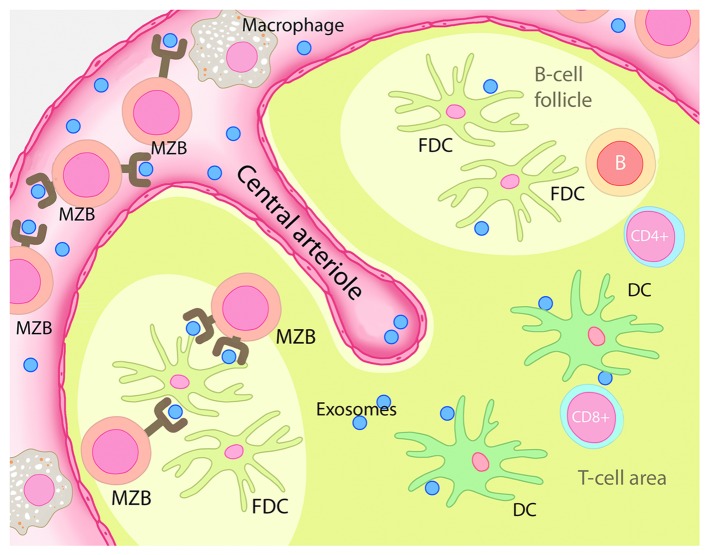
In whole splenocyte cultures, we observed that dexosomes were preferentially taken up by DCs and MZBs in vitro. In vivo, dexosomes elicited significantly weaker CD8+ T-cell responses in MZB-deficient mice.5 MZBs are closely associated with the marginal sinus of the spleen and shuttle blood-borne antigens into the splenic B-cell zone, where they deposit antigens on follicular DCs (FDCs).6 Hence, we propose that intravenously injected dexosomes are taken up by DCs, carrying them into the T-cell zone, and MZBs, shuttling them into the B-cell zone. While DCs initially activate antigen-specific CD4+ and CD8+ T cells toward dexosome-borne antigens, B-cell activation occurs upon contact with FDCs.7 Activated B cells then migrate to the B/T-cell border and interact with CD4+ T cells, boosting CTL responses (Fig. 1). However, it is not clear if the B-cell dependent activation of CD8+ T cells occurs via CD4+ T cells activated by B cells or if B cells act as antigen-presenting cells and directly elicit CD8+ T-cell responses. It has previously been shown that B cells can induce antigen-specific CD4+ and CD8+ T-cell responses in vitro, in a setting in which the B-cell dependent activation of CD8+ T cells relied on previous B-cell priming by CD4+ T cells.8 Moreover, B cells were shown to induce antigen-specific CD4+ T cells and CD8+ T cells in mice lacking functional DCs,9 indicating that B cells can directly activate CD8+ T cells in the presence of CD4+ T-cell help. Clearly, dexosome-induced CD4+ T-cell activation is strongly decreased when B cells are non-functional.10 Nevertheless, it is not known whether cognate antigen interaction is crucial for proficient B-cell help or if cytokines secreted by B cells could have the same effect on T cells.
Figure 1. Dexosome-mediated splenic immunostimulation. Upon intravenous administration, circulating dexosomes are taken up in the spleen either by marginal zone B cells (MZBs)—and hence transported to follicular dendritic cells (FDCs) for B-cell activation—or by dendritic cells (DCs), which activate T lymphocytes in the T-cell zone. B cells can further activate helper T cells at the B/T-cell border to boost subsequent cytotoxic T-lymphocyte responses or directly stimulate CD8+ T cells following CD4+ T-cell priming.
B-cell epitopes on dexosomes not only were beneficial for the induction of a robust immune response but also promoted antineoplastic effects. Indeed, mice immunized with whole protein-loaded dexosomes exhibited reduced tumor growth and improved survival as compared with mice receiving peptide-loaded dexosomes.5 Moreover, higher numbers of cytokine-producing CTLs were detected in the tumors of mice receiving whole protein-loaded dexosomes than in the lesions of mice treated with peptide-loaded dexosomes. Taken together, these observations demonstrate that dexosomes loaded with both T- and B-cell epitopes potently induce CD8+ T cells that are functional even in the tumor microenvironment. We propose that B-cell epitopes should be included in future dexosome-based vaccines, as well as in anticancer vaccines at large, to activate a potent immune response capable of counteracting the immunosuppressive mechanisms generally established by malignant cells.
Disclosure of Potential Conflicts of Interest
S.G. has a patent pending on exosomes for cancer therapy.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24533
References
- 1.Amigorena S. Anti-tumour immunotherapy using dendritic-cell-derived exosomes. Res Immunol. 1998;149:661–2. doi: 10.1016/S0923-2494(99)80035-2. [DOI] [PubMed] [Google Scholar]
- 2.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Näslund TI, Gehrmann U, Qazi KR, Karlsson MC, Gabrielsson S. Dendritic cell-derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol. 2013;190:2712–9. doi: 10.4049/jimmunol.1203082. [DOI] [PubMed] [Google Scholar]
- 6.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr., Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165:1259–65. doi: 10.4049/jimmunol.165.3.1259. [DOI] [PubMed] [Google Scholar]
- 8.Castiglioni P, Gerloni M, Cortez-Gonzalez X, Zanetti M. CD8 T cell priming by B lymphocytes is CD4 help dependent. Eur J Immunol. 2005;35:1360–70. doi: 10.1002/eji.200425530. [DOI] [PubMed] [Google Scholar]
- 9.Gerloni M, Rizzi M, Castiglioni P, Zanetti M. T cell immunity using transgenic B lymphocytes. Proc Natl Acad Sci U S A. 2004;101:3892–7. doi: 10.1073/pnas.0400138101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qazi KR, Gehrmann U, Domange Jordö E, Karlsson MC, Gabrielsson S. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood. 2009;113:2673–83. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]