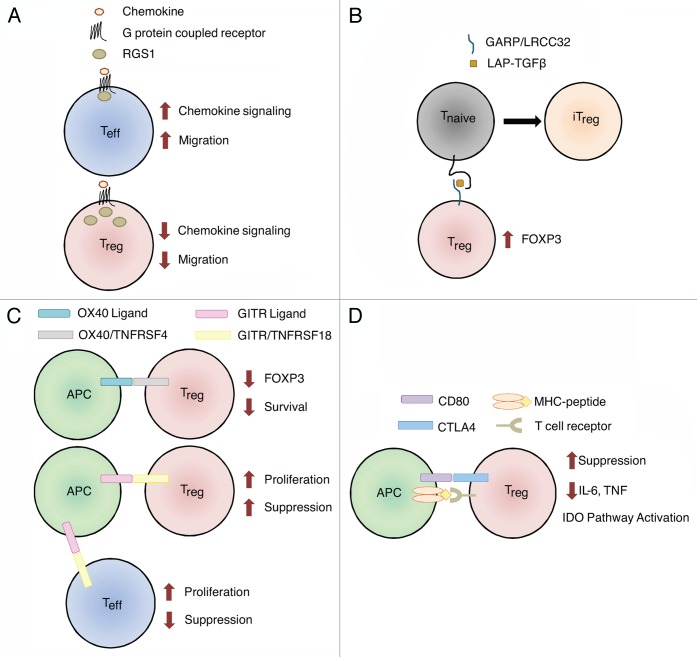
Figure 1. Genes with variants associated with ovarian cancer survival. Normal functions of genes involved in the biology of regulatory T cells (Tregs) are shown. (A) Tregs express more RGS1 than effector T cells (Teffs), resulting in reduced chemotactic migration. SNPs in RGS1 may increase the responsiveness of Tregs to chemotactic signals, allowing them to accumulated within tumors and to establish an immunosuppressive microenvironment. (B) Garpin (GARP) is a surface-bound receptor for latency-associated peptide (LAP)-bound transforming growth factor β (TGFβ). TGFβ-bound GARP can promote the acquisition of a regulatory phenotype by naïve T cells (Tnaïve) cells, hence converting them in induced Tregs (iTregs) and upregulate the transcription factor FOXP3 in Tregs. SNPs in LRRC32 (encoding GARP) may increase its expression levels, stability or activation status, resulting in the propagation of an immunosuppressive phenotype in the microenvironment of mucinous tumors. (C) The ligation of OX40 on the surface of Tregs results in decreased FOXP3 expression and limits their survival. SNPs in TNFRSF4 (encoding OX40) may abrogate this process, resulting in increased Treg survival and functions in mucinous tumors. GITR ligation may affect Tregs directly or indirectly. The ligation of GITR expressed on Tregs increases their proliferation and immunosuppressive functions. The activation of GITR expressed on effector T cells also increases their proliferation, which in turn can prevent the expansion of Tregs. SNPs in TNFRSF18 (encoding GITR) may increase GITR levels or activity, amplifying the immunosuppressive phenotype of mucinous tumors. Alternatively, SNPs in TNFRSF18 may reduce downstream Teff functions, resulting in decreased tumor clearance. (D) CD80 expressed on antigen-presenting cells (APCs) can bind CTLA4 on the surface of Tregs, triggering multiple mechanisms of immunosuppression, including a decreased expression of pro-inflammatory cytokines and the activation of the indoleamine 2,3-dioxygenase (IDO) pathway. SNPs affecting CD80 may increase the affinity of CD80 for CTLA4 in endometrioid tumors, while SNPs in MAD1L1 (which are linked to CD80 expression), may influence the CD80 levels in ovarian cancer patients at large.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.