Abstract
Tumor cells succumb to chemotherapy while releasing ATP. We have found that extracellular ATP attracts dendritic cell (DC) precursors into the tumor bed, facilitates their permanence in the proximity of dying cells and promotes their differentiation into mature DCs endowed with the capacity of presenting tumor-associated antigens.
Keywords: apoptosis, autophagy, calreticulin, CD39, doxorubicin, immunogenic cell death
It has recently become clear that the infiltration of neoplastic lesions by a diverse range of immune effector cells constitutes a critical determinant for the long-term fate of cancer patients. A large number of retrospective clinical studies has demonstrated that the intratumoral abundance of specific lymphoid or myeloid cell populations is endowed—at least in well-defined clinical settings—with robust prognostic and/or predictive value. Driven by these observations, several studies have recently been launched to determine—in a more stringent, prospective manner—the utility of the immune infiltrate for risk stratification and/or for the development of personalized therapies.1,2
As opposed to the majority of chemotherapeutic agents, anthracyclines have the capacity to trigger immunogenic cell death (ICD), hence converting cancer cells (which express a range of tumor-associated antigens) into therapeutic vaccines as they die. Thus, ICD, as induced by anthracyclines and a few other stimuli, elicits an anticancer immune response whereby the host acquires the ability to control the growth of chemoresistant tumor cells.3,4 The emission of immunogenic signals by cancer cells undergoing ICD involves a series of pre-mortem stress responses. In particular, an endoplasmic reticulum (ER) stress response is required for the translocation of the most abundant luminal protein of the ER, calreticulin, on the outer leaflet of the plasma membrane, where it serves as an “eat-me signal” and facilitates the engulfment of portions of dying tumor cells by antigen-presenting cells.3,5 Along similar lines, autophagy is a conditio sine qua non for dying cancer cells to release ATP,6-8 which—by binding to various purinergic receptors (expressed on a wide panel of immune effector cells)—not only operates as a potent chemotactic signal but also stimulates the polarization of T cells toward efficient anticancer immunity.7,9
As a correlate of ICD induced in vivo by the systemic administration of anthracyclines, we observed an early (12–48 h post-chemotherapy) increase in the frequency of tumor-infiltrating myeloid cells. Multicolor immunofluorescence experiments revealed that (1) these cells accumulate in the immediate proximity of dying tumor cells (manifesting the proteolytic activation of caspase-3 as well as nuclear condensation) and that (2) cells with a mature dendritic cell (DC) immunophenotype (i.e., CD11b+CD86+ cells) accumulate relatively early (12 h) post-chemotherapy, while macrophages and neutrophil granulocytes appear comparatively later (48 h).10 The recruitment of these myeloid cell subpopulations turned out to depend on the release of ATP from dying tumor cells (as it was blocked by the expression of the ATP-degrading enzyme CD39 on their surface) as well as on the expression of the purinergic receptor P2Y2 by the host immune system.10 In contrast, P2RX7, another purinergic receptor previously involved in the functional perception of ICD,3 is required for the recruitment of DCs and neutrophils but dispensable for the chemotherapy-induced infiltration of neoplastic lesions by macrophages.10 Thus, ATP and a range of purinergic receptors are required for the chemotactic attraction of myeloid cells into the vicinity of dying tumor cells post-chemotherapy.
Taking advantage of cancer cells engineered to express a variant of green fluorescence protein (GFP) tethered to the inner leaflet of the plasma membrane, we were able to demonstrate that anthracycline-based chemotherapy stimulates the engulfment of tumor cells (or portions thereof) by a population of CD11c+CD11b+Ly6Chigh cells. The FACS-assisted purification of such cells revealed that they efficiently cross-present tumor-associated antigens (such as the model antigen ovalbumin) to CD8+ T cells in vitro, and that they can induce protective anticancer immune responses when adoptively transferred into naïve mice.10 Importantly, the injection of CD11b-blocking antibodies not only inhibited the chemotherapy-induced accumulation of CD11c+CD11b+Ly6Chigh cells in the tumor bed but also reduced the capacity of anthracyclines to exert antineoplastic effects in vivo. In contrast, the depletion of macrophages or DCs had no or only minor effects, respectively, on the efficacy of anthracycline-based chemotherapy.10 Altogether, these results indicate that CD11c+CD11b+Ly6Chigh cells critically contribute to the cross-presentation of tumor-associated antigens in vivo following the administration of ICD-inducing chemotherapeutics.
Given the importance of ATP for the anthracycline-elicited recruitment of CD11c+CD11b+Ly6Chigh cells into the tumor bed, we decided to investigate the role of ATP in the survival and differentiation of DC precursors in more detail. To this aim, we purified CD11c+CD11b+Ly6Chigh leukocytes from the tumor bed two days post-chemotherapy and then adoptively transferred them into malignant lesions developing in distinct hosts. In this setting, the administration of anthracycline-based chemotherapy was required for the optimal survival of adoptively transferred CD11c+CD11b+Ly6Chigh leukocytes within the tumor bed, as well as for their differentiation into CD11c+CD86+MHCII+ DCs. Moreover, the overexpression of CD39 on the surface of tumor cells inhibited the permanence and differentiation of adoptively transferred CD11c+CD11b+Ly6Chigh cells.10 In vitro experiments confirmed the importance of extracellular ATP for the differentiation of CD11c+CD11b+Ly6Chigh cells into CD11c+CD86+MHCII+ DCs. Indeed, in the absence of extracellular ATP (resulting from the overexpression of CD39) as well as in the presence of purinergic receptor inhibitors, bone marrow-derived CD11c+CD11b+Ly6Chigh cells differentiated into neutrophils. Conversely, in the presence of extracellular ATP as released from dying cells, CD11c+CD11b+Ly6Chigh cells acquired a DC-like CD11c+CD86+MHCII+ phenotype,10 suggesting that ATP skews the default differentiation pathway of bone marrow-derived cells (leading to the generation of neutrophils) toward the production of mature DCs.
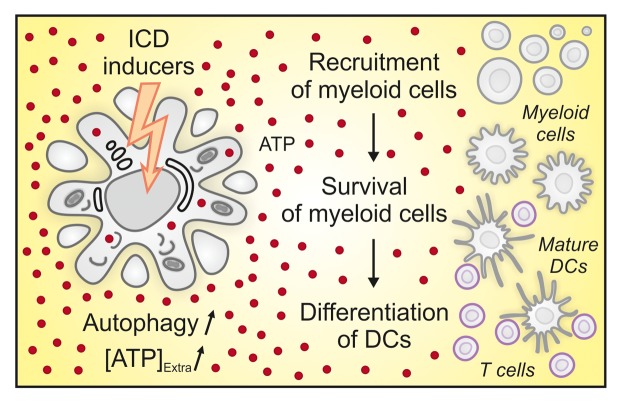
Based on these results, we hypothesize that extracellular ATP may exert three distinct effects on the immune infiltrate following immunogenic chemotherapy (Fig. 1). First, ATP is certainly one of the most important chemotactic factors that bridge cell death to the recruitment of a range of immune effector cells, including DCs and their precursors. Second, ATP may serve as a trophic factor to maintain DCs (and/or their precursors) in the proximity of stressed and dying cancer cells. Third, ATP drives the differentiation of DC precursors into mature antigen-presenting cells exhibiting an immunophenotype similar to that of inflammatory DCs.10 Thus, manipulations designed to preserve the intratumoral levels of ATP, such as the inhibition of ecto-ATPases, may enhance antitumor immune responses by a multipronged positive effect on the recruitment, permanence and differentiation of DC precursors.
Figure 1. Multipronged activity of extracellular ATP during immunogenic chemotherapy. Besides emitting other immunogenic signals, cancer cells succumbing to anthracyclines actively secrete ATP in an autophagy-dependent manner. In turn, extracellular ATP (1) operates as a potent chemotactic factor to favor the local recruitment of distinct populations of myeloid cells, (2) functions as a trophic factor, allowing for the survival and persistence of myeloid cells in the proximity of dying cancer cells and (3) skews the default differentiation pathway of myeloid cells toward the generation of mature inflammatory dendritic cells (DCs), which exert potent antigen-presenting functions. [ATP]Extra, extracellular ATP concentration.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest have been disclosed.
Footnotes
These authors contributed equally to this work.
These authors share senior co-authorship.
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24568
References
- 1.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–33. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 2.Senovilla L, Vacchelli E, Galon J, Adjemian S, Eggermont A, Fridman WH, et al. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology. 2012;1:1323–43. doi: 10.4161/onci.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 4.Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, et al. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–88. doi: 10.4161/onci.1.2.19026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–84. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 6.Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle. 2009;8:3723–8. doi: 10.4161/cc.8.22.10026. [DOI] [PubMed] [Google Scholar]
- 7.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–7. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 8.Michaud M, Sukkurwala AQ, Martins I, Shen S, Zitvogel L, Kroemer G. Subversion of the chemotherapy-induced anticancer immune response by the ecto-ATPase CD39. Oncoimmunology. 2012;1:393–5. doi: 10.4161/onci.19070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013 doi: 10.1016/j.immuni.2013.03.003. In press. [DOI] [PubMed] [Google Scholar]