Abstract
Interferons not only exert a fundamental role during inflammation and immune responses but also modulate the activity of hematopoietic stem cells during homeostatic and demand-adapted hematopoiesis. Identical mechanisms regulate the homeostasis and proliferation of leukemic stem cells (LSCs). Understanding these mechanisms may lead to novel therapeutic approaches against leukemia.
Keywords: interferon, hematopoiesis, leukemia, hematopoietic stem cells, leukemia stem cells
Interferons (IFNs) are mainly produced by immune cells responding to pathogens or malignancies and can be subdivided into two major, functionally distinct classes: Type I and Type II IFNs. Type I IFNs (i.e., IFNα, IFNβ) signal via the IFNα/β receptor, whereas Type II IFN (IFNγ) does so via the IFNγ receptor. Both Type I and Type II IFN receptors are made up of two subunits (i.e., α and β) that—upon engagement—allow for the binding of Janus kinase family members, hence driving the activation of signal transducer and activator of transcription (STAT) family members. The main function of Type I IFNs is to inhibit viral replication. These cytokines are indeed predominantly secreted by plasmacytoid dendritic cells in response to viral nucleic acids. In contrast, IFNγ—which is mainly produced by activated T and natural killer (NK) cells—exerts limited antiviral functions but primarily operates as an immunomodulator and stimulator of monocyte/macrophage activity.1
The mutual interactions between mature immune cells and hematopoietic stem (and progenitor) cells have been addressed only recently.2 An early report by Binder et al. suggested that IFNα/β directly induces a state of transient hematopoietic aplasia in mice acutely infected with the lymphocytic choriomeningitis virus.3 Conversely, recent elegant studies have demonstrated that IFNα induces the proliferation of murine hematopoietic stem cells (HSCs) in vivo. The injection of polyinosinic-polycytidylic acid (poly-I:C), mimicking double-stranded viral RNA and potently inducing IFNα/β production, activated quiescent HSCs in an IFNα/β receptor-dependent manner. Interestingly, the transient activation of HSCs by IFNα did not impair their self-renewal ability, whereas chronic IFNα exposure led to HSC exhaustion due to extensive proliferation.4 These results were confirmed and extended upon the demonstration that interferon regulatory factor 2, a transcriptional repressor of IFNα signaling, preserves the quiescence and multilineage reconstitution capacities of HSCs.4 Therefore, Type I IFNs are important modulators of HSC proliferation and differentiation in response to viral infection.
The role of IFNγ in the function of HSCs is also controversially discussed. In several early studies, IFNγ was shown to induce the apoptotic demise or differentiation of murine HSCs in vivo as well as to suppress the growth and colony-forming potential of human CD34+ stem/progenitor cells in vitro. Conversely, other reports demonstrated that IFNγ potentiates the cytokine-dependent proliferation of human CD34+ stem/progenitor cells in vitro. Furthermore, IFNγ appears to play a fundamental role in the induction of acquired aplasia and anemia of chronic disease (reviewed in ref. 5). These findings were extended by a recent publication showing that IFNγ impairs the self-renewal and proliferative capacities of murine HSCs in vivo.6 On the other hand, recent studies using well-defined mouse models of physiological infection have challenged these findings and have shed new light on the role of IFNγ during demand-adapted hematopoiesis. During a chronic infection with Mycobacterium avium, IFNγ directly activated quiescent HSCs and induced their proliferation. In addition, HSCs from IFNγ-deficient mice displayed a less exhausted phenotype than HSCs from C57BL/6 mice in secondary transplantation experiments, as indicated by their improved re-population capacities.7 Furthermore, in Ehrlichia muris-infected mice, IFNγ facilitated infection-induced myelopoiesis to ensure host defenses by supporting the replenishment of myeloid cells. In addition, in a Plasmodium chabaudi-based murine model of malaria, IFNγ promoted the emergence of a myelo-lymphoid progenitor that generated myeloid cells to constrain microbe spread (reviewed in ref. 8). Altogether, these studies indicate that IFNγ is a potent regulator of HSC function and that the outcome of infection probably depends on the infectious agent itself as well as on the timing and duration of IFNγ stimulation. Of note, the heterogeneous panels of markers used in these studies to identify HSCs and progenitors impede a direct comparison of the results.5
Before the introduction of tyrosine kinase inhibitors (TKIs), IFNα was used as standard therapy for chronic myeloid leukemia (CML). In combination with cytoreductive chemotherapy, the administration of IFNα induced partial or complete cytogenetic responses and significantly prolonged the survival of CML patients.9 Even in the era of TKIs, quiescent, therapy-resistant leukemic stem cells (LSCs) can persist in the bone marrow of CML patients, de facto representing the main cause for disease relapse, drug resistance and therapeutic failure. Therefore, the activation of LSCs with IFNα, followed by the administration of TKIs or other chemotherapeutics, may represent a promising strategy to treat CML patients at risk of relapse.4
We have recently demonstrated in a murine CML model that LSCs are activated and proliferate in response to IFNγ. Adoptively-transferred CML-specific cytotoxic CD8+ T cells (CTLs) produced IFNγ in response to restimulation with leukemia antigens in vivo, and the level of IFNγ produced in this setting depended on the antigen load, i.e., the leukemic load. Therefore, adoptive immunotherapy using leukemia antigen-specific CTLs failed to cure advanced leukemia but paradoxically increased LSC numbers and accelerated the course of CML. The proliferation of LSCs was induced by CTL-derived IFNγ. Leukemia could only be cured by the transfer of CTLs in an early stage of disease, when the levels of leukemic antigens and—as a consequence—IFNγ were low. In addition, the increase in the proliferation of LSCs normally induced by adoptive immunotherapy was limited when IFNγ-deficient CTLs were transferred. Importantly, IFNγ stimulated the proliferation and the colony-forming potential of primary CD34+ stem/progenitor cells isolated from newly diagnosed CML patients, confirming the clinical importance of our findings.10
In summary, both Type I and Type II IFNs directly affect HSC and LSC functions (Fig. 1). However, so far little is known about the interplay between IFNα/β and IFNγ signaling in the regulation of stem cells and the bone marrow stem cell niche during hematopoietic homeostasis, infection and leukemia. To fully understand the implication of these cytokines in hematopoiesis, further investigations will have to elucidate the common and individual roles of Type I and Type II IFNs. This knowledge will allow for the development of novel therapeutic interventions against disorders of hematopoietic homeostasis including leukemia.
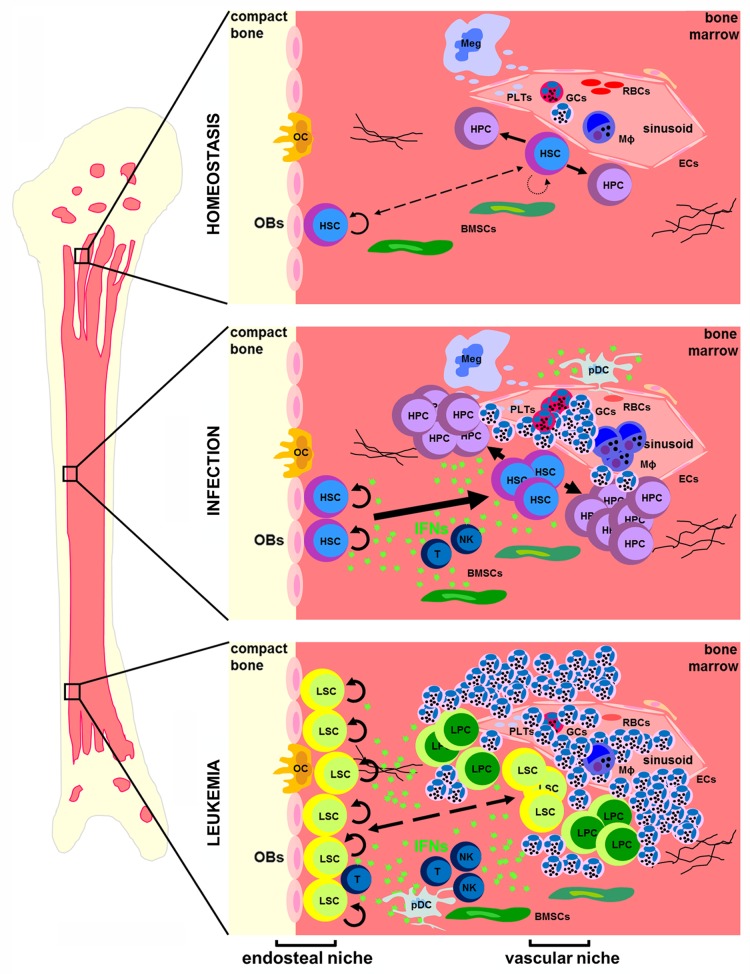
Figure 1. Interferons in the bone marrow during hematopoietic homeostasis, infection and leukemia. During homeostasis, quiescent hematopoietic stem cells (HSCs) in the endosteal niche and activated HSCs in the vascular niche provide a reservoir for the life-long production of all types of mature blood cells. Upon infection, interferons (IFNs) are produced either locally or systemically, leading to the activation and proliferation of HSCs. During leukemia, IFNs are produced in the inflammatory environment of the bone marrow and induce the proliferation of LSCs. BMSC, bone marrow stromal cell; EC, endothelial cell; GC, granulocyte; HPC, hematopoietic progenitor cell; LPC, leukemia progenitor cell; LSC, leukemia stem cell; Meg, megakaryocyte; MΦ, monocyte; NK, natural killer cell; OB, osteoblast; OC, osteoclast; pDC, plasmacytoid dendritic cell; PLT, platelet; RBC, red blood cell; T, T cell.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
These authors contributed equally to this work.
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24572
References
- 1.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 2.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–92. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185:517–30. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010;10:201–9. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- 5.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32:57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bruin AM, Demirel O, Hooibrink B, Brandts CH, Nolte MA. Interferon-gamma impairs proliferation of hematopoietic stem cells in mice. Blood. 2013 doi: 10.1182/blood-2012-05-432906. In press. [DOI] [PubMed] [Google Scholar]
- 7.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–7. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119:2991–3002. doi: 10.1182/blood-2011-12-380113. [DOI] [PubMed] [Google Scholar]
- 9.Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–40. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- 10.Schürch C, Riether C, Amrein MA, Ochsenbein AF. Cytotoxic T cells induce proliferation of chronic myeloid leukemia stem cells by secreting interferon-γ. J Exp Med. 2013;210:605–21. doi: 10.1084/jem.20121229. [DOI] [PMC free article] [PubMed] [Google Scholar]