Abstract
Tumor-associated macrophages (TAMs) and other myeloid cells that infiltrate neoplastic lesions promote tumor progression and are associated with poor patient prognosis. We have recently demonstrated that trabectedin, a licensed and commercially available anticancer agent, is selectively cytotoxic for TAMs and their circulating precursors (monocytes). The macrophage-depleting effect of trabectedin is a key component of its antitumor activity.
Keywords: tumor-associated macrophages, chemotherapy, micro-environment, angiogenesis, marine drug
In most established tumors, incoming monocytes are “educated” by the tumor microenvironment to differentiate into macrophages exhibiting an M2-like functional polarization and hence exerting a number of tumor-supporting functions. Indeed M2 macrophages have been shown to promote tumor cell proliferation and survival, support metastatic dissemination, stimulate angiogenesis and secrete extracellular matrix-remodeling enzymes. Strategies to deplete tumor-associated macrophages (TAMs) or to inhibit their recruitment to neoplastic lesions have been successful in experimental settings and are now considered as a promising therapeutic approach in the clinics.1-6
Trabectedin is an anticancer agent licensed in Europe and several other countries for the second-line treatment of soft tissue sarcoma patients and ovarian carcinoma patients, in combination with liposomal doxorubicin.7-9 Originally extracted from a marine organism, the tunicate Ecteinascidia, it is now synthetically produced by the Spanish company PharmaMar and is the first marine antineoplastic agent to reach marketing.
Trabectedin was initially identified for its potent cytotoxic activity against malignant cells. Trabectedin kills indeed cancer cells and blocks their proliferation by interacting with DNA. Further studies clarified that the mechanism of action of this compound is more complex than that of a conventional cytotoxic agent, as trabectedin affects DNA repair mechanisms as well as the activity of specific transcription factors. With the idea of investigating the effects of trabectedin on non-proliferating cells, we tested it on human circulating monocytes and surprisingly observed that these cells succumbed to trabectedin in 48 h via caspase-dependent apoptosis.10 We next observed that only monocytes and macrophages, among all leukocytes, were susceptible to the cytotoxic effects of trabectedin, a finding that prompted a series of experiments to understand the exquisite selectivity of this agent for mononuclear phagocytes. We eventually demonstrated that trabectedin rapidly (within few hours) triggers the activation of caspase-8 downstream of plasma membrane tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptors (TRAIL-Rs), and that distinct leukocyte subsets express different sets of TRAIL-Rs. In particular, monocytes/macrophages mainly express signaling-proficient TRAIL-Rs, while neutrophils and T lymphocytes preferentially express signaling-incompetent TRAIL-Rs, which are unable to mediate caspase-8 activation in response to agonists, and hence are resistant to trabectedin.10
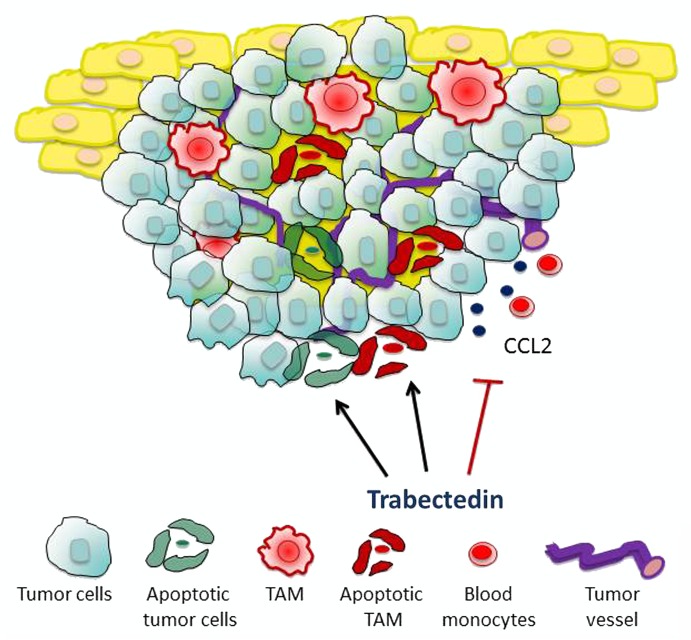
The real issue was to investigate whether trabectedin would be able to kill mononuclear phagocytes in vivo and in the context of cancer. In different mouse tumor models, trabectedin significantly decreased the number of circulating monocytes as well as that of splenic macrophages and TAMs, but had no effect on neutrophils and lymphocytes. To prove that the cytotoxic activity of trabectedin on mononuclear phagocytes is relevant for its antitumor activity, we took advantage of a trabectedin-resistant tumor cell line. Mice bearing trabectedin-resistant tumors were indeed normally sensitive to the antitumor activity of trabectedin, in spite of the fact that the same cancer cells exhibited a pronounced resistance against the drug in vitro.10 We therefore hypothesized that trabectedin exerts major inhibitory effects on TAMs. In line with this interpretation, the adoptive transfer of macrophages to trabectedin-treated mice significantly reinstated tumor growth. Thus, the targeting of macrophages in vivo is a key component of the antitumor activity of trabectedin. Nevertheless, effects other than macrophage depletion may also contribute to the pronounced antineoplastic potential of this drug. Immunohistochemical studies on tumor sections revealed indeed that trabectedin significantly reduces the expression levels of the angiogenic mediator vascular endothelial growth factor (VEGF) and the chemokine CCL2 in tumor vessels.10 Thus, besides direct killing mononuclear phagocytes, trabectedin may limit the recruitment of circulating monocytes into neoplastic lesions and inhibit angiogenesis (Fig. 1).
Figure 1. Mechanisms of action of trabectedin. The marine anticancer agent trabectedin is cytotoxic for tumor-associated macrophages (TAMs) and neoplastic cells. By inhibiting the production of the chemokine CCL2 trabectedin also decreases monocyte recruitment in tumors. The effects of trabectedin on the tumor microenvironment are important for its antitumor activity.
The abundance of circulating monocytes was studied in patients affected by soft tissue sarcomas receiving trabectedin as a standalone therapeutic intervention. In this setting, a decrease in blood monocytes was documented in most patients within a few days after the injection of trabectedin.10 Furthermore, in tumor sections collected before and after neoadjuvant therapy, a dramatic decrease in infiltrating macrophages and vessel density was observed, reinforcing the notion that trabectedin strikes both the neoplastic cell compartment and the tumor microenvironment.
Several questions remain to be addressed. First, does trabectedin independently affect the vessel network or does this effect stem from the trabectedin-induced reduction of pro-angiogenic macrophages? Of note, the macrophage-depleting agent clodronate also inhibits tumor growth in mice (although with a less durable effect) but does not appear to influence the tumor-associated vasculature. Second, is the monocyte-selective cytotoxicity of trabectedin similar in all patients or may individual variability influence the therapeutic efficacy of the drug? A number of pharmacological agents are being studied for their ability to modulate TRAIL-R expression by neoplastic cells, and hence their susceptibility to recombinant TRAIL. Finally, it would be interesting to investigate the feasibility of combination therapies aimed at maximize the effects of trabectedin on myelomonocytic (and perhaps tumor) cells. Along similar lines, the combination of trabectedin with anti-angiogenic drugs may result in improved clinical responses, as it is now well established that TAMs are often responsible for the resistance to these agents.
In summary, our findings delineated a new mechanism of action of a clinically useful and commercially available anticancer agent while providing strong proof-of-concept evidence in support of the therapeutic targeting of macrophages in cancer patients. Trabectedin is currently used in a limited number of oncological indications, often as second-line treatment. Our results open interesting perspectives for the rational exploitation of this peculiar agent in cancer therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest have been disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24614
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–86. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 7.D’Incalci M, Galmarini CM. A review of trabectedin (ET-743): a unique mechanism of action. Mol Cancer Ther. 2010;9:2157–63. doi: 10.1158/1535-7163.MCT-10-0263. [DOI] [PubMed] [Google Scholar]
- 8.Grosso F, Jones RL, Demetri GD, Judson IR, Blay JY, Le Cesne A, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 9.Le Cesne A, Cresta S, Maki RG, Blay JY, Verweij J, Poveda A, et al. A retrospective analysis of antitumour activity with trabectedin in translocation-related sarcomas. Eur J Cancer. 2012;48:3036–44. doi: 10.1016/j.ejca.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–62. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]