Abstract
Toll-like receptor 3 (TLR3) agonists have been extensively used as adjuvants for anticancer vaccines. However, their immunostimulatory effects and precise mechanisms of action in the presence of antineoplastic monoclonal antibodies (mAbs) have not yet been evaluated. We investigated the effect of TLR3 agonists on cetuximab-mediated antibody-dependent cellular cytotoxicity (ADCC) against head and neck cancer (HNC) cells, as well as on dendritic cell (DC) maturation and cross-priming of epidermal growth factor receptor (EGFR)-specific CD8+ T cells. The cytotoxic activity of peripheral blood mononuclear cells (PBMCs) or isolated natural killer (NK) cells expressing polymorphic variants (at codon 158) of the Fcγ receptor IIIa (FcγIIIa) was determined in 51Cr release assays upon incubation with the TLR3 agonist poly-ICLC. NK cell stimulation was measured based on activation and degranulation markers, while DC maturation in the presence of poly-ICLC was assessed using flow cytometry. The DC-mediated cross priming of EGFR-specific CD8+ T cells was monitored upon in vitro stimulation with tetramer-based flow cytometry. TLR3-stimulated, unfractionated PBMCs from HNC patients mediated robust cetuximab-dependent ADCC, which was abrogated by NK-cell depletion. The cytolytic activity of TLR3-stimulated NK cells differed among cells expressing different polymorphic variants of FcγRIIIa, and NK cells exposed to both poly-ICLC and cetuximab expressed higher levels of CD107a and granzyme B than their counterparts exposed to either stimulus alone. Poly-ICLC plus cetuximab also induced a robust upregulation of CD80, CD83 and CD86 on the surface of DCs, a process that was partially NK-cell dependent. Furthermore, DCs matured in these conditions exhibited improved cross-priming abilities, resulting in higher numbers of EGFR-specific CD8+ T cells. These findings suggest that TLR3 agonists may provide a convenient means to improve the efficacy of mAb-based anticancer regimens.
Keywords: ADCC, cetuximab-activated NK cells, cross-priming, EGFR-specific CD8+ T cells, poly-ICLC, TLR3
Introduction
Targeting the epidermal growth factor receptor (EGFR), which is overexpressed in more than 90% of head and neck cancer (HNC) patients, with monoclonal antibodies (mAbs) such as cetuximab is a well-established and clinically effective therapeutic strategy.1-3 If the antitumor efficacy of this mAb solely relied upon the blockade EGFR signaling in tumor cells, abrogating the delivery of proliferative, angiogenic and metastatic signals, one would expect high response rates to cetuximab therapy among EGFR+ cancer patients. However, the response rate to cetuximab among patients bearing solid tumors such as colorectal carcinoma (CRC), non-small cell lung carcinoma (NSCLC) and HNC is only 10–20%, warranting efforts to enhance its antineoplastic activity by means of combinatorial approaches.1,4-9 The immunological mode of action of several antineoplastic mAbs is progressively being recognized.10-13 In this setting, HNC cells may escape recognition by the immune system by downregulating non-classical MHC Class I antigens or owing to polymorphisms in the Fcγ receptor IIIa (FcγRIIIa) expressed by natural killer (NK) cells.12,14,15 Irrespective of the mechanism of resistance, enhancing the immune effects of cetuximab using clinically-relevant immune modifiers and cytokines is a logical approach to improve disease outcome.16-18
Toll-like receptors (TLRs) are expressed by both effector cells of the immune system and cancer cells.19,20 These receptors belong to the large family of pattern-recognition receptors (PRRs), and their primary function is to identify so-called “pathogen-associated molecular patterns” (PAMPs).20,21 The activation of TLRs stimulates innate, and subsequently adaptive, immune responses via the secretion of pro-inflammatory cytokines.20 Moreover, TLR agonists can directly activate immune effectors and hence enhance antitumor immune responses. For instance, it has recently been demonstrated that CpG-containing oligodeoxynucleotides can directly promote the secretion of cytokines by NK cells exposed to antibody-coated tumor cells by activating TLR9.22
The administration of TLR3 agonists to cancer patients induces the secretion of pro-inflammatory cytokines such as tumor necrosis factor α (TNFα), interleukin-12 (IL-12) and interferon γ (IFNγ), which in turn stimulates the recruitment of effector cells to the tumor microenvironment.23-25 Since patients with advanced cancer are typically immunosuppressed, TLR3 agonists may provide a convenient means of enhancing the therapeutic effects of cetuximab and alleviating tumor resistance to mAb-based immunotherapy. Of note, TLR3 agonists have also been shown to exert direct antitumor activity and to suppress metastatic spread in HNC models.26
The TLR3 ligand polyinosinic:polycytidylic acid (polyI:C) was first produced in 1967 and demonstrated a robust ability to stimulate the production of type I IFN.27 However, polyI:C exhibited significant toxicity and the subsequent modification of the compound with carboxycellulose, resulting in the stabilized form known as poly-ICLC, alleviated these initial concerns.28,29 The safety and therapeutic potential poly-ICLC have been tested in several clinical trials involving patients with urological and brain neoplasms, with encouraging results.30-33 Thus, poly-ICLC might also be employed as an immune adjuvant to cetuximab-based immunotherapy in HNC patients.
The objective of this study was to evaluate the effect of poly-ICLC on cetuximab-triggered NK cell-mediated ADCC as well as on dendritic cell (DC) maturation and its impact on the induction of EGFR-specific CD8+ T cells. Furthermore, we wanted to examine the activation profile of NK cells expressing polymorphic variants of FcγRIIIa (at codon 158), which have been correlated with the clinical response to cetuximab-based therapy.34
Results
Expression of TLR3 by HNC cells and direct effects of poly-ICLC
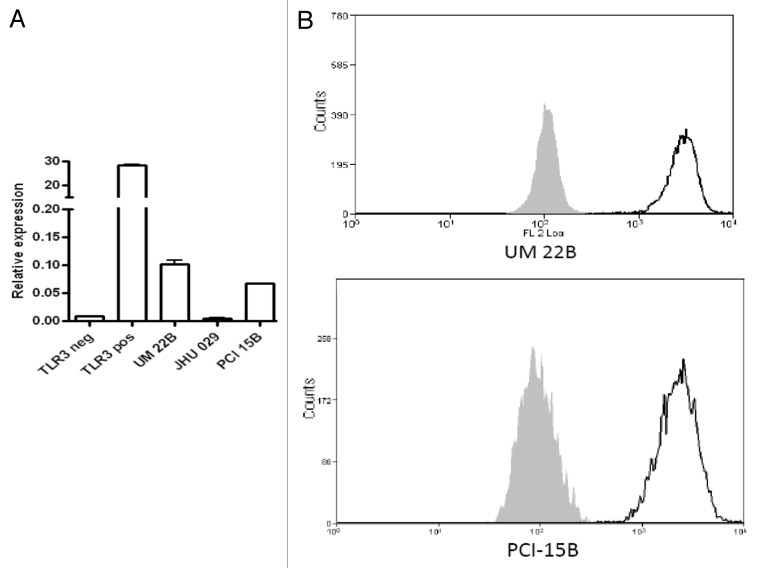
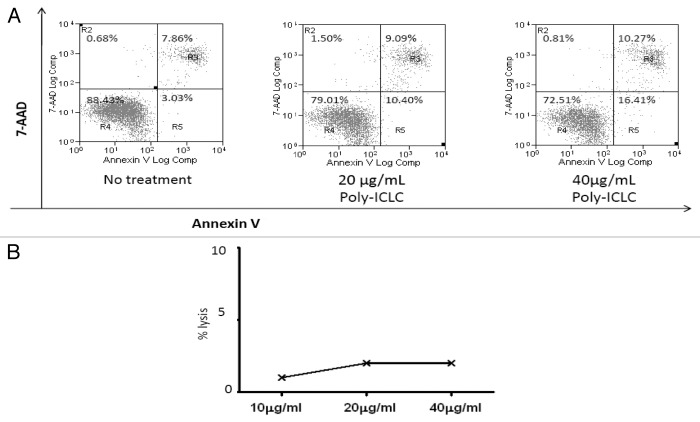
EGFR+ HNC cell lines (UM-22B and PCI-15B) were shown to express TLR3 using quantitative RT-PCR (Fig. 1A) and flow cytometry (Fig. 1B). Next, HNC cells were incubated with poly-ICLC to assess its direct cytotoxic effects. After a stimulation period of 24 h, poly-ICLC induced low levels of apoptosis in TLR3+ HNC cell lines, in a dose-dependent fashion. At a dose of 20 μg/mL or 40 μg/mL, the percentage of apoptotic cells (AnnexinV+) increased indeed from 7.8% (baseline) to 9% (20 μg/mL) and 10.2% (40 μg/mL) (Fig. 2A). We therefore selected the lower dose of poly-ICLC (20 μg/mL) for 51Cr release assays, as this poly-ICLC concentration exerted low background cytotoxicity in the absence of lymphocytes (Fig. 2B).
Figure 1. Expression of TLR3 in by head and neck cancer cells. (A and B) RNA was isolated from head and neck cancer (HNC) UM-22B, PCI-15B and JHU-029 cells as well as by TLR3-negative HEK293 cells, and the corresponding cDNAs were generated. TLR3-transfected HEK293 cells were used as a positive control. TLR3 expression was quantified by qRT-PCR and normalized to the expression of the control gene GUS (coding for β-glucuronidase). Alternatively, TLR3 expression on UM-22B and PCI-15B cells was evaluated by flow cytometry. To this aim, 5 × 105 cells were cultured in DMEM supplemented with L-glutamate, streptomycin and penicillin for 48 h and serum starved for the subsequent 24 h before collection. Cells were then fixed with 2% paraformaldehyde (PFA), permeabilized with 100% methanol and then labeled with an anti-TLR3 monoclonal antibody or isotype-matched antibodies and analyzed by flow cytometry. UM-22B and PCI-15B cells expressed TLR3 at both the mRNA (A) and protein (B) level.
Figure 2. Poly-ICLC exerts a slight pro-apoptotic effect on UM-22B cells but no over toxicity in the absence of PBMC. (A) UM-22B cells were incubated with increasing concentrations of poly-ICLC for 24 h, harvested and then stained with AnnexinV and 7-AAD for flow cytometry. Poly-ICLC slightly increases the percentage of apoptotic cells (shown in bold) in a dose-dependent manner. (B) Alternatively, HNC cells were labeled with 1 mCi 51Cr, washed twice with media and incubated with the indicated concentrations of cetuximab. Finally, supernatants were harvested and radioactivity was quantified on a scintillation counter. Negligible cell lysis was documented when UM-22B cells were incubated with poly-ICLC in the absence of immune effectors.
Poly-ICLC enhances cetuximab-dependent NK cell-mediated ADCC, which correlates with FCGR3A genotype
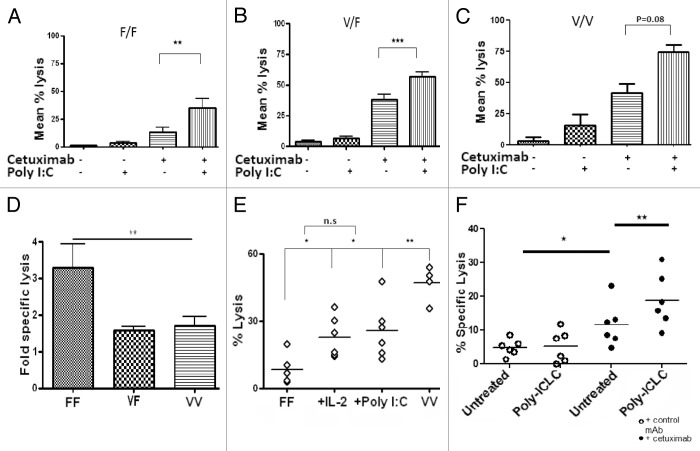
To determine if poly-ICLC-treated lymphocytes would exhibit increased cetuximab-dependent ADCC, unfractionated peripheral blood mononuclear cells PBMCs were incubated with 20 μg/mL poly-ICLC for 18 h and then tested in a classical 51Cr release assay for their ability to lyse HNC cells, in the presence or absence of 10 μg/mL cetuximab or a control IgG1. Poly-ICLC significantly enhanced cetuximab-dependent ADCC by PBMCs irrespective of FcγRIIIa polymorphisms at codon 158 (F/F, p = 0.008l; V/F, p < 0.0001; V/V, p = 0.08) (Fig. 3A–C). Interestingly, the PBMCs from some (approximately 25%) healthy donors failed to respond to poly-ICLC with an increase in their lytic capacity in spite of normal expression levels of TLR3 (data not shown).
Figure 3. Poly-ICLC enhances cetuximab-dependent ADCC by peripheral blood mononuclear cells from healthy donors. (A–E) UM-22B or PCI-15B HNC cells were used as targets in 51Cr release antibody-dependent cellular cytotoxicity (ADCC) assays, using natural killer (NK) cells expressing FcγRIIIa 158F only (F/F), both FcγRIIIa 158F and 158V (V/F) or FcγRIIIa 158V only (V/V). F/F NK cells demonstrated the greatest increase in ADCC upon TLR3 stimulation (p = 0.006). Healthy donor-derived peripheral blood mononuclear cells (PBMCs) were left untreated or treated with 50 μg/mL poly-ICLC for 18 h and then co-cultured for 4 h with 51Cr-labeled UM-22B or PCI-15B cells in the presence of 10 μg/mL cetuximab or isotype-matched IgG1 at an E:T ratio of 50:1. IL-2-treated PBMCs were used as a positive control. Genomic DNA extracted from PBMCs was used to determine the genotype at the FcγRIIIa-coding locus by PCR. (A–C) Effects of poly-ICLC on cetuximab-dependent ADCC using F/F (A), V/F (B) or V/V (C) PBMCs. (D) F/F NK cells derived the greatest functional benefits (approximately 3-fold increase) from poly-ICLC as compared with a 1-fold increase among V/F and V/V cells. (E) The ADCC activity of poly-ICLC-treated F/F PBMCs was significantly enhanced by poly-ICLC, comparing favorably with that of IL-2-treated PBMCs. (F) PBMC harvested from HNC patients were treated with 50 μg/mL poly-ICLC for 18 h prior to incubation with cetuximab-coated, 51Cr-labeled UM-22B or PCI-15B cells at an E:T ratio of 50:1, for 4 h. Supernatants were then harvested and radioactivity was quantified on a scintillation counter. Poly-ICLC enhanced cetuximab-dependent ADCC in PBMCs from 6 out of 23 (26.1%) HNC patients, 11.1% of which were F/F PBMCs, 28.6% V/F and 42.9% V/V PBMCs (p = 0.0028).
When the increase in cetuximab-dependent ADCC induce by poly-ICLC was correlated with FcγRIIIa polymorphic variants, PBMCs expressing FcγRIIIa 158F in homozygosity were found to obtain the most consistent functional improvement from the administration of TLR3 (p = 0.006) (Fig. 3D). We next compared the cetuximab-dependent ADCC of PBMCs expressing FcγRIIIa 158F upon exposure to either 20 μg/mL poly-ICLC or 50 IU/mL IL-2 for 18 h. Notably, poly-ICLC- and IL-2-treated PBMCs showed similar ADCC, which was significantly higher than that of vehicle-treated cells (Fig. 3E).
Poly-ICLC-receiving PBMCs from previously untreated HNC patients with active disease were found to respond to cetuximab with an increased in their capacity to mediate ADCC (Fig. 3F). Among these patients, 11.1% (1/9) expressed FcγRIIIa 158F only, 28.6% (2/7) expressed both FcγRIIIa 158F and FcγRIIIa 158V and 42.9% (3/7) expressed FcγRIIIa 158V only. PBMCs from two patients that did not demonstrate any lytic activity in the presence of cetuximab only became responders when poly-ICLC was included in the assays. Of these patients, one expressed both the FcγRIIIa 158V and 158F variants, while the other expressed FcγRIIIa 158V only, increasing the response rate to cetuximab upon poly-ICLC treatment to approximately 35%.
NK cells mediated poly-ICLC-enhanced, cetuximab-dependent ADCC
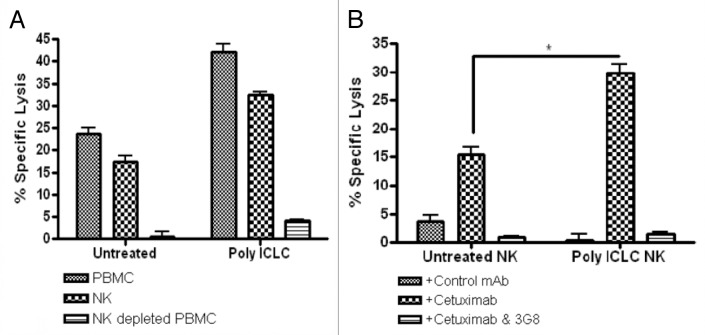
To determine the effector cell(s) of unfractionated PBMCs that would be responsible for the increase in cytotoxicity mediated by poly-ICLC, NK cells were depleted from PMBC preparations. NK cell-depleted PBMCs and unfractionated PBMCs (as a positive control) from the same donors were then used as effector in cetuximab-dependent ADCC assays. Poly-ICLC treated, NK-depleted PBMCs did not mediate appreciable cetuximab-dependent ADCC (Fig. 4A), demonstrating the critical contribution of NK cells to the lysis of cetuximab-coated HNC cells. To validate the importance of NK cells in the lytic activity of unfractionated PBMCs, purified NK cells were obtained by immunomagnetic separation and were used as effectors in in cetuximab-dependent ADCC assays. Purified NK cells mediated indeed robust cetuximab-dependent ADCC, which was significantly enhanced in the presence of poly-ICLC (Fig. 4B). Moreover, the cytotoxicity of NK cells was abolished in the presence of a FcγRIIIa-specific mAb (3G8).
Figure 4. NK cells are the primary effectors of poly-ICLC-stimulated cetuximab-dependent ADCC. (A and B) NK cells were purified from peripheral blood mononuclear cells (PBMCs) harvested from healthy donors using magnetic bead separation. Purified NK cells were treated with 50 μg/mL poly-ICLC for 18 h and then used as effectors in 51Cr release assays involving cetuximab-coated UM-22B or PCI-15B cells (E:T ratio = 10:1). Similarly, NK-depleted PBMCs were treated with poly-ICLC and cetuximab-dependent ADCC was monitored in 51Cr release assay (E:T ratio = 10:1). (A) Poly-ICLC failed to enhance cetuximab-dependent ADCC when the NK-depleted PDMC fraction was used as effector. (B) Poly-ICLC enhanced cetuximab-dependent ADCC when purified NK cells were used as effectors. The lytic activity of PBMCs and NK cells was completely abrogated when an anti-CD16 antibody (3G8) was added to effector cell/target cell co-cultures.
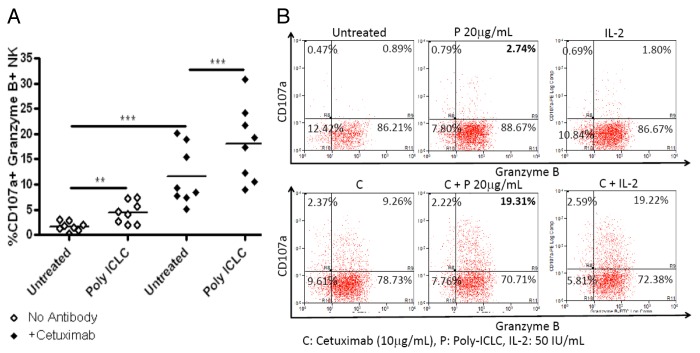
Poly-ICLC promotes NK-cell degranulation
To demonstrate the mechanisms underlying the enhanced lytic potential of TLR3-stimulated NK cells, poly-ICLC-treated PBMCs were evaluated for the expression of degranulation markers CD107a and granzyme B using flow cytometry. CD3negCD56+CD16+ NK cells were significantly more likely to be CD107a+ and granzyme B+ upon exposure to poly-ICLC (p = 0.0019) or cetuximab (p = 0.0009) alone. In addition, the increase in activated CD107a+granzyme B+ NK cells was more pronounced when NK-cell cultures were treated with both poly-ICLC and cetuximab (p = 0.0008) (Fig. 5A), consistent with their greater lytic activity against cetuximab-coated HNC cells. In addition, poly-ICLC-treated PBMCs exhibited a similar percentage of activated CD107a+granzyme B+ NK cells than PBMCs exposed to IL-2. Again, the percentage of activated NK cells was dramatically increased among PBMCs treated with both cetuximab and poly-ICLC as compared with PBMCs receiving poly-ICLC or cetuximab alone (Fig. 5B).
Figure 5. Poly-ICLC plus cetuximab increase the percentage of NK cells characterized by a CD107a+granzymeB+ phenotype. (A and B) Peripheral blood mononuclear cells (PBMCs) harvested from healthy donors were left untreated or treated with 50 μg/mL poly-ICLC for 18 h. Thereafter, PBMCs were co-cultured with PCI-15B cells for 4 h at a 1:1 ratio in the absence or in the presence of 10 μg/mL cetuximab. NK cells were identified upon immunostaining as CD3negCD56+CD16+ cells, and the percentage of CD107a+granzymeB+ NK cells were assessed by flow cytometry. (A) Poly-ICLC and cetuximab alone increase the fraction of CD107a+granzymeB+ NK cells (p = 0.0019 and p = 0.0009, respectively), an effect that is further increased in the presence of both poly-ICLC and cetuximab (p = 0.0008). (B) Poly-ICLC plus cetuximab increased the fraction of CD107a+granzymeB+ NK cells more significantly than poly-ICLC alone, comparing favorably with 50 IU/mL IL-2 (employed as a positive control).
Poly-ICLC-stimulated NK cells enhanced DC maturation and the cross-priming of EGFR-specific CD8+ T cells
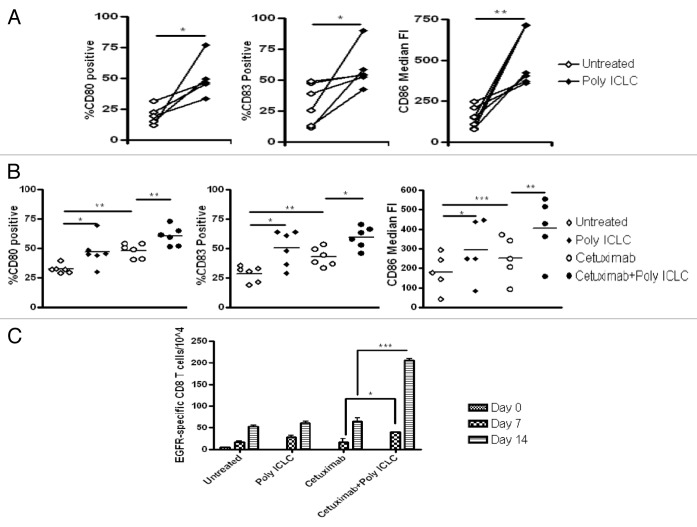
To determine whether poly-ICLC facilitated the priming of EGFR-specific CD8+ T cells in vitro, we first ascertained the effects of poly-ICLC on DC maturation. We have previously described a protocol for generating monocyte-derived DCs (mDCs).11 These DCs are known to express TLR3, a point that we verified by staining mDCs with a TLR3-specific antibody and flow cytometry (data not shown). Next, we incubated mDCs with 50 μg/mL poly-ICLC for 48 h and evaluated the upregulation of the DC maturation markers CD80, CD83 and CD86. As shown in Figure 6A, the stimulation of mDCs with poly-ICLC resulted in a maturation phenotype characterized by increased expression levels of CD80 (p = 0.03), CD83 (p = 0.04) and CD86 (p = 0.004).
Figure 6. Poly-ICLC-treated dendritic cells enhance the cetuximab-dependent induction of EGFR-specific CD8+ T-cells in the presence of NK cells and PCI-15B cells. (A) HLA-A*0201+ dendritic cells (DCs) were treated with 50 μg/mL poly-ICLC for 48 h. The maturation of DCs by poly-ICLC resulted in the upregulation of CD80 (p = 0.03), CD83 (p = 0.04) and CD86 (p = 0.004). Alternatively, HLA-A*0201+ DCs were incubated with natural killer (NK) cells and PCI-15B head and neck cancer (HNC) cells at a 1:1:1 ratio in the presence of medium only, 50 μg/mL poly-ICLC, 10 μg/mL cetuximab or both. (B) Poly-ICLC plus cetuximab induced a significantly higher proportion of DCs expressing CD80, CD83 and CD86 than 10 μg/mL cetuximab or 50 μg/mL poly-ICLC alone (p = 0.001; p = 0.0017; p = 0.002). An in vitro stimulation assay was performed using autologous CD8+ T cells incubated with DCs treated as described above for 7 or 7+7 d. (C) The functional cross-priming effects of DCs to induce EGFR-specific CD8+ T cells following 7-d stimulation were more pronounced when DCs were matured with poly-ICLC plus cetuximab (p = 0.02). A synergistic (nearly 4-fold) increase in the accumulation of EGFR-specific CD8+ T cells was seen in co-cultures treated with poly-ICLC plus cetuximab following a second 7-d-long stimulation period (p < 0.0001).
Next, we co-cultured mDCs with NK cells and PCI-15B HNC cells, alone or in the presence of 20 μg/mL poly-ICLC, 10 μg/mL cetuximab or both. The combination of cetuximab and poly-ICLC induced a significantly higher proportion of DCs expressing high levels of CD80, CD83 and CD86 than cetuximab or poly-ICLC alone (p = 0.001; p = 0.0017; p = 0.002) (Fig. 6B).
The functional significance of DC maturation as induced by poly-ICLC and cetuximab-activated NK cells was assessed by the in vitro stimulation (IVS) of HLA-A*0201 CD8+ T cells. After a 7-d IVS period, there was nearly a 2-fold increase in the frequency of EGFR853–861-specific CD3+CD8+ T cells in the co-cultures exposed to poly-ICLC and cetuximab in combination, as compared with those receiving either drug alone (p = 0.02) (Fig. 6C). Notably, a dramatic (nearly 4-fold) enhancement in the propagation of EGFR853–861-specific CD3+CD8+ T cells was seen in co-cultures treated with poly-ICLC and cetuximab following a second, 7 d-long IVS (p < 0.0001) (Fig. 6C). Therefore, the functional cross-priming activity of DCs was well pronounced in the presence of poly-ICLC and cetuximab-activated NK cells and increased significantly upon repeated IVS.
Discussion
Enhancing host immune responses should be considered as a measure to increase the efficacy of cetuximab-based therapy in HNC patients. With the discovery that TLR agonists function as potent immune adjuvants, promoting innate immunity and the subsequent induction of adaptive immune response has become an attractive alternative in this sense.35 Double stranded RNA is a natural ligand for TLR3 and drives a signaling cascade that results in the production of TH1 cytokines, which are beneficial in the tumor microenvironment.25,36,37 Poly-ICLC, a TLR3 agonist, has been investigated in clinical trials for its ability to boost vaccine-induced immune responses against brain tumors, exhibiting an acceptable toxicological profile.30,33 The role of TLR3 signals in the context of mAb-based therapy, however, has not yet been tested, driving us to launch this preclinical study to evaluate the effects of poly-ICLC on cetuximab-dependent ADCC against HNC cells. The enhancement of cetuximab-dependent ADCC by poly-ICLC is significant because ADCC marks the first step of activation of the immune system during mAb-based immunotherapy. In our model, subsequent steps of activation are promoted by poly-ICLC, owing to its maturing effect on DCs, which eventually results in the activation and recruitment of EGFR specific CD8+ cytotoxic T lymphocytes (CTLs). This strategy might improve disease course among cetuximab-treated HNC patients, since the presence of a strong tumor-specific CTL response has been associated with improved clinical outcomes.38
Our findings suggest for the first time that the use of a TLR3 agonists (poly-ICLC) as an immune adjuvant constitutes a valid means to enhance the antitumor effects of cetuximab against HNC. The expression of TLR3 by immune cells and HNC cell lines was investigated as the TLR3 pathway may per se mediate pro-apoptotic effects in this context.26 Previous studies have reported the intracellular expression of TLR3 by immune cells like DCs, monocytes and NK cells, and these findings were confirmed by our observations.39-41 Similarly, we demonstrated that the EGFR+ HNC cell lines used in our experiments (UM-22B and PCI-15B) express TLR3. This information is clinically relevant as agonists of other TLRs such as TLR4 have been suggested to protect HNC cells from immune attacks.22 In line with results from previous studies,20,26 TLR3 ligation did not mediate any pro-survival effect in HNC cells, further supporting our intent to study the immunostimulatory effects of poly-ICLC on cetuximab-based immunotherapy.18 On the contrary, we documented a modest, dose-dependent pro-apoptotic effect of poly-ICLC on HNC cells.
We had previously established the impact of FcγRIIIa polymorphisms on cetuximab-dependent ADCC against HNC, with the highest lytic effect seen for NK cells expressing FcγRIIIa 158V only, followed by cells expressing both FcγRIIIa 158V and 158F and cells expressing FcγRIIIa 158F only.14 Using 51Cr release assays to determine the effect of poly-ICLC on cetuximab-dependent ADCC, we demonstrated that poly-ICLC stimulates ADCC independent of FcγRIIIa polymorphisms. Such an increase concerned immune cells, as it was observed only in the presence of PBMCs. No measurable cytotoxicity indeed developed when PBMCs were not added to ADCC assays. Furthermore, we demonstrated that the ADCC-stimulating effects of poly-ICLC are primarily mediated by NK cells, as they were abrogated when NK-depleted PBMCs were used as effectors in ADCC assays. NK cells mediated cetuximab-dependent ADCC via the CD16 receptor, as no measurable lysis was seen when an anti-CD16 mAb (3G8) was added to the assays.
To characterize the functional activation of NK cells by poly-ICLC, we employed flow cytometry to evaluate the upregulation of NK-cell degranulation markers (CD107a and granzyme B) in the course of cetuximab-dependent ADCC. The percentage of activated NK cells characterized by a CD107a+granzymeB+ phenotype was modestly increased by poly-ICLC or cetuximab alone, yet augmented to nearly 4-fold in the presence of both agents. Furthermore, the functional activation of NK cells induced by poly-ICLC and IL-2 was similar. While others have analyzed the use of cytokines, such as IL-2, IL-12 and IL-21, to enhance the therapeutic efficacy of mAbs, the clinical utility of this approach is limited by the toxicity of systemic cytokine administration. Agonists of other TLRs, such as TLR7, TLR8 and TLR9, have also been investigated in ongoing or published studies for their immunostimulatory properties.
When cetuximab-dependent ADCC was evaluated on PBMCs obtained from HNC patients, the overall response rate was 26.1%. This rate mirrored the response rate to cetuximab seen in clinical trials involving HNC patients (20–30%), suggesing a correlation between immune responses to cetuximab-based therapy and disease course.1,3,42 Poly-ICLC further enhanced the cytotoxic functions mediated by PMBCs isolated from cetuximab responders. Furthermore, PBMCs (from two distinct HNC patients) that did not demonstrate lytic activity in the presence of cetuximab only became able to mediate ADCC when incubated with poly-ICLC.
The response rate of NK cells from HNC patients expressing FcγRIIIa 158F only to cetuximab was 11.1%, and poly-ICLC was unable to restore cetuximab-dependent ADCC among the non-responders of this group. Overall, the mean percentage of ADCC responses of patient-derived NK cells expressing FcγRIIIa 158F only was 3.9%. Given the extremely low ADCC response rate of NK cells from these HNC patients, we postulate that combination of cetuximab with poly-ICLC might be particularly beneficial in this setting. Enhancing the cetuximab-dependent cytotoxicity of NK cells from HNC patients expressing both FcγRIIIa 158V and 158F or FcγRIIIa 158V only by means of with poly-ICLC was also feasible, since the mean percentage of ADCC responses in this setting was 10.7%.
This hypothesis is in accordance with our immunological model of mAb-based immunotherapy for HNC.3 Since ADCC is an important mechanism used by innate effectors such as NK cells to elicit a first wave of immunogenic death among cancer cells, strategies aimed at enhancing the ADCC response of HNC patients are likely to result in improved adaptive immune responses and therapeutic outcome. To provide evidence in support of the activation of the adaptive immune system by TLR3 agonists combined with cetuximab-mediated immunotherapy, we demonstrated that poly-ICLC and cetuximab induce a robust upregulation of DC maturation markers such as CD80, CD83 and CD86. The maturation of DCs induced by poly-ICLC plus cetuximab had functional downstream consequences, as demonstrated by a synergistic increase in EGFR-specific CD8+ T cells secondary to enhanced DC-mediated cross-priming. Our findings are clinically relevant as they validate the immunological mechanism of action of cetuximab-based immunotherapy against HNC and demonstrate that TLR3 agonists can be used as immune adjuvants to enhance both innate and adaptive HNC-specific immune responses elicited by cetuximab.
In summary, the activation of TLR3 signaling with poly-ICLC exerts a beneficial effect on NK cells, resulting in the increased cetuximab-dependent lysis of HNC cells. Poly-ICLC also enhances DC maturation and the cross-priming of tumor-specific CD8+ cells. Finally, there is a modest, dose-dependent pro-apoptotic effect of poly-ICLC on TLR3+ HNC cell lines. Overall, our study demonstrates for the first time that TLR3 agonists such as poly-ICLC can be employed as immune adjuvants to enhance cetuximab-dependent ADCC against HNC and the consequent elicitation of adaptive antitumor immune responses.
Materials and Methods
Cell culture
Two EGFR+ HNC cell lines (UM-22B and PCI-15B) were cultured at 37 °C and under 5% CO2 in Dulbecco’s modified Eagle’s Medium (DMEM, from Gibco-BRL, Life Technologies, Inc.) supplemented with 5% streptomycin, 2% L-glutamine (Gibco) and 10% fetal bovine serum (FBS, from Hyclone). Blood samples from healthy donors were purchased from the Western Pennsylvania blood bank and blood samples from untreated HNC patients were obtained in accordance to the University of Pittburgh Medical Center Institutional Review Board approval protocol. PBMCs were extracted using a Ficoll-Hypaque gradient (Amersham Biosciences). Highly purified NK cells were obtained from PBMCs using the EasySep NK negative isolation kit (StemCell Technologies, Inc.), according to the manufacturer’s protocol. An NK purity >95% was invariably confirmed by flow cytometry.
Antibodies and flow cytometry
Cetuximab (Erbitux™, Bristol Myers Squibb) was purchased from the Hillman Cancer Center pharmacy. A human IgG1 isotype control was purchased from Enzo Life Science (ALX-804-133). Poly-ICLC (Hiltonol) was supplied by Oncovir Inc. as a courtesy of Dr. Hideho Okada (University of Pittsburgh). PE-conjugated TLR3 mAbs (9039) and PE-conjugated isotype IgG1s (4714) were purchased from eBioscience, while the CD16-specific mAb 3G8 (555404) was obtained from BD Biosciences. The following fluorophore-conjugated antibodies/molecules were used immunostaining prior to flow cytometry: CD3-Alexa 405 (0326), purchased from Life Technologies Inc.; CD16-PE-Cy7 (302015), Granzyme B-FITC (515403), EpCAM-APC (324207), CD11c-PE-Cy7 (301607) and CD86-PE (305405), purchased from Biolegend; CD56-APC (555518), CD8-APC (555369), CD80-FITC (557226), CD83-PE (556855), CD107a-PE (555801), HLA-A*0201-FITC (551285) and 7-AAD(559925) all purchased from BD Pharmingen. Unfractionated PBMCs (1 × 106 cells) or purified NK cells (5 × 105 cells) were treated with 20 μg/mL poly-ICLC for 18 h. Thereafter, cells were incubated with CD56-, CD16- and CD3-specific labeled mAbs in 2% bovine serum albumin (BSA, w:v in PBS) for 30 min at 4 °C. Following two washes in culture medium, cells were fixed with 2% paraformaldehyde (PFA) and analyzed with a Beckman Coulter EPICS XL flow cytometer. Data were analyzed using the Summit 4.3 software (Beckman Coulter).
NK-cell degranulation
For NK-cell degranulation experiments, PBMCs were treated with 20 μg/mL poly-ICLC or cultured in RPMI 1640 medium (Gibco-BRL, Life Technologies, Inc.) without stimulation for 18 h. PBMCs were then co-cultured with PCI-15B cells at 1:1 ratio in the absence or in the presence of 10 μg/mL cetuximab plus brefeldin A (555029, BD Pharmingen) and anti-CD107a or isotype-matched control antibodies for 4 h. PBMCs were then harvested, stained with anti-CD3, CD56 and CD16 mAbs, permeabilized using the BD Cytofix/Cytoperm reagent (BD Pharmingen) and then stained for intracellular granzyme B expression. At analysis, doublet-excluding events were gated for CD3negCD56+CD16+granzyme B+ lymphocytes and analyzed for the percentage CD107a+ cells.
51Cr release assays
PBMCs were pretreated with 20 μg/mL poly-ICLC for 18 h. Next, 1 × 106 HNC cells were labeles with with 1 mCi Na51CrO4 for 1 h at 37°C. Cells were washed twice with medium and incubated with effector cells at an E:T ratio of 50:1 in the presence of 10 μg/mL cetuximab. Four hours later, supernatants were harvested and counted on a Microbeta Trilux scintillation counter (PerkinElmer). Percentage cytotoxicity was calculated using the formula (experimental-spontaneous release)/(maximum-spontaneous release) × 100%; where spontaneous = release from targets incubated with medium alone and maximum = release from targets incubated by 5% Triton X-100 (Sigma Aldrich).
Apoptosis assay
HNC cells (2.5 × 105) were cultured in complete DMEM in T25 flasks for 2 d. Cells were observed under microscopy to ensure adequate growth and no spontanoues cell death and then treated with varying concentrations of poly-ICLC for 24 h. Cells were then harvested and stained with AnnexinV and 7-AAD (BD Pharmingen), according to manufacturer’s instructions. Cytofluorometric acquisition was performed on a Beckman Coulter EPICS XL flow cytometer.
DC culture
After typing for HLA-A*0201, monocytes were isolated from PBMCs by plastic adherence for 2 h at 37 °C. Non-adherent cells were removed and adherent cells were incubated with Aim-V medium (Invitrogen) supplemented with 1000 IU/mL granulocyte macrophage colony-stimulating factor (GM-CSF, fromR&D Systems) 1000 IU/mL IL-4 (from R&D Systems). Three days later, GM-CSF and IL-4 were replenished to the final concentration of 1000 IU/mL. mDCs were harvested on day 6 using 0.25% trypsin-EDTA (25200-072, Life Technologies, Inc.) and co-cultured with NK and PCI-15B cells (ratio 1:1) for 48 h. During this time, co-cultures were left untreated or received 50 μg/mL poly-ICLC, 10 μg/mL cetuximab or both. Cells were then harvested and stained with CD80-FITC plus CD86-PE or CD83-PE antibodies and analyzed on flow cytometry. Doublet-excluding events were gated based on high side scatter and EpCAMnegCD11c+ phenotype and analyzed for the percentage of CD80+ and CD83+ cells as well as for and CD86 mean fluorescence intensity (MFI).
In vitro CD8+ T-cell stimulation
Autologous HLA-A*0201 CD8+ T cells were harvested using the EasySep human T-cell enrichment kit (StemCell Technologies, Inc.), according to the manufacturer’s instructions. CD8+ T cells were then stimulated in vitro with DCs matured as described above either once for 7 d, or twice (14 d in total) in the presence of 20 IU/mL IL-2 (R&D Systems). Wild-type EGFR853–861 was produced by the peptide synthesis facility at the University of Pittsburgh, using the F-moc technology.11,43 The corresponding EGFR853–861 tetramer was synthesized by the NIH Tetramer Facility (Emory University). At the end of the stimulation, CD8+ T cells were harvested and stained with CD3- and CD8-specific antibodies, 7-AAD and the EGFR853–861 tetramer and analyzed by flow cytometry. Doublet-excluding events were gated for viable (7-AADneg) lymphocytes exhibiting a CD3+CD8+ phenotype and analyzed for EGFR853–861 tetramer+/10,000 CD8+ T cells.
Statistical analyses
Significant differences in ADCC by FcγRIIIa phenotype were determined using a Kruskal-Wallis one-way analysis of variance with p values < 0.05 considered as statistically significant. A post-hoc Mann-Whitney non-parametric t-test was performed for differences between groups, with p values < 0.05 considered as statistically significant.
Acknowledgments
This work was supported by National Institute of Health grants R01 DE019727, CA110249 and P50 CA097190. This project used the UPCI Cytometry Facility that is supported in part by award P30 CA047904. Dr. Chwee Ming Lim acknowledged the National Medical Research Council Singapore grant for this work.
Disclosure of Potential Conflicts of Interest
Dr. Andres M Salazar is CEO and scientific director of Oncovir, Inc. The other authors do not have any conflict of interest to disclose.
Footnotes
These authors contributed equally to this work.
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24677
References
- 1.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 2.Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, et al. Phase I study of anti--epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–43. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 3.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–9. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonner JA, Keene KS. Is cetuximab active in patients with cisplatin-refractory squamous cell carcinoma of the head and neck? Nat Clin Pract Oncol. 2007;4:690–1. doi: 10.1038/ncponc0962. [DOI] [PubMed] [Google Scholar]
- 5.Lenz HJ, Van Cutsem E, Khambata-Ford S, Mayer RJ, Gold P, Stella P, et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol. 2006;24:4914–21. doi: 10.1200/JCO.2006.06.7595. [DOI] [PubMed] [Google Scholar]
- 6.Blumenschein GR, Jr., Paulus R, Curran WJ, Robert F, Fossella F, Werner-Wasik M, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol. 2011;29:2312–8. doi: 10.1200/JCO.2010.31.7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.William WN, Jr., Kim ES, Herbst RS. Cetuximab therapy for patients with advanced squamous cell carcinomas of the head and neck. Nat Clin Pract Oncol. 2009;6:132–3. doi: 10.1038/ncponc1321. [DOI] [PubMed] [Google Scholar]
- 8.Lilenbaum RC. The evolving role of cetuximab in non-small cell lung cancer. Clin Cancer Res. 2006;12:4432s–5s. doi: 10.1158/1078-0432.CCR-06-0097. [DOI] [PubMed] [Google Scholar]
- 9.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. FLEX Study Team Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 10.López-Albaitero A, Ferris RL. Immune activation by epidermal growth factor receptor specific monoclonal antibody therapy for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1277–81. doi: 10.1001/archotol.133.12.1277. [DOI] [PubMed] [Google Scholar]
- 11.López-Albaitero A, Mailliard R, Hackman T, Andrade Filho PA, Wang X, Gooding W, et al. Maturation pathways of dendritic cells determine TAP1 and TAP2 levels and cross-presenting function. J Immunother. 2009;32:465–73. doi: 10.1097/CJI.0b013e3181a1c24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–9. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 13.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–5. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 14.López-Albaitero A, Lee SC, Morgan S, Grandis JR, Gooding WE, Ferrone S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58:1853–64. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leibowitz MS, Andrade Filho PA, Ferrone S, Ferris RL. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol Immunother. 2011;60:525–35. doi: 10.1007/s00262-010-0961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhave NS, Carson WE., 3rd Immune modulation with interleukin-21. Ann N Y Acad Sci. 2009;1182:39–46. doi: 10.1111/j.1749-6632.2009.05071.x. [DOI] [PubMed] [Google Scholar]
- 17.Luedke E, Jaime-Ramirez AC, Bhave N, Carson WE., 3rd Monoclonal antibody therapy of pancreatic cancer with cetuximab: potential for immune modulation. J Immunother. 2012;35:367–73. doi: 10.1097/CJI.0b013e3182562d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mani A, Roda J, Young D, Caligiuri MA, Fleming GF, Kaufman P, et al. A phase II trial of trastuzumab in combination with low-dose interleukin-2 (IL-2) in patients (PTS) with metastatic breast cancer (MBC) who have previously failed trastuzumab. Breast Cancer Res Treat. 2009;117:83–9. doi: 10.1007/s10549-008-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jego G, Bataille R, Geffroy-Luseau A, Descamps G, Pellat-Deceunynck C. Pathogen-associated molecular patterns are growth and survival factors for human myeloma cells through Toll-like receptors. Leukemia. 2006;20:1130–7. doi: 10.1038/sj.leu.2404226. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 22.Roda JM, Parihar R, Carson WE., 3rd CpG-containing oligodeoxynucleotides act through TLR9 to enhance the NK cell cytokine response to antibody-coated tumor cells. J Immunol. 2005;175:1619–27. doi: 10.4049/jimmunol.175.3.1619. [DOI] [PubMed] [Google Scholar]
- 23.Diebold SS. Recognition of viral single-stranded RNA by Toll-like receptors. Adv Drug Deliv Rev. 2008;60:813–23. doi: 10.1016/j.addr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Nicodemus CF, Berek JS. TLR3 agonists as immunotherapeutic agents. Immunotherapy. 2010;2:137–40. doi: 10.2217/imt.10.8. [DOI] [PubMed] [Google Scholar]
- 25.Vercammen E, Staal J, Beyaert R. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin Microbiol Rev. 2008;21:13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umemura N, Zhu J, Mburu YK, Forero A, Hsieh PN, Muthuswamy R, et al. Defective NF-κB signaling in metastatic head and neck cancer cells leads to enhanced apoptosis by double-stranded RNA. Cancer Res. 2012;72:45–55. doi: 10.1158/0008-5472.CAN-11-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field AK, Tytell AA, Lampson GP, Hilleman MR. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967;58:1004–10. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci U S A. 2008;105:2574–9. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy HB, Baer G, Baron S, Buckler CE, Gibbs CJ, Iadarola MJ, et al. A modified polyriboinosinic-polyribocytidylic acid complex that induces interferon in primates. J Infect Dis. 1975;132:434–9. doi: 10.1093/infdis/132.4.434. [DOI] [PubMed] [Google Scholar]
- 30.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with alpha-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–6. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin AI, Miyahira AK, Covarrubias A, Teague J, Guo B, Dempsey PW, et al. Toll-like receptor 3-mediated suppression of TRAMP prostate cancer shows the critical role of type I interferons in tumor immune surveillance. Cancer Res. 2010;70:2595–603. doi: 10.1158/0008-5472.CAN-09-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butowski N, Lamborn KR, Lee BL, Prados MD, Cloughesy T, DeAngelis LM, et al. A North American brain tumor consortium phase II study of poly-ICLC for adult patients with recurrent anaplastic gliomas. J Neurooncol. 2009;91:183–9. doi: 10.1007/s11060-008-9705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butowski N, Chang SM, Junck L, DeAngelis LM, Abrey L, Fink K, et al. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01-05) J Neurooncol. 2009;91:175–82. doi: 10.1007/s11060-008-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–8. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–72. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown RA, Razonable RR. A real-time PCR assay for the simultaneous detection of functional N284I and L412F polymorphisms in the human Toll-like receptor 3 gene. J Mol Diagn. 2010;12:493–7. doi: 10.2353/jmoldx.2010.090122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duluc D, Tan F, Scotet M, Blanchard S, Frémaux I, Garo E, et al. PolyI:C plus IL-2 or IL-12 induce IFN-gamma production by human NK cells via autocrine IFN-beta. Eur J Immunol. 2009;39:2877–84. doi: 10.1002/eji.200838610. [DOI] [PubMed] [Google Scholar]
- 38.Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, et al. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451–8. doi: 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 39.Jelinek I, Leonard JN, Price GE, Brown KN, Meyer-Manlapat A, Goldsmith PK, et al. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J Immunol. 2011;186:2422–9. doi: 10.4049/jimmunol.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muzio M, Bosisio D, Polentarutti N, D’amico G, Stoppacciaro A, Mancinelli R, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 41.Perrot I, Deauvieau F, Massacrier C, Hughes N, Garrone P, Durand I, et al. TLR3 and Rig-like receptor on myeloid dendritic cells and Rig-like receptor on human NK cells are both mandatory for production of IFN-gamma in response to double-stranded RNA. J Immunol. 2010;185:2080–8. doi: 10.4049/jimmunol.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–8. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 43.Andrade Filho PA, López-Albaitero A, Gooding W, Ferris RL. Novel immunogenic HLA-A*0201-restricted epidermal growth factor receptor-specific T-cell epitope in head and neck cancer patients. J Immunother. 2010;33:83–91. doi: 10.1097/CJI.0b013e3181b8f421. [DOI] [PMC free article] [PubMed] [Google Scholar]