Abstract
The antineoplastic effects of anthracyclines have been shown to rely, at least in part, on a local immune response that involves dendritic cells (DCs) and several distinct subsets of T lymphocytes. Here, we show that the administration of anthracyclines to mice bearing established neoplasms stimulates the intratumoral secretion of tumor necrosis factor α (TNFα). However, blocking the TNFα/TNF receptor (TNFR) system by three different strategies—namely, (1) neutralizing antibodies, (2) etanercept, a recombinant protein in which TNFR is fused to the constant domain of an IgG1 molecule, and (3) gene knockout—failed to negatively affect the therapeutic efficacy of anthracyclines in three distinct tumor models. In particular, TNFα-blocking strategies did not influence the antineoplastic effects of doxorubicin (a prototypic anthracycline) against MCA205 fibrosarcomas growing in C57BL/6 mice, F244 sarcomas developing in 129/Sv hosts and H2N100 mammary carcinomas arising in BALB/c mice. These findings imply that, in contrast to other cytokines (such as interleukin-1β, interleukin-17 and interferon γ), TNFα is not required for anthracyclines to elicit therapeutic anticancer immune responses.
Keywords: T cells, apoptosis, calreticulin, dendritic cell, immunogenic cell death, interferon γ
Introduction
Although it is commonly assumed that chemotherapeutics eradicate malignant cells as antibiotics kill bacteria, accumulating evidence indicates that successful antineoplastic agents (at least in part) exert therapeutic effects by (re)activating tumor-specific immune responses.1,2 Thus, several anticancer drugs that are nowadays employed in the clinical practice have been shown to elicit a state of cellular stress (eventually translating into cell death) that is accompanied by the emission of so-called “danger-associated molecular patterns” (DAMPs).3-6 An appropriate combination of such DAMPs, encompassing proteins that are exposed on the cell surface as well as soluble factors, converts adaptive responses to stress and cell death into an immunogenic event.4,7
The immunogenicity of anthracycline-induced cell death has been shown to rely on the timely emission of least three distinct DAMPs, namely (1) calreticulin (CRT), which is exposed on the outer leaflet of the plasma membrane early during apoptosis, owing to the activation of an endoplasmic reticulum (ER) stress response;8 (2) ATP, which is secreted into the extracellular space in an autophagy-dependent fashion, along with the activation of caspases and plasma membrane blebbing;9 and (3) high mobility group box 1 (HMGB1), a non-histone chromatin-binding protein that is released by dead cells upon nuclear and plasma membrane permeabilization.10 This spatiotemporally defined combination of DAMPs allows for the recruitment of myeloid cells into the tumor bed and the activation of their inflammasome (which are mediated by purinergic P2RY2 and P2RX7 receptors, respectively), the efficient uptake of tumor-associated antigens by specific myeloid cell subsets (which is stimulated by cell surface-exposed CRT) and optimal antigen presentation (which is promoted by HMGB1). After an initial wave of myeloid cell infiltration (12–72 h post-chemotherapy), various T-cell subsets are recruited into the tumor bed, in particular interleukin-17 (IL-17)-secreting γ/δ T cells (3–5 d post-chemotherapy) and interferon γ (IFNγ)-producing CD8+ α/β T cells (peaking approximately 8 d post-chemotherapy).11-14
The local immune response that is initiated by DAMPs to eventually exert antineoplastic effects is complex. In line with this notion, the blockade of myeloid cell extravasation with CD11b-blocking antibodies as well as the elimination of γ/δ T cells or CD8+ α/β T cells suffices to abolish the therapeutic efficacy of anthracycline-based chemotherapy in vivo. Along similar lines, the genetic or pharmacological inhibition of IL-1β (produced by dendritic cell (DC)-like myeloid cells), IL-17 (secreted by γ/δ T cells) and IFNγ (one of the major cytotoxic factors of CD8+ α/β T cells) is sufficient to abrogate the antineoplastic activity of anthracyclines and other immunogenic chemotherapeutics in rodent models.4,12,15-17
Driven by the discovery that the administration of doxorubicin (a prototypic anthracycline)18 to tumor-bearing mice results in the intratumoral upregulation of tumor necrosis factor α (TNFα),19,20 we investigated the putative contribution of this pleiotropic, multifunctional cytokine21,22 to the efficacy of anticancer immune responses. Surprisingly, we found that blocking the TNFα system by three distinct genetic or pharmacological manipulations fails to affect the chemotherapeutic response of established tumors to anthracyclines.
Results and Discussion
Enhanced TNFα expression in tumors responding to anthracycline-based chemotherapy
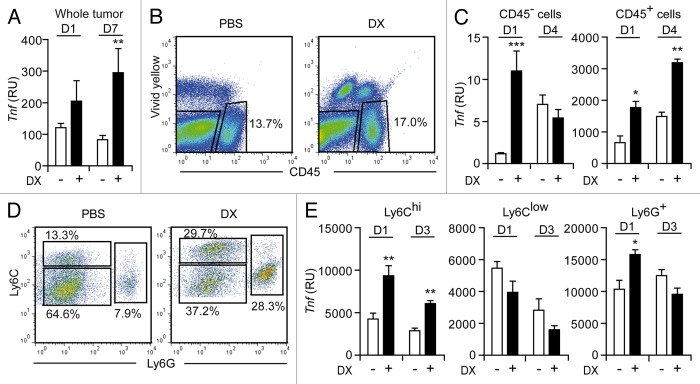
We have previously reported that anthracycline-based promotes the upregulation of TH1-and TH17-related genetic signatures in experimental tumors established in mice. The levels of mRNAs coding for surrogate markers of a TH1 response (such as IFNγ and TNFα) were indeed increased upon the intratumoral administration of doxorubicin.12 Along similar lines, we observed that the Tnf mRNA levels were markedly upregulated in MCA205 fibrosarcomas (established in C57BL/6 mice) 7 d after doxorubicin -based chemotherapy (Fig. 1A). A similar trend could be observed as early as 1 d after the intratumoral administration of doxorubicin, though the threshold for statistical significance was not reached at this time point (Fig. 1A).
Figure 1. Characterization of TNFα production in the tumor microenvironment after immunogenic chemotherapy. (A–E) C57BL/6 mice bearing MCA205 fibrosarcomas (tumor surface 25–45 mm2) were treated with doxorubicin (DX) or an equivalent volume of PBS, as a single intratumoral injection (day 0). (A) Total RNA was extracted from neoplastic lesions collected at day 1 and day 7, and Tnf expression levels were assessed by quantitative RT-PCR. (B–E) As an alternative, tumors were harvested on the indicated day, dissociated into single-cell suspensions and stained with either a CD45-specific (B) or with CD11b-, Ly6C- and Ly6G-targeting antibodies (D). Thereafter, Tnf expression levels were specifically determined among CD45− (C), CD45+ (C), CD11b+Ly6G−Ly6Chi (E), CD11b+Ly6G−Ly6Clow (E) and CD11b+Ly6G+ (E) cells. In (B) and (D), numbers indicate the percentage of cells found in the corresponding gate. Quantitative data on Tnf expression are expressed as relative units upon normalization to Ppia expression levels (RU, means ± SEM; n = 3–8 mice/group). *p < 0.05, **p < 0.01, ***p < 0.001; (unpaired, two-tailed Student’s t-test), as compared with the same cell population isolated on the same day from PBS-treated tumors.
The relative contribution of CD45- (tumor) cells and CD45+ tumor-infiltrating leukocytes (TILs) to the production of TNFα triggered by anthracyclines was determined by performing quantitative RT-PCR on viable cells sorted by cytofluorometry upon immunostaining with a CD45-specific antibody (Fig. 1B). Although both CD45− and CD45+ cells significantly upregulated TNFα at the transcriptional level as early as 1 d after the administration of doxorubicin, on a per-cell basis Tnf mRNA levels were approximately 400-fold higher in TILs than in cancer cells (Fig. 1C). Thus, taking into consideration the relative abundance of CD45+ vs. CD45− cells in the tumor microenvironment, TILs appear to constitute the predominant source of TNFα in established MCA205 fibrosarcomas responding to doxorubicin. Of note, 4 d after chemotherapy, CD45+ (but not CD45−) cells still exhibited increased Tnf mRNA levels as compared with their CD45+ (or CD45−) counterparts obtained from PBS-treated tumors (Fig. 1C). The production of TNFα by TILs exposed to doxorubicin in vivo was temporally coincident with the early influx of inflammatory myeloid cells triggered by immunogenic chemotherapy.11 We therefore compared Tnf mRNA levels in several CD11b+ myeloid cell subpopulations including: Ly6Chi inflammatory monocytes, Ly6Clow cells and Ly6G+ neutrophils (Fig. 1D). Interestingly, at two early time points (1 and 3 days post-chemotherapy), the intratumoral administration of doxorubicin significantly increased Tnf expression by tumor-infiltrating CD11b+Ly6Chi cells, which we have recently shown to operate as antigen-presenting cells (APCs) in situ,11 but not by CD11b+Ly6Clow cells (Fig. 1E). In this setting, Ly6G+ neutrophils exhibited a modest (yet statistically significant) increase in Tnf mRNA levels 1 d, but not 3 d, after immunogenic chemotherapy (Fig. 1E).
Blocking the TNFα system fails to interfere with the recruitment of APCs and their capacity to take up tumor-associated antigens, yet hampers APC maturation
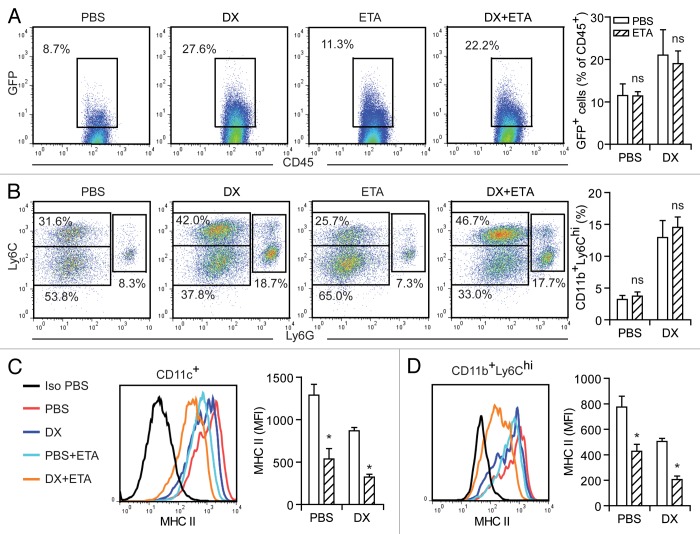
Immunogenic chemotherapies elicit the efficient presentation of tumor-associated antigens, in turn driving potent cytotoxic T-lymphocyte (CTL) responses. To analyze the role of TNFα during antigen presentation, we took advantage of murine CT26 colorectal carcinoma cells engineered to express an eGFP variant that carries consensus sequences for myristoylation plus palmitoylation (MyrPalm-mEGFP), and hence localizes to the inner leaflet of the plasma membrane.23 Thus, we inoculated MyrPalm-mEGFP-expressing CT26 cells in BALB/c mice (allowing us to track the uptake of tumor-associated antigens) and—once neoplastic lesions were established—treated them with a single intratumoral injection of PBS (control conditions) or doxorubicin. In this setting, anthracycline-based chemotherapy enhanced antigen uptake by TILs, an effect that was well pronounced 36 h upon the administration of doxorubicin and was not influenced by the co-administration of etanercept (Fig. 2A), a soluble TNFα decoy molecule (constituted by the TNFα receptor fused to an IgG1 antibody) currently employed for the treatment of several autoimmune diseases.24,25 Along similar lines, etanercept failed to block the recruitment into the tumor bed of CD11b+Ly6Chi cells (Fig. 2B), which are critical for the presentation of tumor-associated antigens in the course of chemotherapy-elicited immune responses.11 TNFα has been reported to operate as a maturation-promoting factor for several human and murine cell types, including DCs.26 In line with this notion, the administration of etanercept along with anthracycline-based chemotherapy inhibited the maturation of CD11c+ as well as CD11b+Ly6Chi cells, as evaluated by the expression on their surface of MHC Class II molecules (Fig. 2C and D). Taken together, these observations suggest that TNFα influences neither the recruitment of APCs to anthracycline-treated tumors nor the ability of these cells to engulf tumor-associated antigens, yet it facilitates APC maturation in an autocrine or paracrine manner.
Figure 2. Role of TNFα in the anthracycline-mediated recruitment, functional activation and maturation of antigen-presenting cells. (A–D) BALB/c mice harboring MyrPalm-mEGFP-expressing CT26 colon carcinomas (tumor surface 25–45 mm2) were treated with doxorubicin (DX) or an equivalent volume of PBS, as a single intratumoral injection (day 0). On the same day, a fraction of mice was initiated on a course of intraperitoneal etanercept (ETA). On day 3, tumors were harvested, dissociated into single-cell suspensions and stained with either a CD45-specific (A) or with CD11b-, CD11c-, Ly6C- and Ly6G-targeting antibodies, alone (B) or combined with antibodies specific for MHC Class II molecules (C and D). (A) reports representative dot plots and quantitative data on the percentage of CD45+ tumor-infiltrating leukocytes (TILs) emitting a GFP-associated fluorescence (indicative of the uptake of tumor-associated antigens). In (B), representative dot plots and quantitative data on the anthracycline-elicited recruitment of CD11b+Ly6G−Ly6Chi, CD11b+Ly6G−Ly6Clow and CD11b+Ly6G+ cells into the tumor bed are illustrated. In (A) and (B), numbers indicate the percentage of cells found in the corresponding gate. (C) and (D) depict representative expression profiles of MHC Class II molecules among CD11c+ and CD11b+Ly6G-Ly6Chi cells, respectively, and the corresponding quantitative data (means ± SEM, n = 3). ns, non-significant; *p < 0.05, (unpaired, two-tailed Student’s t-test), as compared with the same cell population isolated from tumors treated with PBS or DX only (in the absence of ETA).
Normal antineoplastic profile of anthracyclines in spite of TNFα blockade
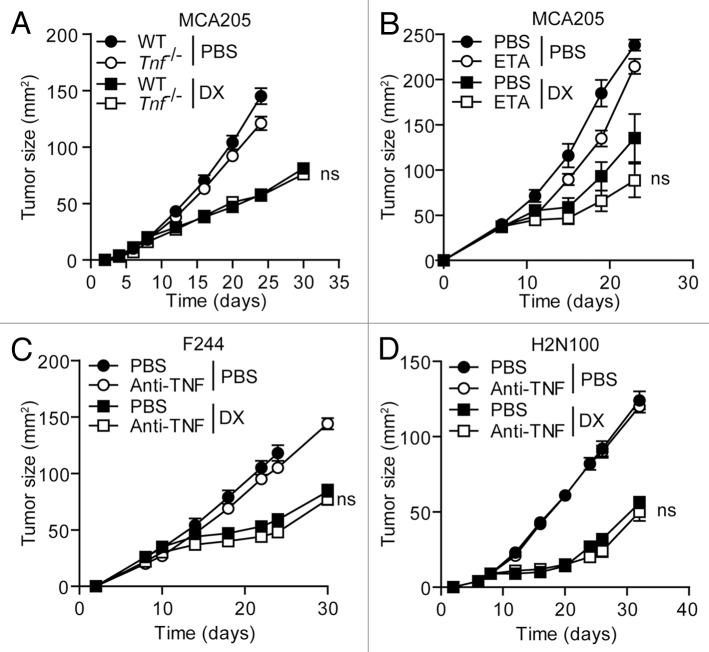
MCA205 fibrosarcomas grew in wild-type and Tnf−/− C57BL/6 mice with virtually overlapping kinetics, and anthracycline-based chemotherapy completely retained its efficacy in the absence of host-derived TNFα (Fig. 3A). Along similar lines, the neutralization of TNFα with etanercept shortly before and continuously after chemotherapy failed to significantly alter the therapeutic efficacy of doxorubicin against MCA205 fibrosarcomas growing in C57BL/6 mice (Fig. 3B). Similar results were obtained when the TNFα system was blocked by the administration of a TNFα-neutralizing antibody. In particular, F244 sarcomas developing in 129/Sv mice as well as H2N100 mammary carcinomas growing in BALB/c mice responded to doxorubicin irrespective of the co-administration of the TNFα-targeting antibody TN3–19.12 (Fig. 3C and D). These findings indicate that TNFα does not influence the responsiveness of tumor-bearing mice to immunogenic chemotherapy.
Figure 3. Influence of TNFα on the therapeutic effects of anthracyclines. (A–D) Tnf−/−(A) or wild type (WT) (A and B) C57BL/6 mice carrying MCA205 fibrosarcomas (tumor surface 25–45 mm2) were treated with doxorubicin (DX) or an equivalent volume of PBS, as a single intratumoral injection (day 8). On the same day, some of the mice were initiated on a course of intraperitoneal etanercept (ETA), for 4 consecutive days. Alternatively, WT 129/Sv mice bearing established F244 sarcomas (C) or BALB/c mice harboring H2N100 mammary carcinomas (D) received DX or an equivalent volume of PBS, as a single intratumoral injection, on day 10 or 8 after the inoculation of tumor cells, respectively. One day prior to chemotherapy, a fraction of mice was initiated on a course of TNFα-neutralizing antibodies (or isotype-matched control antibodies), which were given i.v. on days 9, 10, 14, 17 and 21 (C) or on day 7, 8, 12, 15, 19 and 22 (D). Tumor area was then monitored routinely by means of a common caliper. Results are expresses as means ± SEM (n = 5 mice/group). These experiments were repeated independently twice, yielding comparable results. ns, nonsignificant; (Mann–Whitney U test), as compared with DX-treated WT mice.
Concluding remarks
Here, we present unambiguous evidence indicating that TNFα is dispensable for the therapeutic efficacy of anthracyclines in mice. Indeed, we observed that the blockade of the TNFα system (by means of three different approaches) fails to affect the antineoplastic effects of the prototypic anthracycline doxorubicin in three distinct murine tumor models.
The role of TNFα in cancer immunosurveillance has been the subject of an intense debate. Thus, Tnf−/− mice develop methylcholanthrene (MCA)-induced fibrosarcoma more frequently than their wild-type counterparts.27 Conversely, Tnf−/− mice are protected from the combined carcinogenic effects of the DNA-damaging agent 7,12-dimethylbenz(a)anthracene (DMBA) and 12-O-tetradecanoylphorbol-13-acetate (TPA).28 This apparent discrepancy may reflect the complex biology of carcinogenesis, in which TNFα-driven inflammation and immunosurveillance play antagonist roles.29
The implication of TNFα in anticancer therapy-elicited immune responses also exhibits a considerable degree of context dependency. In a murine model of Simian virus 40 large T antigen (Tag)-driven insulinoma, the adoptive transfer of Tag-specific TH1 cells producing both IFNγ and TNFα has been shown to promote senescence in a TNFα receptor 1 (TNFR1)-dependent fashion.21 Along similar lines, insulinoma cells exposed in vitro to IFNγ and TNFα underwent an irreversible cell cycle arrest that was accompanied by several epigenetic and lysosomal changes associated with cell senescence.21 Furthermore, TNFα has been shown to be required for the rejection of MC57 fibrosarcoma cells by syngeneic mice previously immunized with irradiated cells of the same type.30 Conversely, here we demonstrate that TNFα does not alter the antineoplastic effects of immunogenic chemotherapy. In this setting, a great role can be played by the specificity of distinct tumor models. Indeed, while some tumors are preferentially controlled by innate immune effectors, others are mainly held in check by CD8+ or CD4+ T cells.31-33
Of note, Frances Balkwill’s group has recently demonstrated not only that TNFα is required for the accumulation of F4/80+ macrophages into intraperitoneal ovarian cancer xenografts, but also that there is a correlation between an elevated expression of genes coding for TNFα-related cytokines and the amount of CD68+ cells infiltrating high-grade serous ovarian cancer biopsies.34 We did not investigate directly whether TNFα is required for the anthracycline-driven recruitment of F4/80+ macrophages into MCA205 fibrosarcomas, but neither the accumulation of bulk CD11b+ myeloid cells nor that of inflammatory monocytes (which can differentiate into macrophages or DCs) was hampered by TNFα-blocking maneuvers in our system. Moreover, we have previously shown that the administration of clodronate-loaded liposomes (which efficiently depletes the splenic monocytic/macrophagic cell compartment) fails to affect the antineoplastic potential of anthracyclines,11 arguing against a prominent role for F4/80+ macrophages in the elicitation of therapeutic immune responses by immunogenic chemotherapy.
Anthracycline-elicited anticancer immune responses are mostly mediated by CD8+ T cells, which must produce IFNγ to control tumor growth.35,36 How IFNγ produced by CD8+ T cells exerts antineoplastic effects is currently unknown. Tumors engrafted in mice lacking perforin, a key effector molecule of CD8+ T cells, respond normally to anthracyclines,37 suggesting that classical cytotoxic mechanisms are not involved in the antineoplastic effects of immunogenic chemotherapy. As a possibility, IFNγ-producing CD8+ cells may inhibit tumor growth indirectly, by destroying the tumor vasculature and/or blocking neo-angiogenesis.38-40 Alternatively, such cells may activate sessile macrophages to destroy malignant cells.41 The exact mechanisms through which terminal immune effectors control tumor growth in response to chemotherapy require further exploration.
Materials and Methods
Unless otherwise indicated, chemicals were purchased from Sigma-Aldrich and cell culture products from Gibco-Life Technologies.
Cell lines
Mouse fibrosarcoma MCA205 cells (H-2b), mammary carcinoma H2N100 cells (H-2d), sarcoma F244 cells (derived from 129/Sv mice)42,43 and MyrPalm-mEGFP-expressing colon carcinoma CT26 cells (H-2d),44 were cultured in GlutaMAX™-I-containing RPMI 1640 Medium supplemented with 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, 10 mM HEPES buffer, 100 units/mL penicillin G sodium and 100 μg/mL streptomycin sulfate.
Animal experiments
Female wild-type and Tnf−/− C57BL/6 (H-2b),45 BALB/c (H-2d) and 129/Sv mice were housed in controlled, pathogen-free conditions at either the Institut Gustave Roussy (IGR) or the Peter MacCallum Cancer Centre. Mice were maintained under controlled light cycle (12 h lights ON, 12 h lights OFF), allowed food and water ad libitum, and were invariably used for experiments between 7 and 14 weeks of age. All animal experiments complied with the Federation of European Laboratory Animal Science Association (FELASA) guidelines and were approved either by the IGR Ethics Committee (CEEA IRCIV/IGR n°26, registered with the French Ministry of Research) or by the Peter MacCallum Animal Experimentation Ethics Committee.
Tumor chemotherapy models
For the establishment of syngeneic solid tumors, wild-type and Tnf−/− C57BL/6 mice were inoculated with 8 × 105 MCA205 cells, BALB/c mice with 5 × 105 H2N100 or with 1 × 106 MyrPalm-mEGFP-expressing CT26 cells and 129/Sv mice with 1 × 106 F244 cells s.c. The size of neoplastic lesions was routinely monitored by means of a common caliper, and when tumor surface reached 25–45 mm2 (normally 7–10 d after inoculation, depending on the model), mice received either 2.9 mg/Kg doxorubicin i.t. (as a single injection in 50 μL PBS) or an equivalent volume of solvent. When appropriate, mice also received 50 mg/Kg etanercept or an equivalent volume of solvent i.p. on 4 consecutive days, starting from the day of chemotherapy. Alternatively, mice received 12.5 mg/kg anti-TNF antibodies (clone TN3–19.2) or an equivalent dose of isotype-matched control antibodies i.v. 1 d before chemotherapy, together with chemotherapy as well as 4, 7, 11 and 14 d later.
Flow cytometry
Freshly recovered tumors were cut into small pieces in serum-free GlutaMAX™-I-containing RPMI 1640 medium supplemented with 0.4 Wünsch U/mL Liberase TL (Roche) and 200 U/mL DNase I (Calbiochem) and then transferred to 12-well culture plates and placed at 37 °C for 30 min to promote enzymatic dissociation. Single-cell suspensions were then obtained by filtering through a 70 μm cell strainer. For cell-surface immunostaining, cells were incubated with the following primary antibodies (final concentration = 2 μg/mL; staining temperature = 4 °C; staining time = 25 min): anti-CD45.2 (104), anti-CD11b (M1/70), anti-CD11c (N418), anti-Ly6C (AL-21) all from BD PharMingen; anti-I-A/I-E (M5/114.15.2), anti-Ly6G (1A8) from BioLegend. To identify live cells, the LIVE/DEAD® Fixable Yellow Dead Cell Stain Kit (Molecular Probes-Life Technologies) was employed. Cytofluorometric assessments and cell sorting were performed on a LSR II flow cytometer or on a FACSAria™ cell sorter (both from Becton Dickinson) and cytofluorometric data were analyzed by the FlowJo software (Tree Star, Inc.).
Quantitative RT-PCR
Total RNA was obtained from whole neoplastic lesions by means of the Maxwell® 16 Tissue LEV Total RNA Purification Kit (Promega), while total RNA was extracted from FACS-sorted cells with the RNeasy Micro Kit (Qiagen), following the manufacturer’s instructions. Up to 2 μg total RNA from each sample was then reverse transcribed by means of the SuperScript III Reverse Transcriptase (Life Technologies), random primers (Promega) and the Deoxynucleoside Triphosphate Set, PCR grade (Roche), in the presence of the RNaseOUT™ recombinant ribonuclease inhibitor (Life Technologies). Tnf expression levels were quantified by means of a dedicated TaqMan® Gene Expression Assay kit (Applied Biosystems), using the Universal Master Mix II (with UNG) (Life Technologies) and a StepOnePlus™ Real-Time PCR System (Applied Biosystems). Quantitative RT-PCR data were invariably normalized to the expression levels of the housekeeping gene peptidylprolyl isomerase A (Ppia) by means of the 2−ΔCt method.
Statistical analyses
Unless otherwise indicated, results are expressed as means ± SEM or means ± SD, as appropriate. Representative data from at least two independent experiments are shown. Unpaired, two-tailed Student’s t-tests were used to compare normally distributed data, while non-parametric Mann-Whitney U tests were employed for tumor growth curves. Statistical analyses were performed by means of Prism 5 (GraphPad software), p values < 0.05 were considered as statistically significant.
Acknowledgments
We thank colleagues from the animal facility and flow cytometry platform of the IGR for technical support as well as Dr. Stephen R. Mattarollo (Diamantina Institute, University of Queensland, Australia) for fruitful discussion. G.K. and L.Z. are supported by the European Commission (ArtForce); European Research Council; Agence National de la Recherche (ANR); Ligue Nationale contre le Cancer; Fondation pour la Recherche Médicale (FRM); Institut National du Cancer (INCa); Association pour la Recherche sur le Cancer (ARC), LabEx Immuno-Oncologie; Fondation de France; Fondation Bettencourt-Schueller; AXA Chair for Longevity Research; Cancéropôle Ile-de-France; Paris Alliance of Cancer Research Institutes (PACRI) and Cancer Research for Personalized Medicine (CARPEM). Y.M. and H.Y. were supported by China Scholarship Council (CSC). Y.M. and L.G. are supported by the LabEx Immuno-Oncologie. M.J.S. was supported by a National Health and Medical Research Council (NH&MRC) Australia Fellowship and Program Grant and by a grant from the Victorian Cancer Agency.
Glossary
Abbreviations:
- APC
antigen-presenting cell
- CRT
calreticulin
- CTL
cytotoxic T lymphocyte
- DAMP
damage-associated molecular pattern
- DC
dendritic cell
- DMBA
7,12-dimethylbenz(a)anthracene
- ER
endoplasmic reticulum
- HMGB1
high mobility group box 1
- IFNγ
interferon γ
- MCA
methylcholanthrene
- TIL
tumor-infiltrating leukocyte
- TNFα
tumor necrosis factor α
- TNFR
TNFα receptor
- TPA
12-O-tetradecanoylphorbol-13-acetate
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
These authors contributed equally to this work and share senior co-authorship.
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24786
References
- 1.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–33. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 2.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 3.Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780–8. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- 4.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 5.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 6.Tait SW, Green DR. Mitochondria and cell signalling. J Cell Sci. 2012;125:807–15. doi: 10.1242/jcs.099234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–90. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–7. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 10.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–41. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senovilla L, Vacchelli E, Galon J, Adjemian S, Eggermont A, Fridman WH, et al. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology. 2012;1:1323–43. doi: 10.4161/onci.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medina-Echeverz J, Berraondo P. Colon cancer eradication after chemoimmunotherapy is associated with intratumoral emergence of proinflammatory myeloid cells. Oncoimmunology. 2012;1:118–20. doi: 10.4161/onci.1.1.18049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011;71:4809–20. doi: 10.1158/0008-5472.CAN-11-0753. [DOI] [PubMed] [Google Scholar]
- 16.Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, et al. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–88. doi: 10.4161/onci.1.2.19026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, et al. Trial Watch: Chemotherapy with immunogenic cell death inducers. OncoImmunology. 2012;1:179–88. doi: 10.4161/onci.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudek AM, Garg AD, Krysko DV, De Ruysscher D, Agostinis P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 2013 doi: 10.1016/j.cytogfr.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24:1297–305. doi: 10.1016/j.cellsig.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222–5. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–5. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 22.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 23.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–6. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 24.Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006;355:704–12. doi: 10.1056/NEJMct055183. [DOI] [PubMed] [Google Scholar]
- 25.Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept Psoriasis Study Group Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–22. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 26.Brunner C, Seiderer J, Schlamp A, Bidlingmaier M, Eigler A, Haimerl W, et al. Enhanced dendritic cell maturation by TNF-alpha or cytidine-phosphate-guanosine DNA drives T cell activation in vitro and therapeutic anti-tumor immune responses in vivo. J Immunol. 2000;165:6278–86. doi: 10.4049/jimmunol.165.11.6278. [DOI] [PubMed] [Google Scholar]
- 27.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652–6. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–31. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 29.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baxevanis CN, Voutsas IF, Tsitsilonis OE, Tsiatas ML, Gritzapis AD, Papamichail M. Compromised anti-tumor responses in tumor necrosis factor-alpha knockout mice. Eur J Immunol. 2000;30:1957–66. doi: 10.1002/1521-4141(200007)30:7<1957::AID-IMMU1957>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 31.DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, et al. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell. 2011;19:72–85. doi: 10.1016/j.ccr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18:485–98. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 34.Kulbe H, Chakravarty P, Leinster DA, Charles KA, Kwong J, Thompson RG, et al. Australian Ovarian Cancer Study Group A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72:66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 36.Obeid M, Tesniere A, Panaretakis T, Tufi R, Joza N, van Endert P, et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 37.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 38.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–27. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 39.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellone M, Calcinotto A, Corti A. Won’t you come on in? How to favor lymphocyte infiltration in tumors. Oncoimmunology. 2012;1:986–8. doi: 10.4161/onci.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–4. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107:1547–52. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steigemann P, Wurzenberger C, Schmitz MH, Held M, Guizetti J, Maar S, et al. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell. 2009;136:473–84. doi: 10.1016/j.cell.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Körner H, Riminton DS, Strickland DH, Lemckert FA, Pollard JD, Sedgwick JD. Critical points of tumor necrosis factor action in central nervous system autoimmune inflammation defined by gene targeting. J Exp Med. 1997;186:1585–90. doi: 10.1084/jem.186.9.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]