Abstract
In addition to the well-established effector functions of IgGs, including direct cytotoxicity and antibody-dependent cellular cytotoxicity, some populations of IgGs may exert anti-inflammatory effects. Here, we describe a population of antibodies that form in the natural course of metastatic cancer and contain glycans that terminate with sialic acid. We demonstrate that both the titer of these antibodies and their level of sialylation are relatively stable throughout the progression of metastatic melanoma. The sialylation pattern of these antibodies somehow correlates with their specificity for tumor-associated antigens, as IgGs targeting several antigens associated with infectious agents are relatively poor of sialic acid. We also show that some antibodies targeting the melanoma-associated antigen NY-ESO-1 bind to the human C-type lectin CD209 (DC-SIGN). We propose that these antibodies are candidate anti-inflammatory antibodies. The presence of anti-inflammatory antibodies in cancer patients may explain, at least in part, why tumors persist and spread in the host despite strong tumor-specific humoral responses. The elucidation of the cellular and molecular pathways involved in the induction of anti-inflammatory antibodies specific for tumor-associated antigens and their function may yield important insights into how tumors evade immune detection and progress.
Keywords: anti-inflammatory, DC-SIGN, glycosylation, HER-2/neu, IgG, NY-ESO-1, sialic acid, tumor immunity
Introduction
Antibodies are an important component of the immune system. One of the questions that have puzzled cancer immunologists for years is why tumors are not commonly rejected by the immune system even though most patients exhibit antibodies targeting their own cancer.1 Autoantibodies specific for tumor-associated antigens (TAAs) have been documented in patients affected by most common human malignancies.2,3 These antibodies have been attributed both diagnostic and prognostic relevance. In some instances, the presence of such autoantibodies implicates indeed decreased recurrence-free or overall survival, while in other settings it portends a survival advantage. These observations support the conceptual framework known as “cancer immunoediting,” as originally proposed in 2002 by Lloyd Old and coworkers.4 This theory proposes that the immune system plays a dual role in cancer. On one hand, the immune system can suppress tumor formation by destroying (pre)malignant cells or inhibiting their growth. On the other hand, the immune system operates a selection whereby tumors become ever fitter to survive and spread in the host. In fact, the tumor microenvironment itself may drive this selection process by producing immunoregulatory cytokines such as transforming growth factor β (TGFβ) and interleukin (IL)-10, by promoting the enzymatic activity of indolamine 2,3 dioxygenase, or by recruiting and stimulating the function of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs).5,6 In addition, intratumoral T cells might be subjected to direct immunosuppressive interactions, such in the case of a variety of tumors (including ovarian cancer, renal cell carcinoma and melanoma) that express B7 protein family members.7-9 The role of antibodies in cancer immunoediting, if any, is largely undefined.
Most of the autoantibodies specific for TAAs are of the IgG class.2 The antigen-binding portion of IgG molecules reside in the unique combination of the variable regions of the heavy and light chains. Conversely, the effector functions of IgGs are imparted by their constant region (CH2), as known as constant fragment (Fc). The N-linked glycosylation of the CH2 region via N297 is highly heterogeneous, involving a large variety of terminal sugars.10 These terminal glycans influence the binding of IgGs to complement components and to both activating and inhibitory Fc receptors. For example, terminal galactose residues promote the binding of IgGs to C1q, thus increasing complement-mediated cytotoxicity.11 Along similar lines, the absence of core fucose results in the enhancement of antibody-dependent cellular cytotoxicity (ADCC) upon the improved binding of IgGs to activating FcγRIIIa receptors.12,13 Conversely, Fc glycans that terminate in sialic acid (Sia) exhibit a markedly reduced propension to trigger ADCC, owing to a decreased affinity for FcγRIIIa14 and—in some cases—may also have a reduced affinity for cognate antigens.15 The terminal sialylation of Fc with α2,6-linked Sia (α2,6-Sia) has been proposed to be primarily responsible for the anti-inflammatory effects of intravenous immunoglobulin (IVIG), possibly due to the interaction of infused IgGs with the C-type lectin dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) on antigen-presenting cells and the attendant upregulation of inhibitory FcγRIIb receptors.16,17 The anti-inflammatory effect stemming from the interaction of sialylated IgGs with antigen-presenting cells has been referred to as “sialic acid switch.” Thus, the post-translational modification of N-linked glycans at N297 may change the effector functions of IgG from inflammatory to anti-inflammatory.18-20
In order to evaluate whether the human humoral response to TAAs might involve candidates for the sialic acid switch, we screened blood samples from patients bearing metastatic neoplasms (primarily melanoma and breast carcinoma) for the presence of autoantibodies specific for two common TAAs, the cancer-testis antigen NY-ESO-1 and ERBB2, also known as HER2. Positive samples were then assessed for their sialic acid content, and selected antibodies were affinity-purified and evaluated for the sialylation of Fc regions and functional activity. We found that a substantial fraction of autoantibodies specific for NY-ESO-1 and HER2 are sialylated, as demonstrated by their binding to the Sambucus nigra agglutinin (SNA). In addition, a fraction of anti-NY-ESO-1 antibodies were found to be specifically sialylated in an α2,6 linkage site on galactose via N-linked glycosylation, presumably at N297 on the Fc. These antibodies can bind DC-SIGN and therefore represent candidate anti-inflammatory antibodies.
Results
Autoantibodies specific for NY-ESO-1 and HER2 are a common and stable finding in metastatic melanoma and breast cancer patients
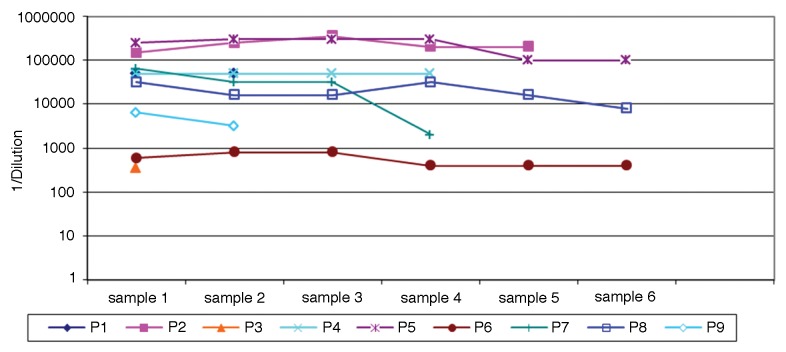
We screened 168 serum samples from 80 patients with metastatic melanoma for antibodies targeting the NY-ESO-1 antigen, nine of which were positive in this respect (Table 1). Conversely, none among 33 healthy individuals was positive for NY-ESO-1-targeting autoantibodies. In melanoma patients, antibodies specific for NY-ESO-1 generally had a very high titer (>1/32,000). Of note, none of the patients initially bearing NY-ESO-1-specific autoantibodies seroconverted in the course of the observation period (Fig. 1). Likewise, the seroconversion of patients that initially had no NY-ESO-1-targeting antibodies was not observed in the course of our study (data not shown). The longitudinal evaluation of anti-NY-ESO-1 antibody titers on several of these patients documented consistent changes in a single patient. Otherwise, anti-NY-ESO-1 antibody titers were relatively stable throughout the observation period (Fig. 1).
Table 1. Autoantibodies targeting NY-ESO-1 and HER2 in cancer patients and healthy subjects.
| Diagnosis | Antigen | # Screened* | # Positive | % Positive | Titer** |
|---|---|---|---|---|---|
| Melanoma |
NY-ESO-1 |
80 |
9 |
11.5 |
350, 600, 1600, 32K, 50K, 50K, 64K, 150K, 250K |
| Healthy |
NY-ESO-1 |
33 |
0 |
0 |
n.a. |
| HER2+ breast cancer |
HER2 |
82 |
3 |
3.7 |
3K, 3K, 25K |
| HER2− breast cancer |
HER2 |
29 |
1 |
3.4 |
25K |
| Breast cancer |
HER2 |
59 |
4 |
6.8 |
600, 25K, 25K, 25K |
| Healthy | HER2 | 12 | 0 | 0 | n.a. |
Represents unique patients; **expressed as 1/dilution, at entry into the study. n.a., not available.
Figure 1. Anti-NY-ESO-1 antibody titers in the course of this study. Titers are expressed as 1/dilution. Sample numbers indicate clinical visit in the course of treatment and vary from patient to patient. The minimum interval between sequential samples was one month. The maximum time interval between entry into the study and study completion was 16 mo.
One hundred-70 patients with breast carcinoma were screened for antibodies specific for HER2, and only 6 patients were found to be positive. Anti-HER2 autoantibodies were found in patients bearing HER2+ as well as HER2- tumors, although the number of patients exhibiting anti-HER2 antibodies was too low to determine whether the development of anti-HER2 antibodies would be related to HER2 expression on tumor cells at the time of entry into the study. No anti-HER2 antibody responses were detected among 12 cancer-free individuals. Because samples from breast cancer patients were available at a single time point, we did not have the opportunity to serially study HER2-specific antibodies in this cohort. In addition, limited amounts of serum (approximately 200 μL) were incompatible for the affinity purification of antibodies specific for HER2.
Antibodies targeting NY-ESO-1 and HER2 commonly contain high levels of Sia, as measured by SNA lectin analysis
In order to determine the proportion of anti-NY-ESO-1 IgGs that are sialylated, we quantified by ELISA IgGs that has previously been fractionated on SNA lectin-containing columns (Table 2). Among 9 serum samples containing anti-NY-ESO-1 autoantibodies, five had a Sia content higher than 5% at entry into the study. In samples from two patients exhibiting high levels of anti-NY-ESO-1 antibodies, one with uterine cancer and one with lung adenocarcinoma, the fraction of sialylated IgGs was 45% and 24%, respectively. The Sia content in IgGs specific for HER2 was generally low, as samples from only 2 out of 6 patients had a Sia content higher than 5%. Interestingly, in two patients with uterine and lung cancers, the Sia content of HER2-specific autoantibodies was 18% and 79%, respectively.
Table 2. Sialylation of IgGs targeting tumor-associated antigens*.
| Patient ID | Diagnosis | N° | Specificity | % SNA+ |
|---|---|---|---|---|
| P1 |
Melanoma |
2 |
NY-ESO-1 |
7 |
| P2 |
Melanoma |
5 |
NY-ESO-1 |
2–4 |
| P3 |
Melanoma |
1 |
NY-ESO-1 |
5 |
| P4 |
Melanoma |
4 |
NY-ESO-1 |
2–3 |
| P5 |
Melanoma |
6 |
NY-ESO-1 |
9–23 |
| P6 |
Melanoma |
6 |
NY-ESO-1 |
14–19 |
| P7 |
Melanoma |
4 |
NY-ESO-1 |
18–28 |
| P8 |
Melanoma |
6 |
NY-ESO-1 |
19–21 |
| P9 |
Melanoma |
2 |
NY-ESO-1 |
2 |
| 3C3 |
Uterine cancer |
1 |
NY-ESO-1 |
45 |
| 4G1 |
Lung cancer |
1 |
NY-ESO-1 |
19 |
| 1C9 |
Breast cancer |
1 |
HER2 |
12 |
| 1E9 |
Breast cancer |
1 |
HER2 |
4 |
| 1G7 |
Lung cancer |
1 |
HER2 |
79 |
| 1H3 |
Breast cancer |
1 |
HER2 |
3 |
| 4E3 |
Breast cancer |
1 |
HER2 |
4 |
| 5G1 |
Breast cancer |
1 |
HER2 |
5 |
| 6D1 |
Breast cancer |
1 |
HER2 |
34 |
| 6G11 | Uterine cancer | 1 | HER2 | 18 |
* Data are expressed as percent of material eluted from Sambucus nigra agglutinin (SNA)-containing columns with respect to total material recovered (range of values obtained over N° study visits). Each value within ranges has been obtained by calculations based on a minimum of two independent experiments and triplicate assessments within each assay.
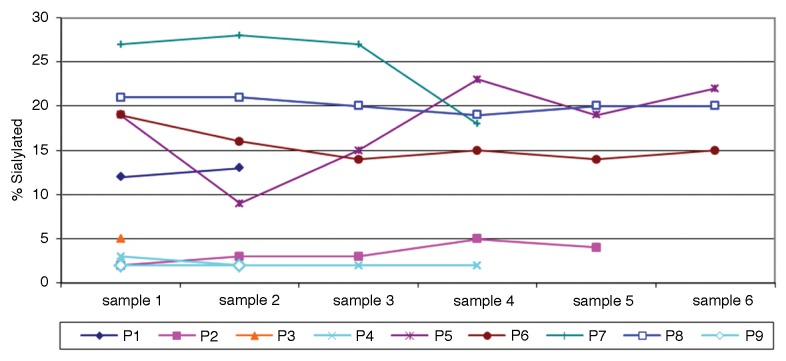
The analysis of serial samples obtained from patients exhibiting NY-ESO-1-targeting antibodies demonstrated that the level of sialylated IgGs was relatively stable in each patient (Fig. 2). The levels of Sia+ IgGs specific for NY-ESO-1 could be stratified into two groups, namely, ≤5% or ≥10%, which—for purposes of presentation—we indicate as low and high, respectively. As illustrated in Figure 2, we observed essentially no crossovers from one group to the other over time. Specifically, both highly and poorly sialylated NY-ESO-1-specific IgGs remained so over the course of this study.
Figure 2. Fraction of sialic acid-containing (Sia+) anti-NY-ESO-1 IgGs over time. Values are expressed as percent of NY-ESO-1 antibody that partitioned into the Sambucus nigra agglutinin (SNA) lectin-positive fraction. Sample numbers indicate clinical visit number in the course of treatment. Intervals vary from patient to patient, with a minimum of 1 mo.
Metastatic melanoma patients do not exhibit a global increase in the sialylation of the IgG repertoire
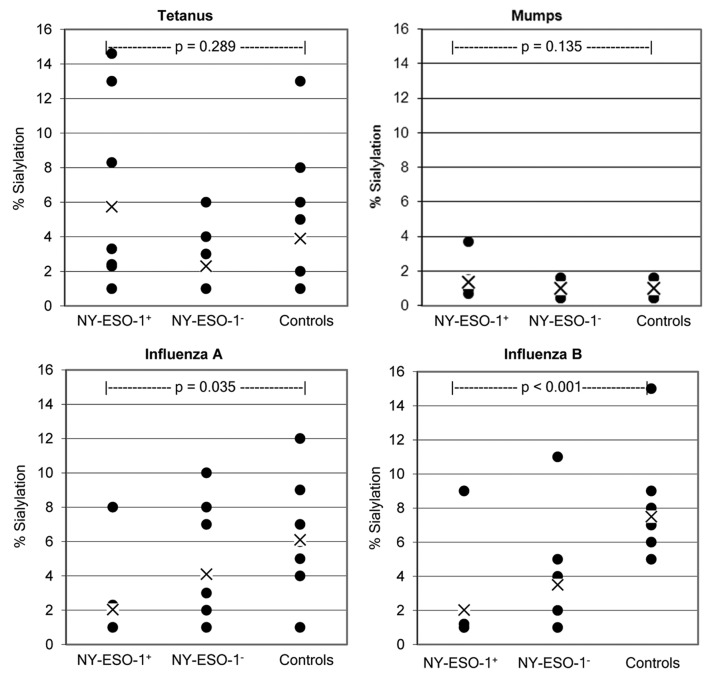
We tested SNA lectin-enriched and depleted IgG fractions against a variety of common antigens in order to investigate whether an increased IgG sialylation is a generalized feature of metastatic cancer patients. Figure 3 shows the percentage of sialylated IgGs specific for different antigens (as obtained in melanoma patients). In particular, IgGs specific for influenza A, influenza B, mumps, and tetanus toxoid-associated epitopes exhibited a lower overall Sia content than IgGs targeting NY-ESO-1. Additionally, at the level of individual patients, the percent sialylation of IgGs specific for infectious agents-associated antigens never exceed the Sia content of anti-NY-ESO-1 autoantibodies. The overall level of sialylation of IgGs targeting mumps and tetanus toxoid-related antigens did not differ between NY-ESO-1+ and NY-ESO-1− patients (p = 0.135) nor between NY-ESO-1+ patients and healthy individuals (p = 0.289). However, we observed a statistically significant difference in the Sia content of IgGs specific for influenza A-associated antigens between NY-ESO-1+ patients and healthy subjects (p = 0.035), as well as a significant difference in Sia+ IgGs targeting influenza B-related antigens between both NY-ESO-1+ or NY-ESO-1− patients and healthy individuals (p < 0.001). These data suggest that while IgGs specific for NY-ESO-1 contain a high fraction of sialylated species, this does not reflect an increased sialylation of the IgG repertoire. We also tested the total sialyltransferase activity in serum samples from these patients, finding no correlation between this parameter and the level of sialylated NY-ESO-1-specific IgGs (data not shown).
Figure 3. Comparison of sialylation levels on IgGs specific for NY-ESO-1 or antigens related to infectious agents. Data points represent average values rounded to whole figures for each patient. Each patient was analyzed in triplicate wells per assay, and assays were performed as two independent experiments. X = mean of groups. Patient groups are NY-ESO-1+ (n = 8) and NY-ESO-1− (n = 10) melanoma patients. Controls are healthy human subjects (n = 10).
The Fc of affinity purified autoantibodies specific for NY-ESO-1 contains glycans that terminate with α2,6-Sia
Some Fab regions of IgGs can be subject to N-linked glycosylation and terminate with Sia because of the unique amino acid sequences of the variable regions of the IgG heavy and light chains. In order to examine the relative distribution of glycans that terminate in α2,6-Sia among the Fab and Fc regions of IgGs specific for NY-ESO-1, we digested affinity purified anti-NY-ESO-1 IgGs with papain and analyzed the resulting fragments by SNA lectin-based immunoblotting. Figure 4 shows representative results for the samples of 8 patients exhibiting high levels of anti-NY-ESO-1 antibodies. A band co-migrating with the approximately 28 KDa Fc fragment was identified in all samples tested. Although SNA lectin blots are not quantitative, the total amount of sialylated material (both Fc and Fab) that we observed corresponded to Sia estimates as obtained with ELISAs. In addition, in several cases (patients P5, P7, P8) the Fc was the predominant species to terminate with α2,6-Sia, while in other cases (patients P2, P4 and P6), the Fc region turned out to be the only sialylated species. In several cases, a species of a molecular mass of approximately 75 KDa was visualized by SNA-based immunoblotting, presumably corresponding to papain-resistant material.21 This was particularly true for highly sialylated IgGs, suggesting that elevated levels of sialylation on IgGs may prevent the access of papain to the their polypeptide backbone. An extended incubation in papain (up to 18 h) did not improve the resolution of this species, and its identity was not further characterized. Because of limited amounts of samples and the lack of a suitable matrix for the affinity purification of HER2-specific antibodies, we were only able to analyze the reactivity of total sialylated IgGs in this setting. SNA lectin blots of papin-digested IgGs from these samples revealed the sialylation of both Fab and Fc species, a pattern resembling that of antibodies specific for NY-ESO-1 (data not shown). Taken together, these data demonstrate that the Fc regions of affinity purified anti-NY-ESO-1 IgGs and antibodies specific for HER2 contain glycan structures that terminate with α2,6-Sia.
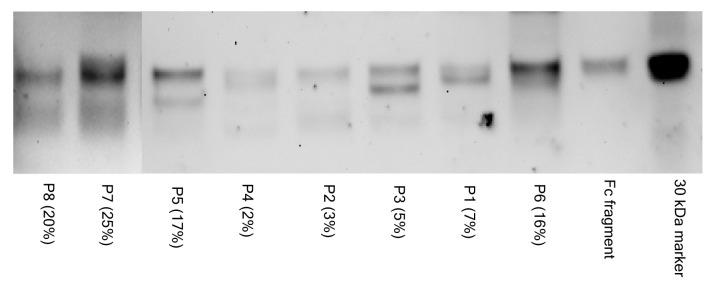
Figure 4. SNA lectin blot of Fc and Fab fragments from affinity purified anti-NY-ESO-1 IgGs. The percentage of sialic acid-containing (Sia+) IgGs observed in the course of the study is indicated in parentheses. The material analyzed by lectin blot consisted of pools of samples from the same patient that had been independently affinity purified. The image is a composite of two separate blots, as identified by the vertical line. The 30 KDa biotinylated marker and the commercially available Fc fragment (approximately 28 KDa) are shown for comparison. Lectin blot assays were done independently in duplicate instance, resulting in essentially identical findings. Each lane was loaded with approximately 1 μg of material.
Intact affinity purified anti-NY-ESO-1 antibodies bind human DC-SIGN
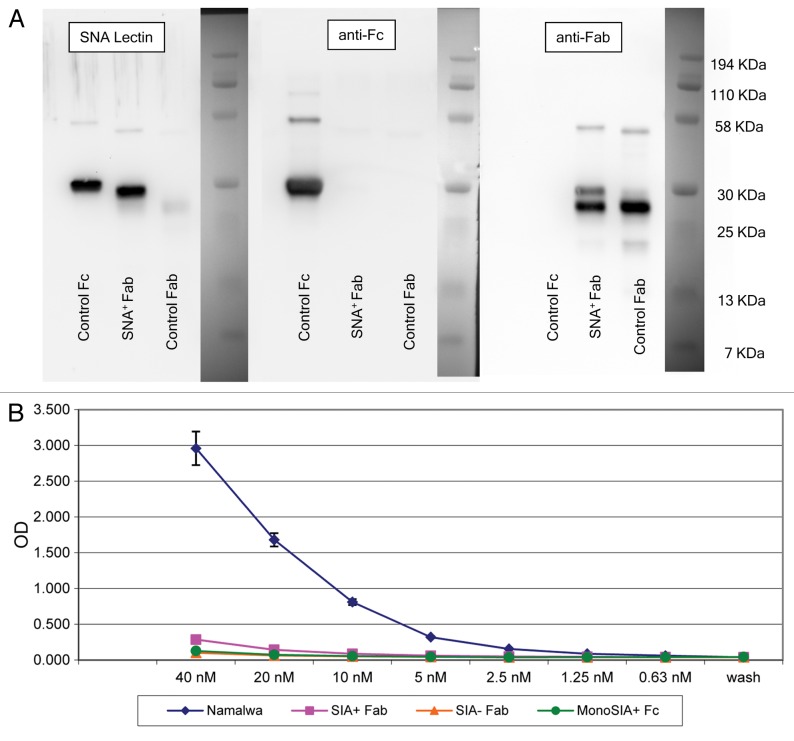
The recent demonstration that sialylated Fcs bind to and activate DC-SIGN prompted us to investigate whether affinity purified anti-NY-ESO-1 sialylated IgGs would likewise bind DC-SIGN. To this aim, we generated a recombinant, soluble human DC-SIGN and used it as a binding probe for affinity purified anti-NY-ESO-1 antibodies. Soluble DC-SIGN bound known DC-SIGN ligands such as intercellular adhesion molecule (ICAM)-2 and ICAM-3, but not unrelated molecules such as CD28 and CTLA-4 (data not shown). In order to exclude the possibility that the binding to DC-SIGN would be due to Sia+ Fabs, we prepared Sia+ Fab fragments from human IVIG by SNA lectin chromatography upon papain digestion, followed by the depletion of Fc fragments and intact IgGs with protein A. Figure 5A shows western and SNA lectin blots of Sia+ Fab fragments. The Sia+ Fab preparation showed minimal reactivity when probed with anti-Fc antibodies and —as expected—manifested as a single reactive species of approximately 25 KDa when probed with the SNA lectin. The results of binding experiments performed with these Sia+ Fab fragments and soluble DC-SIGN are shown in Figure 5B. As predicted, Sia+ Fab, Sia− Fab (asialo-Fab), and monosialylated Fc fragments bound minimally to soluble DC-SIGN, whereas the 4E10 monoclonal antibody, which contains a high amount of di-sialylated Fc glycans22,23 exhibiting a considerable binding. A more detailed characterization of sDC-SIGN and its binding to di-sialylated Fc fragments exceeded the scope of this manuscript and is to be published elsewhere (Oaks et al., in preparation).
Figure 5. Sialic acid-containing (Sia+) Fab fragments do not bind soluble DC-SIGN. (A) Western and lectin blots of purified Sambucus nigra agglutinin (SNA)+ Fab fragments from human IgG. SNA+ Fab fragments were probed with the SNA lectin or antibodies specific for human Fc or Fab fragments. Control Fab and Fc fragments were obtained commercially. Only a minor fraction of control Fab fragments terminates with α2,6-Sia, as defined by lectin blotting (left panel). Conversely, the control Fc fragment contains Sia, as defined by positive signal in the lectin blot. This control Fc is likely to contain largely monosialylated glycoforms, as only a minor fraction is retarded on SNA lectin columns (data not shown), and does not bind soluble DC-SIGN (sDC-SIGN, see below). The two species identified in the Fab fragments obtained from IVIG (right panel) may represent different glycoforms of Sia+ Fabs. In support of this, the digestion of this material with sialidase results in a complete absence of either of the two species in SNA lectin blots, and peptide-N-glycosidase digests of Sia+ Fabs migrate as a single faster species when probed with anti-Fab antibodies (data not shown). The preparation is operationally free of Fc components (middle panel). (B) ELISA binding of SNA+ Fab fragments from IVIG. Diamonds, intact 4E10 positive controls; Squares, Sia+ Fabs; triangles, Sia− (asialo) Fabs; circles, monosialylated Fcs. Please notice that monosialylated control Fc fragments do not bind sDC-SIGN, as they are largely devoid of di-sialylated glycans, consistent with above blotting experiments. Wash = no protein added, control for background binding. Fc and 4E10 binding was detected with horseradish peroxidase (HRP)-conjugated anti-Fc antibodies, while Fab binding was detected with HRP-conjugated anti-Fab antibodies.
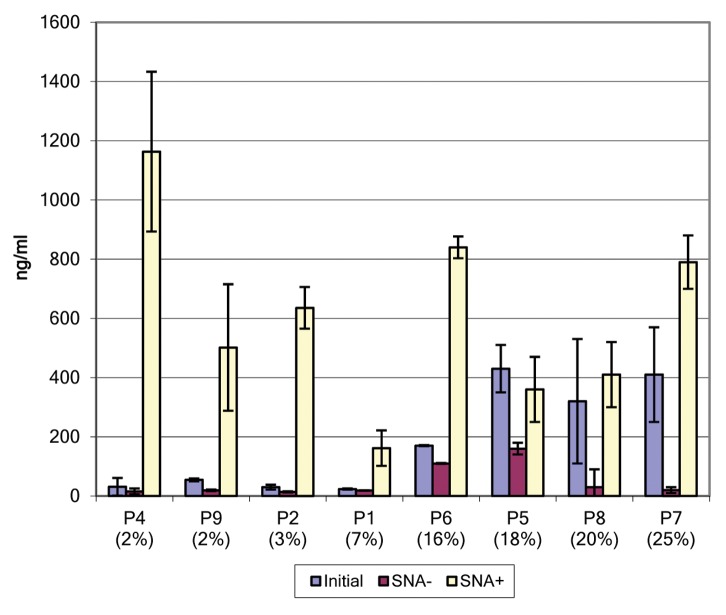
Having established that the binding of IgGs to DC-SIGN is independent of Fab (and its sialylation state), we evaluated the binding of intact, affinity purified anti-NY-ESO-1 antibodies to DC-SIGN (Fig. 6). We observed a clear relationship between the percentage of sialylated antibodies as measured by ELISA and the extent of binding to soluble DC-SIGN. In order to determine whether the binding activity of affinity purified anti-NY-ESO-1 antibodies to DC-SIGN is limited to their sialylated fraction, we evaluated SNA+ and SNA− antibody fractions in binding assays. These analyses demonstrate that the SNA+ antibody fraction is enriched for species that binds soluble DC-SIGN, whereas the SNA− fraction exhibited a markedly DC-SIGN-binding ability. This was true for each patient analyzed. Indeed, the SNA+ IgG fraction of all samples showed the highest DC-SIGN binding potential, the SNA- fraction has a markedly reduced binding activity, and non-fractionated samples bound DC-SIGN to intermediate levels. These data suggest that the DC-SIGN-binding fraction of anti-NY-ESO-1 IgGs from melanoma patients is largely limited to the population of antibodies whose Fc terminates with α2,6-Sia.
Figure 6. Binding of affinity purified anti-NY-ESO-1 antibodies to soluble DC-SIGN. Selected samples (based on material availability) were applied to wells coated with soluble DC-SIGN (sDC-SIGN). Bound IgGs were estimated by comparing the titration of samples on fixed amounts of DC-SIGN, and interpolated from a standard curve generated from IgGs that were directly bound to microwells. Lavender boxes = intact affinity purified IgGs; purple boxes = Sambucus nigra agglutinin (SNA)-lectin depleted IgG (SNA−); yellow boxes = SNA-enriched fraction of IgG (SNA+). Error bars represent standard deviations as obtained from hextuplicate determinations. Representative data from one out of two independent assays are reported.
Discussion
We have identified high-titer autoantibodies specific for two different tumor-associated antigens that contain post-translational modifications terminating with α2,6-Sia. Sia+ IgGs represent a consistent finding in that, when present, they are relatively stable within individual patients. These autoantibodies also appear to be selectively sialylated, as antibodies specific for antigens associated with infectious agents contain lower levels of Sia. Many of the Sia modifications are located within the glycan structure of the Fc region of IgG heavy chains, and they allow these antibodies to bind human DC-SIGN. We propose that the glycan signature of these antibodies can confer them anti-inflammatory effector functions.
A large body of evidence indicated that anticancer immune responses involve the formation of antibodies to autologous TAAs. These include antigens that are overexpressed by tumor cells, antigens resulting from frame-shift mutations, viral antigens, stem cell antigens, ganglioside-like antigens, and cancer-testis antigens (reviewed in refs. 2 and 3). One of the best studied TAAs is v-erb-b2 erythroblastic leukemia viral oncogene homolog 2 (ERBB2, best known as HER2). Autoantibodies specific for HER2 have been identified in patients bearing a variety of cancers including breast,24,25 lung26,27 and colon28 carcinoma. The frequency of HER2-specific antibodies among breast cancer patients, independent of HER2 expression by the tumor, is reported to be in the range of 11–21%.2,24 When analyzed independently of tumor grade, stage and HER2 expression, we observed anti-HER2 antibodies in 4.7% (8 of 170 subjects) breast carcinoma patients. The low frequency of breast cancer patients bearing HER2-specific antibodies in our study may be due to differences in our patient population, such as HER2 expression status. Interestingly, patients with autoantibodies specific for HER2 do not appear to obtain clinical benefits from them, although it has previously been shown that these autoantibodies can mediate antineoplastic effects similar to those exerted by trastuzumab.29 The apparent absence of clinical benefits in patients bearing HER2-specific autoantibodies is perhaps due to differences in the glycosylation pattern of Fc fragments, directly influencing effector functions.
Autoantibodies specific for the cancer-testis antigen NY-ESO-1 have been detected in patients affected by a large variety of neoplasms, including multiple myeloma,30 melanoma31 as well as esophageal,32,33 breast,27 lung,26,27,34 liver,35,36 prostate,37 ovarian38 and colorectal27 cancer. Unlike HER2-specific autoantibodies, antibodies targeting NY-ESO-1 appear to have a diagnostic and a prognostic value. For instance, a statistically significant survival disadvantage have been observed in prostate cancer patients bearing NY-ESO-1-specific antibodies, as compared with seronegative patients.37 Similarly, the production of NY-ESO-1-targeting autoantibodies is more prevalent among patients with advanced esophageal cancer32,33 and aggressive multiple myeloma.30 The de novo formation of antibodies specific for NY-ESO-1 has been reported in approximately 12% of melanoma patients tested.31 Our results, indicating that 10% of metastatic melanoma patients exhibit NY-ESO-1-specific antibodies, are consistent with this report. We are unaware of studies correlating the de novo production of autoantibodies specific for NY-ESO-1 and clinical outcome in melanoma patients. However, our data and previous findings indicating that that such autoantibodies—when present—can be very abundant31 imply that NY-ESO-1-specific antibodies lack the ability to control oncogenesis and/or tumor progression.
The wide variety of N-linked glycans found on immunoglobulin molecules endows them with functional features that play a critical role in antigen binding as well as in effector functions. Thus, N-linked glycans can promote the solubility of antibodies, stimulate their secretion, increase their half-life in the extracellular space and/or enhance their binding to complement components as well as to cellular receptors (reviewed in ref. 10). Over 30 different IgG glycoforms have been identified so far,10,14 and this extensive diversity provides the basis for a multiplicity of effector functions. The development of ever more refined analytical techniques, in particular those coupling liquid chromatography and mass spectroscopy, has resulted in a relatively clear description of how IgG glycosylation varies in human health and disease (reviewed in ref. 39). For example, it is now clear that IgG glycosylation is a dynamic process that varies with age and sex as well as in pathophysiological conditions such as pregnancy and various diseases. Fc N-glycans are generally arranged in a biantennary structure, most of which is fucosylated. A portion of these glycans carry a bisecting N-acetylglucosamine, and some antennae terminate with α2,6-Sia. It is well established that there is a global increase in the sialylation (including α2,6-sialylation) of proteins in cancer patients.40 Consistent with these findings, β-galactoside α2,6-sialyltransferase (ST6Gal I) expression is increased in the setting of cancer.41 In part, these changes may be due to increased levels of hepatic ST6Gal I transferase as a component of the acute phase inflammatory response.42 However, the levels of total serum sialyltransferase were not elevated in our patient population, argue against this as a mechanism for increased Fc sialylation. We also observed a somehow selective increase in the levels of sialylation of anti-NY-ESO-1 antibodies, but not of antibodies specific for infectious agent-related antigens. Taken together, these results argue against a global increase in the Sia content of IgGs in cancer patients. In this regard, our data are consistent with recent findings by Kasermann et al., who observed a variable enrichment or depletion of autoantibodies and IgGs specific for infectious agent-associated antigens among SNA fractionated IVIG.43
Most studies on the sialylation of IgGs produced in response to natural antigen exposure (de novo humoral responses) have relied on identification and purification of sialylated antibodies by SNA lectin chromatography and/or enzyme-linked lectin assays (ELLA). One of the shortcomings of this approach for the analysis of Fc glycans is that SNA lectin binds both Fab and Fc glycans.23,44-46 This issue becomes particularly problematic for the study of polyclonal IgG populations, as the glycosylation of variable regions on Fab fragments may be in the order of 20% (based on sequence database entries).47 According to experimental estimates, the glycosylation of variable regions ranges from 15 to 25%,10,48 while approximately 12% Fab glycans terminate with α2,6-Sia, as estimated by SNA lectin chromatography.23 In the case of pooled immunoglobulins, this represents a nearly 10-fold excess of sialylated Fab glycans as compared with their di-sialylated Fc counterparts.23,45 Thus, our initial estimates of sialylation for IgGs targeting NY-ESO-1 and HER2 did not discriminate between Fab and Fc sialylation, but rather represented the total abundance of IgGs with glycans terminating in α2,6-Sia. Nevertheless, data obtained from SNA lectin blots, although semi-quantitative, demonstrated a predominance of Sia+ Fcs over Sia+ Fabs in all samples.
Fc sialylation seems to be a dynamic process in the course of humoral immune responses against conventional antigens as well as autoantigens. Shortly after immunization with bovine serum albumin, the sialylation of Fc glycans was markedly reduced in mice,49 presumably due to reduced availability of terminal galactose, the penultimate sugar for terminal α2,6-Sia. Consistent with this, a decrease in the levels of Sia+ Fcs of antibodies specific for citrullinated proteins and proteinase 3 has been observed in patients affected by rheumatoid arthritis and Wegener’s granulomatosis, respectively.50,51 Conversely, remission from these diseases has been shown to correlate with an increased expression of α2,6 sialyltransferase and an attendant increase in sialylated antigen-specific IgGs.50-52
Limited data are available regarding Fc sialylation outside the clinical setting of autoimmune disease. An increased sialylation of the whole serum, as well as of IgG Fc glycans, has been reported in three patients with ovarian cancer,53 but the IgG specificity was not described. In the context of alloimmunity, Wuhrer et al. have described an increased sialylation of Fc glycans in affinity purified pathogenic IgG1 alloantibodies specific for human platelet-associated antigens.54 These data are consistent with the increased sialylation of Fc fragments that we observed in cancer patients, and support the notion that Fc sialylation is regulated during the humoral response to a variety of antigens. Very little is known, however, about the kinetics and functional activities of such antibodies.
Much of what we know about the functional consequences of Fc glycosylation on the immune system comes from the seminal studies on IVIG by Ravetch and collaborators (reviewed in refs. 18, 19 and 55). Although it was originally used in the clinics only as an antibody replacement therapy, IVIG has now been cleared by US regulatory agencies for use in patients affected by a variety of inflammatory diseases including idiopathic thrombocytopenia purpura, Guillain–Barre syndrome, Kawasaki’s disease and chronic inflammatory demyelinating neuropathy.56,57 IVIG is also commonly used to treat the rejection of solid allotransplants,58,59 as well as in antibody reduction protocols for patients with high levels of HLA-specific antibodies awaiting transplantation.60 The precise mechanisms underpinning the anti-inflammatory properties of IVIG have been the subject of intense debate. The notion emerging from the body of work performed by the Ravetch’s group is that the anti-inflammatory activity of IVIG resides within a small population of immunoglobulins bearing Fc glycans that terminate with α2,6-Sia.61 In support of this concept, the depletion of sialylated glycans from IVIG by SNA lectin chromatography abrogates its anti-inflammatory effects in a variety of animal and ex vivo models.62 Moreover, IgG1 Fcs enzymatically engineered to be highly sialylated recapitulate the anti-inflammatory actions of IVIG.62 These sialylated immunoglobulins exert anti-inflammatory effects via a specific C-type lectin, namely DC-SIGN, which is expressed on cells of the monocytic compartment.17 The interaction of sialylated Fc fragments with DC-SIGN results in the production of IL-33, in turn inducing the expansion of IL-4-secreting basophils and stimulating the expression of the inhibitory FcγRIIB receptor on macrophages.16 Thus, sialylated IgG Fc fragments may serve as a “switch” that promotes the establishment of an anti-inflammatory environment where antigens are located (e.g., the tumor site)
The anti-NY-ESO-1 antibodies described in this work also bound DC-SIGN. This finding supports our hypothesis that sialylated anti-NY-ESO-1 antibodies function as anti-inflammatory mediators. Hence, the concept of cancer immunoediting may not be restricted to the cellular response to tumors, but extend to components of the humoral immune system. We propose that anti-inflammatory antibodies, such as those described here, may be central for understanding the interactions between tumors and the immune system and yield important information on the genesis, progression and dissemination of cancer.
We realize that our study was limited by the low frequency of cancer patients bearing TAA-specific antibodies in our cohort. However, the consistency of our findings throughout serial samples from these patients supports the contention that the glycan signature of TAA-targeting antibodies is stable within a given patient. Our research activity currently aims at defining the frequency and function of sialylated TAA-specific antibodies and further characterizing their clinical relevance. We believe that these investigations will improve our understanding of the dynamic interactions between tumors and the host and may provide important clues about the mechanisms whereby cancers evade detection and elimination by the immune system.
Materials and Methods
Subjects and blood samples
Blood samples were obtained under the oversight of the Aurora Health Care Institutional Review Board. Patient samples for NY-ESO-1-related studies were obtained in the course of therapy and recruited through our high-dose IL-2 treatment unit. All patients enrolled had metastatic melanoma and had failed prior conventional chemotherapy. Samples for HER2-related studies were from patients with a prior histologic diagnosis of breast carcinoma and were obtained through the biorepository program of Aurora Health Care. As this is a shared resource biorepository, only limited amounts of blood and limited clinical information were available for these patients. Samples from patients with metastatic uterine and lung cancer were also obtained from our biorepository. Serum was used for NY-ESO-1-related assays and plasma for HER2-related studies.
Peptides, antibodies, antigens and lectins
A 40-mer (ESO1–40) from NY-ESO-1 was used as target antigen for anti-NY-ESO-1 ELISA. This sequence represents a dominant B-cell epitope, as it is recognized by sera from a wide spectrum of cancer patients.31 The peptide (MQAEGRGTGGSTGDADGPGGPGIPDGPGGNAGGPGEAGAT) was synthesized and HPLC purified by New England Peptide. The same peptide was immobilized on a Sepharose Sulfolink resin (Thermo Pierce, 20402) and used for the affinity purification of anti-NY-ESO-1 antibodies. An irrelevant peptide, hereafter referred to as CAT-4 (CYEQPKYTPEKETLE), was used as a reference for the non-specific binding of human IgGs in ELISAs. The anti-HER2 hybridoma clone 520C9.C3B10T was purchased from the American Type Culture Collection (ATCC, HB-8696). 520C9 cells were grown in serum-free medium (Invitrogen, 2045-076), and culture supernatants were purified by protein L chromatography (Thermo Pierce, 20510). The 4E10-Namalwa antibody was used as a positive control in soluble DC-SIGN-binding assays. 4E10 is a recombinant human monoclonal antibody specific for HIV-1 gp41 and was purchased from Polymun Scientific (AB004). The rationale for its use as a control antibody in DC-SIGN-binding assays is that 4E10 is devoid of Fab glycans but rich in Fc glycans. The liquid chromatography-mass spectroscopy (LC-MS)-assisted analysis of 4E10 glycans has confirmed a high proportion of mono- and di-sialylated terminal glycans at N297.23 Control Fab and Fc fragments were purchased from Jackson Laboratories (009-000-007 and 009-000-008, respectively). Horseradish peroxidase (HRP)-conjugated anti-human Fab antibodies were purchased from Abcam (98535). HRP-conjugated antibodies specific for human IgG Fcs were purchased from Jackson Laboratories (709-035-098) and Abcam (ab98567). Biotinylated mouse anti-human IgGs (clone G18-145) (product 555785) were purchased from BD Biosciences. Antigens from influenza A, influenza B and mumps viruses were purchased from Microbix Systems (EL-13-02, EL-14-03 and EL-06-02, respectively) and were plated at 200 ng/well for ELISAs. The tetanus toxoid was purchased from Sanofi Pasteur (49281-820-10) and was used at a 1/100 dilution for coating ELISA plates. Agarose-conjugated SNA lectin (AL-1303) and biotinylated SNA lectin (B-1305) were purchased from Vector Laboratories.
Cell lines
The HER2 overexpressing cell line SK-BR-3 was purchased from ATCC (HTB-30) and maintained in McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS). Lysates of subconfluent SK-BR-3 cell cultures were prepared by repeated freeze-thawing in lysis buffer (50 mM Tris pH 7.8, 150 mM NaCl and 1% Triton X100), centrifuged to remove insoluble material, filtered, adjusted to 5 mg/mL total protein content and used as target antigens in ELISAs described below.
Affinity purification of IgG and lectin chromatography
IgGs were purified from serum samples by means of Nab Protein A/G spin columns, as per manufacturer’s instructions (Thermo-Pierce, 89954). Residual IgMs and IgAs were minimized by means of the Melon Gel (Thermo Pierce, 45212). IgGs obtained from these columns were quantified and tested for contaminating IgMs and IgAs by ELISA. Mean IgM content was 0.3% ± 0.5%, and mean IgA content was 2.4% ± 1.2%. Purified IgGs were then run through an affinity column to which the NY-ESO-1 peptide had previously been immobilized, washed with 100 volumes of binding buffer (PBS), and eluted with IgG Elution Buffer (Thermo-Pierce, 21004), pH 2. SNA lectin chromatography was performed on 50 μg input purified IgG in SNA binding buffer (25 mM HEPES pH 8, 150 mM NaCl, 1 mM CaCl2 and 1 mM MnCl2) containing 50 μL washed SNA-agarose resin. Binding was performed overnight with rotation at 4 °C. The entire non-bound fraction was collected after centrifugation for 2 min at 1000 G. The resin was washed three times in 100 volumes of binding buffer, and the SNA-bound material was eluted in 500 mM lactose at 45 °C for 1 h with periodic agitation. The absence of IgG binding to unconjugated agarose was confirmed in separate experiments (data not shown).
ELISA
Autoantibodies specific for NY-ESO-1 and HER2 were detected by ELISA. The NY-ESO-1 assay was a modification of an assay originally described by Zeng et al.31 Briefly, 3 pmoles of ESO1–40 were added to the wells of Maxisorp plates (Nunc, 468667) in coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.1) and incubated overnight at 4 °C. Control wells containing the CAT-4 peptide and antigen-free wells were included in each serum dilution tested. Wells were then blocked by the addition of the SuperBlock reagent (Thermo-Pierce, 37515) for 1 h at room temperature. Serum samples were diluted in SuperBlock reagent supplemented with 5% FBS and incubated for 1 h at room temperature with shaking. For the initial ELISA screen, serial 2-fold serum dilutions from 1/200 through 1/1600 were performed. Wells were washed three times and a 1/5000 dilution of biotinylated anti-human IgGs was added for 1 h. After washing, a 1/40,000 dilution of streptavidin-HRP (Invitrogen, 43-4323) was added, incubated for 20 min and washed. Color development was performed by the addition of 100 μL 3,3′,5,5′-tetramethylbenzidine (Thermo-Pierce, 34021). The reaction was terminated with the addition of 0.5N sulfuric acid, and OD at 540 nm was read on an Emax spectrophotometer (Molecular Devices). Reactions that exhibited an OD at least twice higher than that of antigen-free wells and wells containing the CAT-4 peptide were considered as positive. All samples were tested in duplicate assessments and in at least three independent experiments. The quantification of of IgGs from SNA lectin-fractionated material was performed by the interpolation of OD based on a standard curve obtained with human IgGs (R&D, 1-001-A) detected as described above.
Autoantibodies specific for HER2 were detected using a sandwich ELISA modified from Ward et al.28 Maxisorp wells were coated with 200 ng/well of monoclonal anti-HER2 antibodies overnight in coating buffer, blocked in SuperBlock reagent as described above, and then incubated with 250 μg SK-BR-3 lysate for 1 h at room temperature. Lysates prepared from chinese hamster ovary (CHO) cells were used as controls for non-specific binding. Plasma samples were diluted in SuperBlock reagent supplemented with 2.5% FBS and normal mouse serum (Jackson Immunoresearch, 015-000-120), and incubated for 1 h at room temperature. Bound human IgG was detected as outlined above, and OD values were corrected by the subtraction of values obtained with CHO lysates. Reactions that exhibited an OD at least twice higher than that of antigen-free wells and wells containing the irrelevant CHO cell lysate were considered as positive.
Lectin immunoblotting
Lectin blotting of affinity purified anti-NY-ESO-1 IgGs digested with papain was used to evaluate Fc fragments for α2,6-Sia content. To this end, 3 μg affinity purified IgGs were digested on immobilized papain (Pierce Fab Micro Preparation Kit, 44685), according to the manufacturer’s instructions. Digestion products were separated by PAGE on NuPAGE 12% Bis-Tris Gel (Invitrogen, NP0341BOX) in a discontinuous buffer system on a Novex Mini Cell (Invitrogen, EI0001). The separated components were electroblotted onto polyvinylidene fluoride (PVDF) membranes and probed with biotinylated SNA lectin (2 μg/mL) for 1 h at room temperature, followed by incubation with 32 ng/mL streptavidin-HRP (Invitrogen). The blots were developed by means of the Super Signal West Chemiluminescent Substrate (Thermo-Pierce, 34080), according to the manufacturer’s instructions, and visualized on an UltraLum image analyzer. Images relative to Western and lectin blotting are composites of blots obtained at different time points in the course of this study.
Molecular cloning of soluble human DC-SIGN
Human soluble DC-SIGN (sDC-SIGN) was cloned by standard PCR methods into the bacterial expression vector pET100/D-TOPO (Invitrogen, K100-01), essentially as described by Su et al.63 The template for PCR was the human CD209-coding full-length cDNA clone purchased from OriGene (SC304915 NM_021155.2). The PCR product was cloned directionally into pET100/D-TOPO (Invitrogen, K100-01). Transformants were sequenced bi-directionally on an Applied Biosystems 3500 Genetic Analyzer in order to ensure in-frame cloning relative to the T7 promoter and PCR fidelity. Recombinant clones were expressed in Escherichia coli strain BL21 Star™ (DE3) obtained in the form of competent cells from Invitrogen (44-0049). The purification of sDC-SIGN-6xHis fusion was performed on Ni columns, essentially as described elsewhere.63 For ligand binding assays, recombinant sDC-SIGN (200 ng/well) was coated onto Maxisorp wells overnight at 4 °C, blocked in Carb-Free Block reagent (Vector Labs, SP-5040) for 1 h, and then incubated with intact IgG samples in TBS containing 0.5% Tween 20 and 1 mM CaCl2 for 2 h at room temperature. Bound immunoglobulins were detected by means of a cocktail of HRP-conjugated donkey anti-human IgGs and HRP-conjugated goat anti-human IgG Fcs, each diluted 1/100,000 in wash buffer, developed and read as described above.
Preparation of sialylated Fab fragments
Sia+ Fab fragments were prepared from human IVIG (Privigen, CSL Behring AG, NDC 44206-436-05). To this aim, 500 mg IVIG was exchanged into digestion buffer (20 mM sodium phosphate, pH 7.0, 10 mM EDTA, 20 mM cysteine) with the use of a desalting column (Thermo-Pierce, 89889). The entire contents were added to 1 mL of washed and packed immobilized papain (Thermo-Pierce, 20344) and were incubated for 6 h at 37 °C with rotation. The slurry was centrifuged at 1500 G, and the supernatant was applied directly to a protein A chromatography column (BioRad UNOsphereSUPrA, 156-0218) equilibrated with 0.5X PBS (Thermo-Pierce, 28372). The flow-through was collected, buffer was exchanged into SNA binding buffer (25 mM Hepes pH 8, 150 mM NaCl, 1 mM each CaCl2 and MnCl2), and the entire contents were added to 2 mL packed SNA-agarose. The material was bound in batch for 30 min at room temperature, and the resin was washed with 20 volumes of binding buffer and then eluted by the addition of 0.5 M lactose in binding buffer. A second elution with 0.5 M lactose in 0.2 M acetic acid was performed and the combined contents of elutions were neutralized by the addition of 1/10 volume 1 M Tris (pH 9). The buffer was exchanged with PBS, and the SNA+ fraction was then reapplied to a protein A column (as above) in order to remove residual intact IgGs or Fc fragments. The product was analyzed by Western and lectin blotting to verify purity and reactivity.
Statistical analyses
The statistical analysis of ELISA data was performed by means of SigmaPlot v. 11, using Kruskal–Wallis one way analysis of variance on ranks. The Tukey test was used for all pairwise multiple comparisons.
Acknowledgments
The authors thank the physicians (Jonathan Treisman and John Hanson) and Nina Garlie and her staff of the Immunotherapy Program for assistance with obtaining blood samples from melanoma patients. We thank Judy Tjoe, Natalie Polinske and Matt Tector for help with obtaining samples through the Aurora Health Care Biorepository (ORBIT), and Eric Ballacer and Blake Shaffer for technical assistance. We also thank John Richards and Karen Michel for DNA sequencing of recombinant plasmids. This work was funded by a grant through the Northwest Mutual Life Insurance Foundation. Samuel Taylor was the recipient of Research Internship Award from the Medical Staff of Aurora St. Luke’s Medical Center.
Glossary
Abbreviations:
- α2,6-Sia
α2,6-linked Sia
- ADCC
antibody-dependent cellular cytotoxicity
- DC-SIGN
dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin
- ELLA
enzyme-linked lectin assay
- Fab
antigen-binding fragment
- FBS
fetal bovine serum
- Fc
crystalizable fragment
- IVIG
intravenous immunoglobulin
- LC-MS
liquid chromatography-mass spectroscopy
- OD
optical density
- sDC-SIGN
soluble DC-SIGN
- Sia
sialic acid
- Sia+Fab
sialylated Fab
- Sia+Fc
sialylated Fc
- Sia+
sialylated
- SNA
Sambucus nigra agglutinin
- TAA
tumor-associated antigen
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24841
References
- 1.Sahin U, Türeci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A. 1995;92:11810–3. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–44. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobold S, Lütkens T, Cao Y, Bokemeyer C, Atanackovic D. Autoantibodies against tumor-related antigens: incidence and biologic significance. Hum Immunol. 2010;71:643–51. doi: 10.1016/j.humimm.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Radoja S, Rao TD, Hillman D, Frey AB. Mice bearing late-stage tumors have normal functional systemic T cell responses in vitro and in vivo. J Immunol. 2000;164:2619–28. doi: 10.4049/jimmunol.164.5.2619. [DOI] [PubMed] [Google Scholar]
- 6.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 7.Wei S, Curiel T, Coukos G, Liu R, Zou W. Inhibitory B7 family members in human ovarian carcinoma. Adv Exp Med Biol. 2008;622:261–71. doi: 10.1007/978-0-387-68969-2_21. [DOI] [PubMed] [Google Scholar]
- 8.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 9.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–66. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 10.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 11.Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog. 2005;21:1644–52. doi: 10.1021/bp050228w. [DOI] [PubMed] [Google Scholar]
- 12.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–40. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 13.Satoh M, Iida S, Shitara K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin Biol Ther. 2006;6:1161–73. doi: 10.1517/14712598.6.11.1161. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–3. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 15.Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol. 2007;44:1524–34. doi: 10.1016/j.molimm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–3. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105:19571–8. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. J Exp Med. 2007;204:11–5. doi: 10.1084/jem.20061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol. 2010;30(Suppl 1):S9–14. doi: 10.1007/s10875-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 20.Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Ann N Y Acad Sci. 2012;1253:170–80. doi: 10.1111/j.1749-6632.2011.06305.x. [DOI] [PubMed] [Google Scholar]
- 21.Raju TS, Scallon BJ. Glycosylation in the Fc domain of IgG increases resistance to proteolytic cleavage by papain. Biochem Biophys Res Commun. 2006;341:797–803. doi: 10.1016/j.bbrc.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Stadlmann J, Pabst M, Kolarich D, Kunert R, Altmann F. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics. 2008;8:2858–71. doi: 10.1002/pmic.200700968. [DOI] [PubMed] [Google Scholar]
- 23.Stadlmann J, Weber A, Pabst M, Anderle H, Kunert R, Ehrlich HJ, et al. A close look at human IgG sialylation and subclass distribution after lectin fractionation. Proteomics. 2009;9:4143–53. doi: 10.1002/pmic.200800931. [DOI] [PubMed] [Google Scholar]
- 24.Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol. 1997;15:3363–7. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- 25.Goodell V, Waisman J, Salazar LG, de la Rosa C, Link J, Coveler AL, et al. Level of HER-2/neu protein expression in breast cancer may affect the development of endogenous HER-2/neu-specific immunity. Mol Cancer Ther. 2008;7:449–54. doi: 10.1158/1535-7163.MCT-07-0386. [DOI] [PubMed] [Google Scholar]
- 26.Chapman CJ, Murray A, McElveen JE, Sahin U, Luxemburger U, Türeci O, et al. Autoantibodies in lung cancer: possibilities for early detection and subsequent cure. Thorax. 2008;63:228–33. doi: 10.1136/thx.2007.083592. [DOI] [PubMed] [Google Scholar]
- 27.Stockert E, Jäger E, Chen YT, Scanlan MJ, Gout I, Karbach J, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–54. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward RL, Hawkins NJ, Coomber D, Disis ML. Antibody immunity to the HER-2/neu oncogenic protein in patients with colorectal cancer. Hum Immunol. 1999;60:510–5. doi: 10.1016/S0198-8859(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 29.Montgomery RB, Makary E, Schiffman K, Goodell V, Disis ML. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res. 2005;65:650–6. [PubMed] [Google Scholar]
- 30.van Rhee F, Szmania SM, Zhan F, Gupta SK, Pomtree M, Lin P, et al. NY-ESO-1 is highly expressed in poor-prognosis multiple myeloma and induces spontaneous humoral and cellular immune responses. Blood. 2005;105:3939–44. doi: 10.1182/blood-2004-09-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng G, Aldridge ME, Wang Y, Pantuck AJ, Wang AY, Liu YX, et al. Dominant B cell epitope from NY-ESO-1 recognized by sera from a wide spectrum of cancer patients: implications as a potential biomarker. Int J Cancer. 2005;114:268–73. doi: 10.1002/ijc.20716. [DOI] [PubMed] [Google Scholar]
- 32.Akcakanat A, Kanda T, Koyama Y, Watanabe M, Kimura E, Yoshida Y, et al. NY-ESO-1 expression and its serum immunoreactivity in esophageal cancer. Cancer Chemother Pharmacol. 2004;54:95–100. doi: 10.1007/s00280-004-0768-3. [DOI] [PubMed] [Google Scholar]
- 33.Akcakanat A, Kanda T, Tanabe T, Komukai S, Yajima K, Nakagawa S, et al. Heterogeneous expression of GAGE, NY-ESO-1, MAGE-A and SSX proteins in esophageal cancer: Implications for immunotherapy. Int J Cancer. 2006;118:123–8. doi: 10.1002/ijc.21219. [DOI] [PubMed] [Google Scholar]
- 34.Türeci O, Mack U, Luxemburger U, Heinen H, Krummenauer F, Sester M, et al. Humoral immune responses of lung cancer patients against tumor antigen NY-ESO-1. Cancer Lett. 2006;236:64–71. doi: 10.1016/j.canlet.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, et al. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332–41. doi: 10.1158/1078-0432.CCR-04-0181. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura S, Nouso K, Noguchi Y, Higashi T, Ono T, Jungbluth A, et al. Expression and immunogenicity of NY-ESO-1 in hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21:1281–5. doi: 10.1111/j.1440-1746.2006.04271.x. [DOI] [PubMed] [Google Scholar]
- 37.Fosså A, Berner A, Fosså SD, Hernes E, Gaudernack G, Smeland EB. NY-ESO-1 protein expression and humoral immune responses in prostate cancer. Prostate. 2004;59:440–7. doi: 10.1002/pros.20025. [DOI] [PubMed] [Google Scholar]
- 38.Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–83. [PubMed] [Google Scholar]
- 39.Huhn C, Selman MH, Ruhaak LR, Deelder AM, Wuhrer M. IgG glycosylation analysis. Proteomics. 2009;9:882–913. doi: 10.1002/pmic.200800715. [DOI] [PubMed] [Google Scholar]
- 40.Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J. 1997;14:569–76. doi: 10.1023/A:1018580324971. [DOI] [PubMed] [Google Scholar]
- 41.Varki A, Kannagi R, Toole BP. Glycosylation Changes in Cancer. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Betozzi CR, et al. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2009:617-32. [PubMed] [Google Scholar]
- 42.Kaplan HA, Woloski BM, Hellman M, Jamieson JC. Studies on the effect of inflammation on rat liver and serum sialyltransferase. Evidence that inflammation causes release of Gal beta 1 leads to 4GlcNAc alpha 2 leads to 6 sialyltransferase from liver. J Biol Chem. 1983;258:11505–9. [PubMed] [Google Scholar]
- 43.Käsermann F, Boerema DJ, Rüegsegger M, Hofmann A, Wymann S, Zuercher AW, et al. Analysis and functional consequences of increased Fab-sialylation of intravenous immunoglobulin (IVIG) after lectin fractionation. PLoS One. 2012;7:e37243. doi: 10.1371/journal.pone.0037243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalziel M, McFarlane I, Axford JS. Lectin analysis of human immunoglobulin G N-glycan sialylation. Glycoconj J. 1999;16:801–7. doi: 10.1023/A:1007183915921. [DOI] [PubMed] [Google Scholar]
- 45.Stadlmann J, Pabst M, Altmann F. Analytical and Functional Aspects of Antibody Sialylation. J Clin Immunol. 2010 doi: 10.1007/s10875-010-9409-2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guhr T, Bloem J, Derksen NI, Wuhrer M, Koenderman AH, Aalberse RC, et al. Enrichment of sialylated IgG by lectin fractionation does not enhance the efficacy of immunoglobulin G in a murine model of immune thrombocytopenia. PLoS One. 2011;6:e21246. doi: 10.1371/journal.pone.0021246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther. 2007;7:1401–13. doi: 10.1517/14712598.7.9.1401. [DOI] [PubMed] [Google Scholar]
- 48.Holland M, Yagi H, Takahashi N, Kato K, Savage CO, Goodall DM, et al. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim Biophys Acta. 2006;1760:669–77. doi: 10.1016/j.bbagen.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 49.Lastra GC, Thompson SJ, Lemonidis AS, Elson CJ. Changes in the galactose content of IgG during humoral immune responses. Autoimmunity. 1998;28:25–30. doi: 10.3109/08916939808993842. [DOI] [PubMed] [Google Scholar]
- 50.van de Geijn FE, Wuhrer M, Selman MH, Willemsen SP, de Man YA, Deelder AM, et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther. 2009;11:R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Espy C, Morelle W, Kavian N, Grange P, Goulvestre C, Viallon V, et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s) Arthritis Rheum. 2011;63:2105–15. doi: 10.1002/art.30362. [DOI] [PubMed] [Google Scholar]
- 52.Scherer HU, van der Woude D, Ioan-Facsinay A, el Bannoudi H, Trouw LA, Wang J, et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62:1620–9. doi: 10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- 53.Saldova R, Royle L, Radcliffe CM, Abd Hamid UM, Evans R, Arnold JN, et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 2007;17:1344–56. doi: 10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- 54.Wuhrer M, Porcelijn L, Kapur R, Koeleman CA, Deelder A, de Haas M, et al. Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. J Proteome Res. 2009;8:450–6. doi: 10.1021/pr800651j. [DOI] [PubMed] [Google Scholar]
- 55.Lux A, Aschermann S, Biburger M, Nimmerjahn F. The pro and anti-inflammatory activities of immunoglobulin G. Ann Rheum Dis. 2010;69(Suppl 1):i92–6. doi: 10.1136/ard.2009.117101. [DOI] [PubMed] [Google Scholar]
- 56.Katz U, Shoenfeld Y, Zandman-Goddard G. Update on intravenous immunoglobulins (IVIg) mechanisms of action and off- label use in autoimmune diseases. Curr Pharm Des. 2011;17:3166–75. doi: 10.2174/138161211798157540. [DOI] [PubMed] [Google Scholar]
- 57.Katz U, Achiron A, Sherer Y, Shoenfeld Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun Rev. 2007;6:257–9. doi: 10.1016/j.autrev.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 58.Jordan SC, Vo AA, Tyan D, Nast CC, Toyoda M. Current approaches to treatment of antibody-mediated rejection. Pediatr Transplant. 2005;9:408–15. doi: 10.1111/j.1399-3046.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 59.Jordan SC, Vo AA, Toyoda M, Tyan D, Nast CC. Post-transplant therapy with high-dose intravenous gammaglobulin: Applications to treatment of antibody-mediated rejection. Pediatr Transplant. 2005;9:155–61. doi: 10.1111/j.1399-3046.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 60.Jordan SC, Toyoda M, Vo AA. Intravenous immunoglobulin a natural regulator of immunity and inflammation. Transplantation. 2009;88:1–6. doi: 10.1097/TP.0b013e3181a9e89a. [DOI] [PubMed] [Google Scholar]
- 61.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–3. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 62.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–6. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su SV, Hong P, Baik S, Negrete OA, Gurney KB, Lee B. DC-SIGN binds to HIV-1 glycoprotein 120 in a distinct but overlapping fashion compared with ICAM-2 and ICAM-3. J Biol Chem. 2004;279:19122–32. doi: 10.1074/jbc.M400184200. [DOI] [PubMed] [Google Scholar]