Abstract
To better understand how warming, increased precipitation and their interactions influence community structure and composition, a field experiment simulating hydrothermal interactions was conducted at an annual forb dominated desert steppe in northern China over 2 years. Increased precipitation increased species richness while warming significantly decreased species richness, and their effects were additive rather than interactive. Although interannual variations in weather conditions may have a major affect on plant community composition on short term experiments, warming and precipitation treatments affected individual species and functional group composition. Warming caused C4 grasses such as Cleistogenes squarrosa to increase while increased precipitation caused the proportions of non-perennial C3 plants like Artemisia capillaris to decrease and perennial C4 plants to increase.
Introduction
Concurrent with increases in atmospheric CO2 and other greenhouse gases, global mean temperature has increased by 0.76°C since the 1850s and will further likely increase by 2.0–4.5°C by the end of this century [1]. Meanwhile, precipitation regimes are expected to be asymmetrically distributed, potentially causing mean global precipitation to increase by 7% [2]. Temperature and precipitation are important abiotic factors that directly and/or indirectly affect plant physiological processes and influence the growth, phenology, adaptive strategies, and productivity of individual plant species [3], [4]. Temperature and precipitation may also affect interactions among plants, their distributions, alter interspecific relationships [5]–[8], and often plant community structure and composition [9]–[11], ecosystem structure and function [12]–[17], and potentially feedbacks with climate change [18], [19]. Therefore, understanding the effect of hydrothermal changes on plant community structure and composition is crucial for evaluating the possible consequences of climate change on terrestrial ecosystems and may help inform regulatory policies to cope with climate change.
Evidence both from theoretical and empirical approaches has demonstrated that climatic changes, such as warming and precipitation change, profoundly affect plant community structure and composition [3], [8]–[11], [20]–[22]. Experimental warming can alter plant community structure and composition by altering competitive interactions and dominance hierarchies of different plant species or functional groups [6], [8], [9], [20], [23], [24]. For example, climate warming can increase the proportion of herbaceous plant species on arctic tundra [21], reduce the biomass of most species on a montane meadow [9], [25], or result in the loss of plant species [11], [26]. A variety of experiments have also examined plant community responses to changes in precipitation amounts [10], [27], [28], precipitation frequency [27], [29], [30], and precipitation seasonality [31], [32]. These studies provide abundant evidence showing climatic warming and changes in precipitation can strongly influence community structure and function.
However, most of these studies have focused on the effects of individual climate change drivers on plant communities, while the potential for additive or interactive effects of multiple environmental factors on plant communities remains unclear [28], [33]. The effects of multifactorial treatments differ from those of a single-factor treatment. For example, warming-induced reductions in soil water availability may limit positive effects of warming and increased precipitation on plant community and ecosystem processes [9], [34], [35]. Conversely, increased precipitation can decrease soil temperature and attenuate high-temperature stress induced by warming [36]. Meta-analyses have demonstrated the interactive effects of warming and increased precipitation on Net Primary Productivity (NPP) are small [37] or not significant [38]. This uncertainty highlights the importance of examining ecosystem responses to multiple abiotic factors [33]. But until now, few field experiments [9], [23], [33], [39]–[42] have factorially manipulated both temperature and precipitation.
The Intergovernmental Panel on Climate Change (IPCC) [1] reported that climate change will initially affect temperate steppe ecosystems because semiarid temperate steppe at high latitudes is one of the ecosystems which is most vulnerable to climate change [8], [20], [43]. Desert steppe located in northern China is an important part of the Eurasian grassland biome, which covers a total area of about 8.8 million ha, supports biodiversity, provides ecosystem services, and contributes to the socio-economic development of the region [44]. Temperatures in this region have increased substantially during the past 50 years [45] and are projected to experience “much greater than average” increases in the future [46]. Also, an increase in precipitation amount has been predicted in northern China [1]. A modeling study has shown temperature and precipitation are predicted to increase by 3°C and 30–100 mm, respectively [47]. Most research on climate change effects in the region has been on typical steppe [8], [41], [43], [45], [48]–[50]. Little attention has been paid to the response of desert steppe to climate change. Here our aim was to understand the impacts of climatic changes on desert steppe and dominant plant species.
To examine the interactive effects of temperature and precipitation on the plant community, we conducted a field experiment with warming manipulated by infrared heaters and increased precipitation simulated by watering at an annual forb dominated desert steppe in Damao County, Inner Mongolia since May 2011. Previous studies have demonstrated that water availability is a key factor regulating ecosystem responses to warming and increased precipitation in steppe system [43], [48]. Thus, changes in water availability resulting from warming and increased precipitation in the semiarid steppe will likely determine plant community composition. Given that increased precipitation improves soil water availability, we hypothesized that increased precipitation would promote both plant growth and species richness. Warming stimulates evapotranspiration and decreases soil water availability [9], [34], [48], exacerbating water stress, thus our second hypothesis was that warming would suppress plant growth and decrease species richness. Additionally, previous studies have shown additive effects of warming and increased precipitation [23], [48]. Third we hypothesized that warming and increased precipitation would independently affect plant community composition. Testing these hypotheses may provide new insights on the response of desert steppe to climate change.
Materials and Methods
Ethics Statement
All observational and field studies at the experimental site were undertaken with relevant permissions from the owners– Meteorological Bureau of Damao County, Inner Mongolia. The location is not privately-owned or protected in any way and the field studies did not involve endangered or protected species.
Experimental Site
The experiment was conducted at a desert steppe (41°38′38.3″N, 110°19′53.3″E; 1409 m a.s.l.), in Damao County, Inner Mongolia, China. This site had not been grazed for 21 years. The region's typical continental climate is strongly influenced by the arid Mongolian airflow. The long-term (1978–2007) mean annual temperature was 4.6°C while the monthly mean temperature varied from –14.1°C in January to 21.4°C in July. Mean annual precipitation was about 255.2 mm with 67.6% distributed in the growing season (June to August). The soil at the experimental site is classified as chestnut according to Chinese classification, with mean bulk density of 1.23 g cm–3 and pH of 7.4. A calcium laminated layer lies at 20–30 cm depth below ground [51]. In generally, the desert steppe is dominated by a perennial C3 grass (Stipa klemenzii), an annual C3 forb (Neopallasia pectinata), an annual C3 sub-shrub (Artemisia capillaris) and a perennial C4 grass (Cleistogenes squarrosa). Our study site has a history of anthropogenic disturbance and is instead dominated by the annual forb (Neopallasia pectinata).
Experimental Design
A randomized complete block design with two warming treatments (unwarmed [T0], warmed [T2]) and three watering treatments (ambient precipitation [W0], precipitation increased by 15% [W15], and precipitation increased by 30% [W30]) was manipulated since May 2011. The six treatment combinations were each replicated three times. Eighteen 2×2 m2 plots were arranged in 3×6 matrix (3 blocks with one of each treatment randomized within each block) with 1 m buffer separating adjacent plots. All the warmed plots were heated continuously (24 h/day, starting on 8 June 2011) by IR lamps (GHT220-800, Beijing Sanyuan Huahui Electric Light Source Co. Ltd., Chaoyang, Beijing, China) suspended 1.3 m above the ground. The lamp was 1.0 m long, 800 W at full electrical power fixed in a metal heater. A ‘dummy’ heater with the same shape and size as the infrared heater was mounted in the control plot to simulate the shading effects [34], [49]. A wooden square frame (2 m long and 0.2 m wide) was fastened to the ground along the sides of plot (0.1 m emerged aboveground and 0.1 m buried in soil) to prevent lateral movement of water and nutrients between individual plots and their surroundings. Based on the local long-term (1978–2007) monthly mean precipitation, watering volumes of each plot were 22.29 L, 39.41 L and 43.40 L in the W15 treatment plots and 44.14 L, 78.83 L and 86.36 L in the W30 treatment plots from June to August, respectively. Taking into account the frequency of precipitation in the growing season, we manipulated increased precipitation treatments once a week. All treatments were conducted only during the growing season each year.
Meteorological Measurements
An air temperature and humidity monitoring instrument (HOBO Pro v2 Temp/RH, Onset Computer Corporation, Bourne, MA, USA) was mounted in a radiation shield at 40 cm above the ground in the center of each plot. Measurements taken every 2 seconds were averaged for each thirty minute period. In each plot, a thermocouple (HOBO S-TMB-M006, Onset Computer Corporation, Bourne, MA, USA) at 5 cm below the ground surface and a humidity transducer (HOBO S-SMA-M005, Onset Computer Corporation, Bourne, MA, USA) at 0–20 cm below the surface were installed to measure soil temperature and soil moisture content, respectively. Data were also recorded by an automatic data logger (HOBO H21-002, Onset Computer Corporation, Bourne, MA, USA) every 30 minutes. Meteorological data from June to August in 2011 and 2012 were obtained from an Automatic Meteorological Observation Instrument located 250 m northwest of the experimental site.
Plant Community Characteristics
We measured plant community characteristics at the peak of plant biomass in late August in both years. A permanent 1×1 m2 quadrat was established at the center of each plot and vegetation characteristics in the quadrat were measured in 2011 and 2012. Plants were divided into different functional groups on the basis of life form (grasses, forbs, legumes, and sub-shrubs), photosynthetic pathway (C3 and C4 plants), and life history (annual/biennial and perennial plants) (Table 1). Ground coverage was visually estimated. A 1×1 m2 quadrat frame with 100 sub-grids (0.1×0.1 m2) was used to measure the coverage of each species. The coverages of different functional groups and the entire community were summed up by the species coverages. From the estimates of canopy coverage per species and functional group, we determined the relative coverage of each (i.e. percent cover). The individual numbers of each species and the plant community in the frame were counted. We determined the Species richness (S), Shannon-Wiener index (H′), and Pielou evenness index (J′) for each plot. The Pielou evenness index was calculated as: J′ = H′/ln(S), where H’ is the Shannon-Wiener index and calculated as: H′ = –∑Piln(Pi), where Pi is the proportional number of species i and S is the species richness in the community.
Table 1. The plant species, life form (LF), photosynthetic pathway (PP), life history traits (LH) and the percent cover of plant species within the quadrats (1 × 1 m2).
| Species | LF | PP | LH | Percent cover (%) | |
| 2011 | 2012 | ||||
| Cleistogenes squarrosa | Grass | C4 | Perennial | 12.27 | 13.74 |
| Stipa klemenzii | Grass | C3 | Perennial | 6.31 | 7.23 |
| Tribulus terrestris | Forb | C4 | Annual | 0.08 | 2.38 |
| Chenopodium glaucum | Forb | C4 | Annual | / | 0.95 |
| Neopallasia pectinata | Forb | C3 | Annual | 49.78 | 14.77 |
| Erodium stephanianum | Forb | C3 | Annual | 4.67 | 0.48 |
| Heteropappus altaicus | Forb | C3 | Perennial | 2.33 | 2.46 |
| Convolvulus ammannii | Forb | C3 | Perennial | 0.26 | 0.08 |
| Allium bidentatum | Forb | C3 | Perennial | 0.52 | 0.40 |
| Ixeris chinensis | Forb | C3 | Perennial | / | 0.32 |
| Salsola collina | Forb | C4 | Annual | 2.25 | 2.38 |
| Iris tenuifolia | Forb | C3 | Perennial | / | 0.24 |
| Scorzonera divaricata | Forb | C3 | Perennial | 0.09 | 0.16 |
| Astragalus galactites | Legume | C3 | Perennial | 9.68 | 2.54 |
| Lagochilus ilicifolius | Sub-shrub | C3 | Perennial | 0.69 | 0.16 |
| Artemisia capillaries | Sub-shrub | C3 | Annual | 10.29 | 51.07 |
| Caragana stenophylla | Sub-shrub | C3 | Perennial | 0.78 | 0.64 |
Statistical Analysis
Three-way ANOVAs were carried out to test the main and interactive effects of warming, increased precipitation, and year on community composition (the percent cover of individual species and functional groups), and community structure (species richness, Shannon diversity, and Pielou evenness). One-way ANOVA with a Duncan’s multiple range tests was used to test the statistical significance in the mean values of the treatments. Statistical significances of all tests were set at P<0.05. All statistical analyses were conducted with SPSS 17.0 software (SPSS Institute Incorporated, Chicago, Illinois, USA), and all the data were normally distributed as determined by the Shapiro-Wilk W statistic [52] prior to statistical analysis.
Results
Environmental Factors
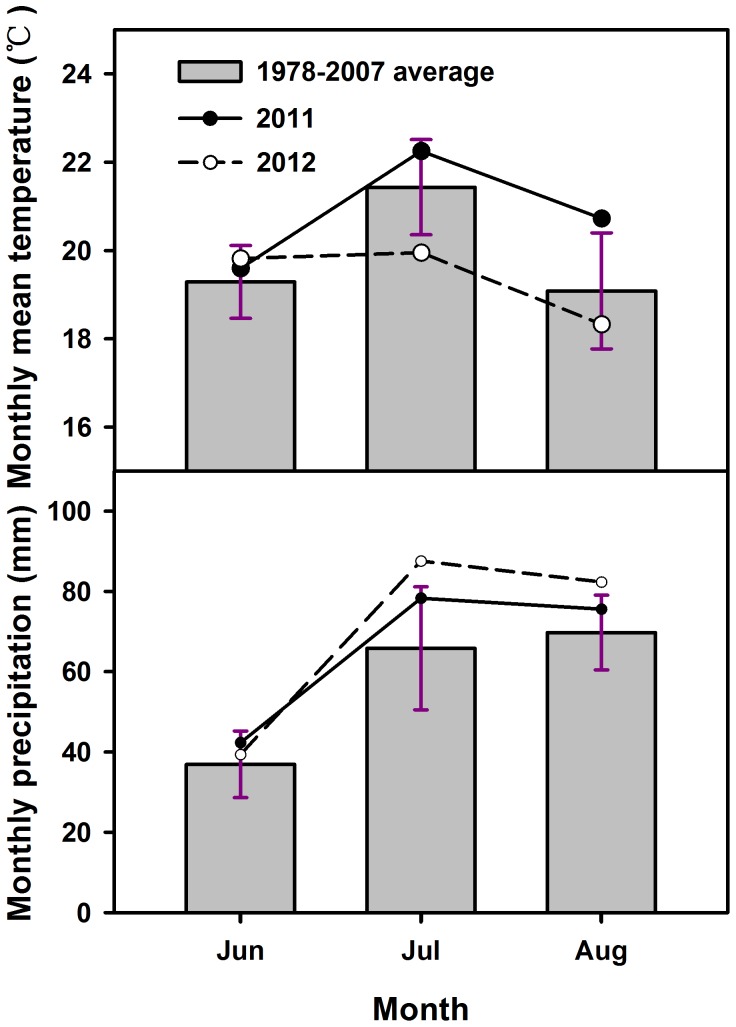
Compared to the 30-year averages, weather conditions at our experimental site were unusually hot and wet and unusually cool and wet in the 2011 and 2012 growing seasons, respectively (Fig. 1).
Figure 1. Monthly average temperature (top) and total monthly precipitation (bottom) in the growing season.
Gray bars indicate the regional monthly averages (mean ± SE) from 1978 to 2007.
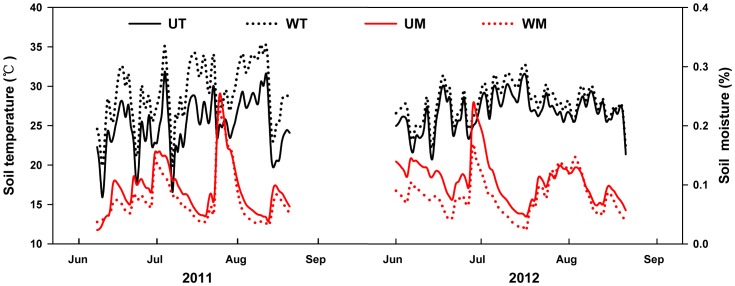
During the two growing season periods, the soil temperature showed strong interannual variation at 5 cm deep (P<0.01; Fig. 2). The soil temperatures in the warmed and control plots in 2011 were 5.18°C and 2.66°C higher than those in 2012, respectively. Warming induced by infrared heaters elevated the mean soil temperature by 4.10°C (2011, P<0.01) and 1.58°C (2012, P<0.01), but reduced the mean soil moisture (0–20 cm, v/v) by 1.66% (2011, P<0.05) and 2.63% (2012, P<0.01) (Fig. 2).
Figure 2. Daily mean soil temperature and soil moisture during the growing season in 2011 and 2012.
UT, soil temperature in unwarmed plot; WT, soil temperature in warmed plot; UM, soil moisture in unwarmed plot; WM, soil moisture in warmed plot.
Percent Cover of Dominant Species
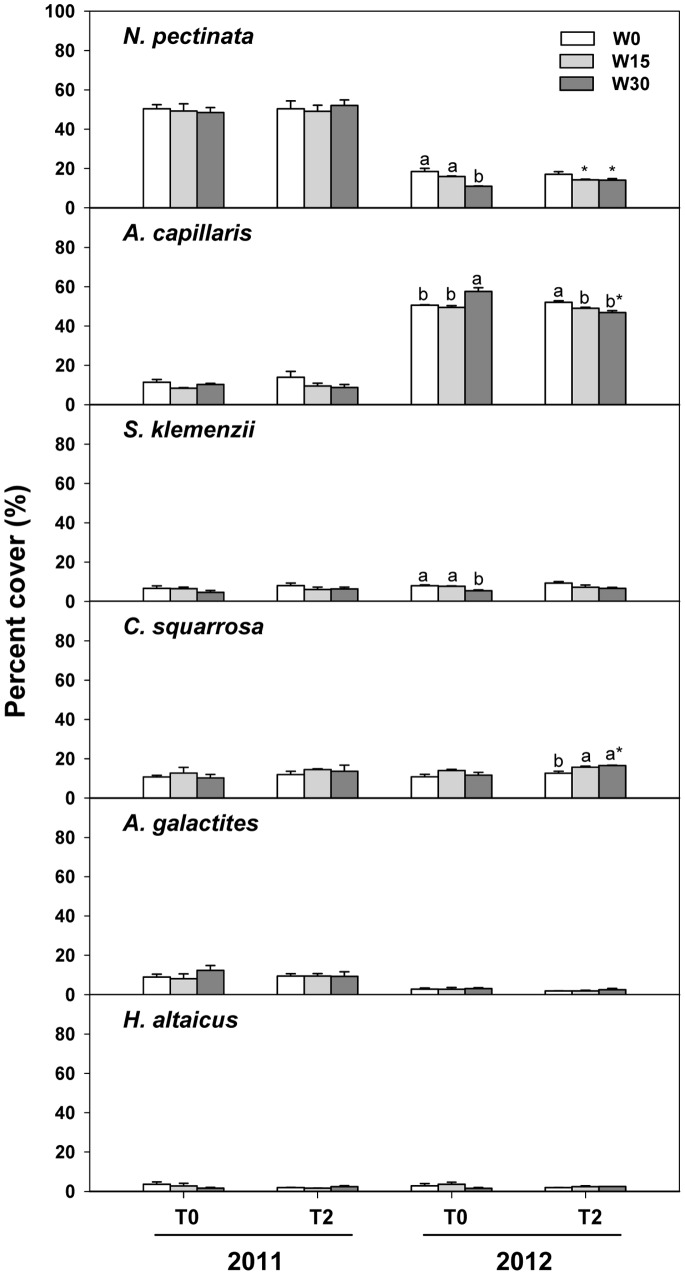
Warming significantly increased the percent cover of C. squarrosa (F 1, 24 = 7.122, P<0.05) while it had no significant effects on other species (Table 2; Fig. 3). Increased precipitation had significant impacts on the percent cover of A. capillaris (F 2, 24 = 4.718, P<0.05) and S. klemenzii (F 2, 24 = 6.192, P<0.01). Increased precipitation (W15 vs. W0) significantly decreased the percent cover of A. capillaris by 9.07% (P<0.01), and increased precipitation (W15 and W30 vs. W0) also reduced the percent cover of S. klemenzii by 13.75% (P = 0.095) and 27.83% (P<0.01), respectively.
Table 2. Results (P-values) of three-way ANOVAs on the effects of temperature, precipitation, year and their interactions on the percent cover of dominant species.
| Sources of Variation | df | N. pectinata | A. capillaris | S. klemenzii | C. squarrosa | A. galactites | H. altaicus |
| T | 1 | 0.668 | 0.124 | 0.135 | 0.013 | 0.476 | 0.200 |
| P | 2 | 0.259 | 0.019 | 0.007 | 0.075 | 0.441 | 0.495 |
| Y | 1 | <0.001 | <0.001 | 0.062 | 0.179 | <0.001 | 0.802 |
| T×P | 2 | 0.357 | 0.001 | 0.265 | 0.459 | 0.570 | 0.083 |
| T×Y | 1 | 0.677 | 0.020 | 0.814 | 0.735 | 0.783 | 0.742 |
| P×Y | 2 | 0.292 | 0.121 | 0.811 | 0.731 | 0.738 | 0.524 |
| T×P×Y | 2 | 0.984 | 0.073 | 0.977 | 0.937 | 0.485 | 0.898 |
Abbreviations: T, temperature; P, precipitation; Y, year; N. pectinata, Neopallasia pectinata; A. capillaris, Artemisia capillaris; S. klemenzii, Stipa klemenzii; C. squarrosa, Cleistogenes squarrosa; H. altaicus, Heteropappus altaicus; A. galactites, Astragalus galactites. Significant level: at <0.05 in bold.
Figure 3. Effects of warming and increased precipitation on the percent cover of dominant species in 2011 and 2012.
N. pectinata, Neopallasia pectinata; A. capillaris, Artemisia capillaris; S. klemenzii, Stipa klemenzii; C. squarrosa, Cleistogenes squarrosa, H. altaicus, Heteropappus altaicus; A. galactites, Astragalus galactites. Values are the means ± SE of three replications. Different lowercases indicate significant difference among different precipitation treatments in the same temperature treatment within the same year (p<0.05); * indicates significant difference between unwarmed and warmed treatments in the same precipitation treatment within the same year (p<0.05).
We observed shifts in species relative contributions to the plant community between years (Table 2; Fig. 3). The percent cover of A. capillaris increased from 10.29% in 2011 to 51.07% in 2012 (F 1, 24 = 2722.381, P<0.001), while the percent cover of N. pectinata (from 49.78% to 14.77%; F 1, 24 = 677.013, P<0.001) and A. galactites (from 9.68% to 2.54%; F 1, 24 = 71.925, P<0.001) decreased during the same period (Table 1 & 2; Fig. 3). We failed to detect significant differences in the percent cover of S. klemenzii (F 1, 24 = 3.847, P = 0.062), C. squarrosa (F 1, 24 = 1.914, P = 0.179), and H. altaicus (F 1, 24 = 0.064, P = 0.802) between years.
We detected an interactive effect of warming and water addition treatments on the percent cover of only one plant species, A. capillaris (F 2, 24 = 10.155, P<0.01). In addition, the effects of warming on the percent cover of A. capillaris varied with year (F 1, 24 = 6.254, P<0.05; Table 2). Warming increased the percent cover of A. capillaris by 7.02% in 2011 while decreased that by 6.05% in 2012. We did not observe any interactions among warming, increased precipitation, and year on the percent cover of other dominant species (Table 2).
Percent Cover of Functional Groups
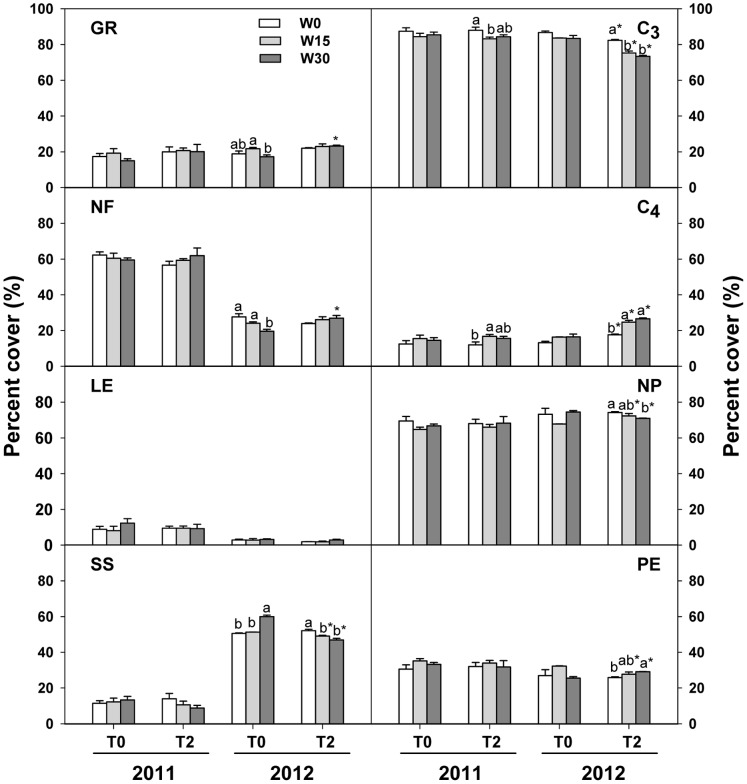
Experimental warming increased the percent cover of grasses by 17.8% (F 1, 24 = 8.912, P<0.01) and decreased the percent cover of sub-shrubs by 8.8% (F 1, 24 = 10.753, P<0.01) while it had no effect on that of forbs (F 1, 24 = 0.032, P = 0.859) or legumes (F 1, 24 = 0.418, P = 0.524; Table 3). Increased precipitation did not affect any life form functional groups. In contrast, there were significant shifts in functional groups between years (Table 3; Fig. 4). For example, we observed several functional groups increasing (grasses, P = 0.046; sub-shrubs, P<0.001) from 2011 to 2012 while forbs (P<0.001) and legumes (P<0.001) decreased during the same period. The relative contributions of forbs (F 2, 24 = 5.995, P<0.01) and sub-shrubs (F 2, 24 = 12.708, P<0.001) to community cover were both significantly affected by the interactions of warming and increased precipitation (Table 3). Increased precipitation (W15 and W30 vs. W0) decreased the percent cover of forbs by 5.99% and 12.08% in unwarmed plots while increased that by 6.14% and 10.51% in warmed plots. In contrast, increased precipitation (W15 and W30 vs. W0) enhanced the percent cover of sub-shrubs by 2.53% and 18.17% in unwarmed plots while increased that by 9.86% and 15.85% in warmed plots (Fig. 4).
Table 3. Results (P-values) of three-way ANOVAs on the effects of temperature, precipitation, year and their interactions on the percent cover of different functional groups.
| Sources of Variation | df | GR | NF | LE | SS | C3 | C4 | NP | PE |
| T | 1 | 0.006 | 0.859 | 0.524 | 0.003 | <0.001 | <0.001 | 0.612 | 0.612 |
| P | 2 | 0.230 | 0.901 | 0.376 | 0.377 | <0.001 | <0.001 | 0.047 | 0.047 |
| Y | 1 | 0.046 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| T×P | 2 | 0.298 | 0.008 | 0.633 | <0.001 | 0.120 | 0.120 | 0.332 | 0.332 |
| T×Y | 1 | 0.862 | 0.158 | 0.846 | 0.070 | <0.001 | <0.001 | 0.911 | 0.911 |
| P×Y | 2 | 0.933 | 0.396 | 0.797 | 0.176 | 0.204 | 0.204 | 0.977 | 0.977 |
| T×P×Y | 2 | 0.975 | 0.877 | 0.416 | 0.144 | 0.545 | 0.545 | 0.256 | 0.256 |
Abbreviations: T, temperature; P, precipitation; Y, year; GR, grasses; NF, nongraminous forbs; LE, legumes; SS, sub-shrubs; C3, C3 plants; C4, C4 plants; NP, non-perennial (annual/biennial) plants; PE, perennial plants. Significance level: P<0.05 in bold.
Figure 4. Effects of warming and increased precipitation on the percent cover of different functional groups in 2011 and 2012.
GR, grasses; NF, nongraminous forbs; LE, legumes; SS, sub-shrubs; C3, C3 plants; C4, C4 plants; NP, non-perennial (annual/biennial) plants; PE, perennial plants. Values are the means ± SE of three replications. Different lowercases indicate significant difference among different precipitation treatments in the same temperature treatment within the same year (p<0.05); * indicates significant difference between unwarmed and warmed treatments in the same precipitation treatment within the same year (p<0.05).
The percent cover of C3 and C4 plants were both significantly affected by temperature (F 1, 24 = 31.513, P<0.001), precipitation (F 2, 24 = 16.796, P<0.001), and year (F 1, 24 = 40.592, P<0.001), but the warming effects varied by year (F 1, 24 = 22.762, P<0.001; Table 3). Increased precipitation negatively affected the percent cover of C3 plants while increased the relative contributions of C4 plants. In 2011, there was no significant warming effect on C3 plants or C4 plants. But in 2012, warming significantly decreased the percent cover of C3 plants by 9.56% (P<0.01) but increased that of C4 plants by 54.91% (P<0.01; Fig. 4).
Neither perennial plants nor non-perennial plants differed between the two temperature treatments (F 1, 24 = 0.265, P = 0.612; Table 3). In contrast, increased precipitation (F 2, 24 = 3.492, P<0.05) had significant effects on perennial plants and non-perennial plants. Increased precipitation (W15 vs. W0) elevated the percent cover of perennial plants by 12.21% (P<0.05) while increased that of non-perennial plants by 4.94% (P<0.05; Fig. 4). We observed differences between years for perennial and non-perennial plants (F 1, 24 = 19.647, P<0.001). The percent cover of perennial plants decreased from 32.79% in 2011 to 27.87% in 2012 (P<0.01), while the percent cover of non-perennial plants increased from 67.21% in 2011 to 72.13% in 2012 (P<0.01). We failed to detect any interactions among warming, increased precipitation, and year on perennial plants or non-perennial plants (Table 3).
Plant Community Diversity
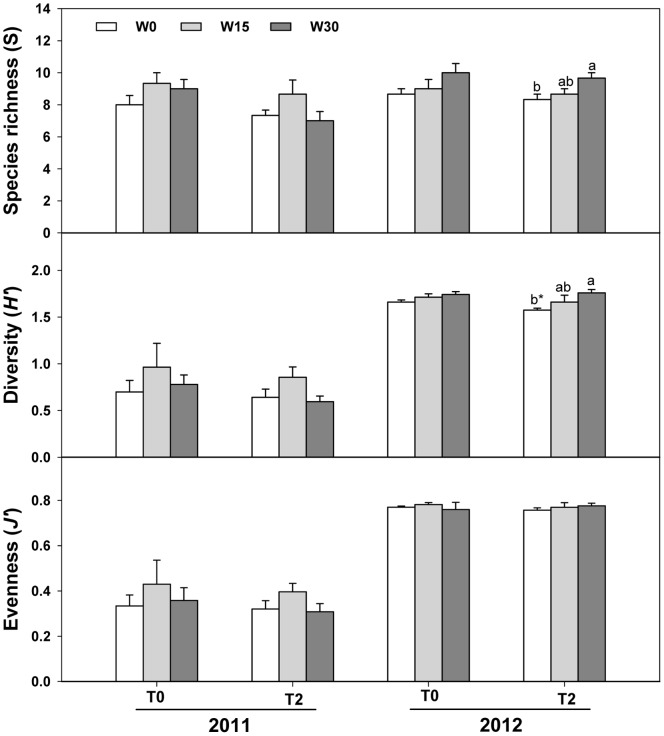
Experimental warming reduced species richness by 8.72%, but it did not influence H’ or J’ across the two years. Increased precipitation also had no effect on H’ or J’ across the two years, but increased species richness (Table 4; Fig. 5). In 2011, warming reduced species richness by 14.49% while increased precipitation had no significant effect on species richness. In 2012, species richness was increased as precipitation increased and there was a significant difference between W0 treatment andW30 treatment (Fig. 5).
Table 4. Effects (P-values) of temperature, precipitation, year and their interactions on plant species richness, Shannon diversity and Pielou evenness by three-way ANOVAs.
| Source of variation | df | S | H’ | J’ |
| T | 1 | 0.028 | 0.192 | 0.487 |
| P | 2 | 0.044 | 0.118 | 0.232 |
| Y | 1 | 0.013 | <0.001 | <0.001 |
| T×P | 2 | 0.603 | 0.997 | 0.986 |
| T×Y | 1 | 0.221 | 0.524 | 0.564 |
| P×Y | 2 | 0.047 | 0.152 | 0.393 |
| T×P×Y | 2 | 0.603 | 0.727 | 0.862 |
Abbreviations: T, temperature; P, precipitation; Y, year; S, species richness; H’, Shannon diversity; J’, Pielou evenness. Significant P-values at 0.05 level are bolded.
Figure 5. Effects of warming and increased precipitation on species richness (S) of the community, Shannon-Wiener index (H’), and Pielou evenness (J’) (mean ± SE) in 2011 and 2012.
T0, unwarmed; T2, warmed; W0, ambient precipitation; W15, precipitation increased by 15%; W30, precipitation increased by 30%. Different lowercases indicate significant difference among different precipitation treatments in the same temperature treatment within the same year (p<0.05); * indicates significant difference between unwarmed and warmed treatments in the same precipitation treatment within the same year (p<0.05).
In the annual forb dominated community, diversity measures (S, 10.22%, P<0.05; H’, 122.37%, P<0.01; and J’, 113.89%, P<0.01) were greater in 2012 than 2011.We failed to detect any interactions among warming, increased precipitation, and year on H’ or J’ across the two years (Table 4).
Discussion
Combined Effects of Weather Condition and Simulated Hydrothermal Treatments on Community Dynamics
In semiarid steppe, precipitation is the main factor limiting plant growth [32], [48]. During the experiment, ambient precipitation was larger than the 30 year mean precipitation, which resulted in more productivity and enormous interannual variations of measured variables during the two years’ experiment was maintained. Interannual variability of community structure and function affected by annual weather conditions have been reported across different regions [53], [54]. Also, weather conditions often interact with hydrothermal treatments in experiments affecting community dynamics [40], [55], [56], sometimes eclipsing treatment effects to a certain extent [42], [57]. We documented significant interannual variability in measured abiotic response variables (e.g. soil temperature and moisture) illustrating that the effects of weather conditions can be enormous and may exceed treatment effects. Since our water additions occurred during a sequence of years with greater than average precipitation, effects of water additions are likely to be conservative.
In addition to precipitation amount, precipitation frequency and intensity are also important for plant growth and should be taken into account under future climate change scenarios [1], [58]. But in this study, artificial precipitation frequency was once a week to simplify the treatment. Precipitation intensity was artificially changed once a month to match monthly historic averages, which would affect plant growth and community structure and composition. A limit to this type of experimental manipulation, however, is that water additions on a sunny day (low humidity, high winds, higher evapotranspiration) may be effectively less than a same addition during a natural rain event when humidity is likely higher and evapotranspiration is likely lower.
Effects of Warming and Increased Precipitation on Community Structure
Experimental warming decreased species richness primarily by reducing the prevalence of annual forb species. Other climate change experiments have also detected reductions in richness [11], [57], [59], but others observed no effects on species richness [55], [56]. Klein et al. [11] attributed species loss induced by warming to heat stress and litter accumulation. Yang et al. [41] concluded that warming indirectly affected species richness by altering soil water availability. In this region, species loss is expected to be the result of direct and indirect effects of warming. For instance, some psychrophilic C3 plants (e.g. Allium bidentatum) would disappear as the result of heat stress. Moreover, they are affected by reduction of soil water availability owing to warming. Since the lost species persist in seed bank and will germinate in plots with added soil moisture, such effects are likely ephemeral and should be examined in long-term.
Unlike the negative effects of warming on species richness, increased precipitation enhanced species richness in this study (Fig. 5). For instance, the annual C4 forb- Chenopodium glaucum appeared in wetter plots. This finding supports the results reported in other grassland ecosystems [10], [41], [55]. Increased precipitation may directly affect species richness through altering soil moisture and can relieve warming-induced heat stress by reducing soil temperature. Thus, increased precipitation plays a positive role in maintaining the stability of grassland ecosystem.
Neither warming nor increased precipitation had significant effects on the diversity index and evenness, while strong interannual variations were detected in this study (Table 4; Fig. 5). Our two year experiment is a relatively short duration which may be insufficient for detecting changes in many species, especially perennial plants. Thus, we recommend caution in interpreting the effects of short-term weather manipulations as an indicator of the effects of long-term climatic shifts. Also, treatment effects on species diversity might be under-estimated or over-estimated because treatment plots are typically small and cannot involve all species or eliminate the effects of surrounding species. Overall, hydrothermal treatment effects on community structure are small and may be difficult to predict from field experiments at small spatial and temporal scales [42], [60]. Since interannual variation in weather is so extreme, experimental design may require manipulations over a decade or more to reveal the consequences of climate change.
Effects of Warming and Increased Precipitation on Functional Groups
Because of their intrinsic hydrothermal sensitivity, functional groups can show different speeds and amplitudes of their responses to environmental change which results in shifts in their competitive abilities and dominance hierarchies among individual species or functional groups [4], [9], [41], [42]. This may also affect community structure and composition. In our experiment, the community was dominated by forbs, C3 plants, and non-perennial plants in 2011. But by 2012, the dominant group was replaced by sub-shrubs and the relative contributions of C4 plants and non-perennial plants were significantly elevated (Fig. 4). The expansion of sub-shrubs in 2012 mainly resulted from overgrowth of A. capillaris, whose percent coverage increased nearly four times relative to that in 2011 (Fig. 3). The increased percent coverage of C. squarrosa resulted in the enhancement of C4 species in 2012.
The result showing that experimental warming and increased precipitation both significantly affect community composition confirms observations in other grassland communities [57], [61], [62]. Annual or biennial plants respond rapidly to environmental change. In this study, however, increased precipitation decreased the percent cover of non-perennial plants, which may be due to the considerable growth of perennial C4 plants (e.g. C. squarrosa) under increasing moisture conditions. The ability of C4 plants to flourish relative to C3 species in warmed and increased precipitation plots lends support to the conclusion that C4 plants have a competitive advantage in warmer and wetter climate scenarios [13], [42]. This was similar to results in other grassland ecosystems [4], [42], [63]. Such changes in plant functional groups may alter the response characteristics of communities and ecosystems to future climate change [64].
Effects of Warming and Increased Precipitation on Dominant Species
Dominant species, as the most prevalent species in the community, strongly affect biotic conditions and are often key drivers of community dynamics [6]. Responses of dominant species to environmental factors sometimes mirror the entire community [23]. For instance, N. pectinata and A. capillaris dominated our community in 2011 and 2012, respectively (Fig. 1 & 3), and their responses to hydrothermal changes are consistent with community dynamics. As annual plants, N. pectinata and A. capillaris effectively sensed and responded to hydrothermal changes, and they could take full advantage of scarce resources (such as water) to quickly grow and reproduce which allowed them to dominate the community.
In ecosystems with high environmental stress, environmental conditions are the primary limiting factors [65]. Because of their different competitive abilities between species, weather conditions and hydrothermal treatments had different impacts on species, which influenced the composition of functional groups and community. When other resources are not limiting, warming often promotes plant growth by stimulating metabolism and enhancing photosynthetic rates. But in this study, most species grew well when precipitation increased and less in warmed plots (Fig. 3). That may be because changes in soil water availability induced by warming can offset the positive warming effects in this semiarid region where precipitation is often the most limiting factor. Increased precipitation promoted the growth of A. capillaris and S. klemenzii but decreased their relative contributions to plant community, which may be caused by growth of other species.
Conclusions
In this study, plant community composition in the desert steppe was altered under simulated climatic changes. The effects of increased precipitation influenced species-, functional group-, and community-level responses more than the effects of warming but variation in weather condition had a greater effect than either treatment. These results demonstrate that future climatic warming if coupled with added moisture may not have drastic negative effects on community structure. Overall, soil moisture was the dominant factor affecting species richness, the percent cover of species and functional groups. Significant differences in percent cover among individual species and functional groups under different hydrothermal treatments were observed. Increased precipitation promoted the growth of C4 plants such as C. squarrosa but decreased the percent cover of annual Artemisia plants like N. pectinata and A. capillaris, while warming caused C4 grasses to increase and C3 sub-shrubs to decrease; and their effects on plant community composition were additive rather than interactive.
Acknowledgments
Authors thank Jian Song, Yu Wang, Hui Wang, Yaohui Shi, Zhixiang Yang, and Xiaomin Lu for the field assistance, Feng zhang for providing meteorological data, and Lingfeng Mao for the valuable advice on the manuscript.
Funding Statement
This research was jointly supported by State Key Development Program of Basic Research (2010CB951303), Strategic Priority Research Program - Climate Change: Carbon Budget and Related Issues of the Chinese Academy of Sciences (XDA05050408) and State Key Laboratory of Vegetation and Environmental Change (2011zyts09). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.IPCC (2007) Climate Change 2007: The Physical Science Basis. Summary for Policymakers. Cambridge University Press, New York.
- 2. Weltzin JF, Bridgham SD, Pastor J, Chen JQ, Harth C (2003) Potential effects of warming and drying on peatland plant community composition. Global Change Biology 9: 141–151. [Google Scholar]
- 3. Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, et al. (2003) Fingerprints of global warming on wild animals and plants. Nature 421: 57–60. [DOI] [PubMed] [Google Scholar]
- 4. Sherry RA, Weng ES, Arnone JA, Johnson DW, Schimel DS, et al. (2008) Lagged effects of experimental warming and doubled precipitation on annual and seasonal aboveground biomass production in a tallgrass prairie. Global Change Biology 14: 2923–2936. [Google Scholar]
- 5. Klanderud K (2005) Climate change effects on species interactions in an alpine plant community. Journal of Ecology 93: 127–137. [Google Scholar]
- 6. Klanderud K, Totland O (2005) Simulated climate change altered dominance hierarchies and diversity of an alpine biodiversity hotspot. Ecology 86: 2047–2054. [Google Scholar]
- 7. Klanderud K, Totland O (2007) The relative role of dispersal and local interactions for alpine plant community diversity under simulated climate warming. Oikos 116: 1279–1288. [Google Scholar]
- 8. Niu S, Wan S (2008) Warming changes plant competitive hierarchy in a temperate steppe in northern China. Journal of Plant Ecology 1: 103–110. [Google Scholar]
- 9. Harte J, Shaw R (1995) Shifting dominance within a montane vegetation community - results of a climate-warming experiment. Science 267: 876–880. [DOI] [PubMed] [Google Scholar]
- 10. Knapp AK, Fay PA, Blair JM, Collins SL, Smith MD, et al. (2002) Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 298: 2202–2205. [DOI] [PubMed] [Google Scholar]
- 11. Klein JA, Harte J, Zhao XQ (2004) Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau. Ecology Letters 7: 1170–1179. [Google Scholar]
- 12. Hooper DU, Vitousek PM (1997) The effects of plant composition and diversity on ecosystem processes. Science 277: 1302–1305. [Google Scholar]
- 13. Tilman D, Knops J, Wedin D, Reich P, Ritchie M, et al. (1997) The influence of functional diversity and composition on ecosystem processes. Science 277: 1300–1302. [Google Scholar]
- 14. Zhou G, Wang Y, Wang S (2002) Responses of grassland ecosystems to precipitation and land use along the Northeast China Transect. Journal of Vegetation Science 13: 361–368. [Google Scholar]
- 15. Aerts R, Cornelissen JHC, Dorrepaal E (2006) Plant performance in a warmer world: general responses of plants from cold, northern biomes and the importance of winter and spring events. Plant Ecology 182: 65–77. [Google Scholar]
- 16. Morgan JA, Milchunas DG, LeCain DR, West M, Mosier AR (2007) Carbon dioxide enrichment alters plant community structure and accelerates shrub growth in the shortgrass steppe. Proceedings of the National Academy of Sciences of the United States of America 104: 14724–14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morgan JA, LeCain DR, Pendall E, Blumenthal DM, Kimball BA, et al. (2011) C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature 476: 202–205. [DOI] [PubMed] [Google Scholar]
- 18. Liston GE, McFadden JP, Sturm M, Pielke RA (2002) Modelled changes in arctic tundra snow, energy and moisture fluxes due to increased shrubs. Global Change Biology 8: 17–32. [Google Scholar]
- 19. Chapin FS, Sturm M, Serreze MC, McFadden JP, Key JR, et al. (2005) Role of land-surface changes in Arctic summer warming. Science 310: 657–660. [DOI] [PubMed] [Google Scholar]
- 20. Chapin FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA (1995) Responses of arctic tundra to experimental and observed changes in climate. Ecology 76: 694–711. [Google Scholar]
- 21. Arft AM, Walker MD, Gurevitch J, Alatalo JM, Bret-Harte MS, et al. (1999) Responses of tundra plants to experimental warming: Meta-analysis of the international tundra experiment. Ecological Monographs 69: 491–511. [Google Scholar]
- 22. Thuiller W, Lavorel S, Araujo MB, Sykes MT, Prentice IC (2005) Climate change threats to plant diversity in Europe. Proceedings of the National Academy of Sciences of the United States of America 102: 8245–8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kardol P, Campany CE, Souza L, Norby RJ, Weltzin JF, et al. (2010) Climate change effects on plant biomass alter dominance patterns and community evenness in an experimental old-field ecosystem. Global Change Biology 16: 2676–2687. [Google Scholar]
- 24. Munson SM, Webb RH, Belnap J, Hubbard JA, Swann DE, et al. (2012) Forecasting climate change impacts to plant community composition in the Sonoran Desert region. Global Change Biology 18: 1083–1095. [Google Scholar]
- 25. Valpine PD, Harte J (2001) Plant responses to experimental warming in a Montane meadow. Ecology 82: 637–648. [Google Scholar]
- 26. Gedan KB, Bertness MD (2009) Experimental warming causes rapid loss of plant diversity in New England salt marshes. Ecology Letters 12: 842–848. [DOI] [PubMed] [Google Scholar]
- 27. Fay PA, Carlisle JD, Danner BT, Lett MS, McCarron JK, et al. (2002) Altered rainfall patterns, gas exchange, and growth in grasses and forbs. International Journal of Plant Sciences 163: 549–557. [Google Scholar]
- 28. Dukes JS, Chiariello NR, Cleland EE, Moore LA, Shaw MR, et al. (2005) Responses of grassland production to single and multiple global environmental changes. PLoS Biology 3: 1829–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fay PA, Kaufman DM, Nippert JB, Carlisle JD, Harper CW (2008) Changes in grassland ecosystem function due to extreme rainfall events: implications for responses to climate change. Global Change Biology 14: 1600–1608. [Google Scholar]
- 30. Swemmer AM, Knapp AK, Snyman HA (2007) Intra-seasonal precipitation patterns and above-ground productivity in three perennial grasslands. Journal of Ecology 95: 780–788. [Google Scholar]
- 31. Bates JD, Svejcar T, Miller RF, Angell RA (2006) The effects of precipitation timing on sagebrush steppe vegetation. Journal of Arid Environments 64: 670–697. [Google Scholar]
- 32. Chimner RA, Welker JM, Morgan J, LeCain D, Reeder J (2010) Experimental manipulations of winter snow and summer rain influence ecosystem carbon cycling in a mixed-grass prairie, Wyoming, USA. Ecohydrology 3: 284–293. [Google Scholar]
- 33. Shaw MR, Zavaleta ES, Chiariello NR, Cleland EE, Mooney HA, et al. (2002) Grassland responses to global environmental changes suppressed by elevated CO2 . Science 298: 1987–1990. [DOI] [PubMed] [Google Scholar]
- 34. Wan S, Luo Y, Wallace LL (2002) Changes in microclimate induced by experimental warming and clipping in tallgrass prairie. Global Change Biology 8: 754–768. [Google Scholar]
- 35.Bell JE, Sherry R, Luo YQ (2010) Changes in soil water dynamics due to variation in precipitation and temperature: An ecohydrological analysis in a tallgrass prairie. Water Resources Research 46: doi: 10.1029/2009WR007908.
- 36. Xu Z, Zhou G, Li H (2004) Responses of chlorophyll fluorescence and nitrogen level of Leymus chinensis seedling to changes of soil moisture and temperature. Journal of Environmental Sciences 16: 666–669. [PubMed] [Google Scholar]
- 37. Wu ZT, Dijkstra P, Koch GW, Penuelas J, Hungate BA (2011) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Global Change Biology 17: 927–942. [Google Scholar]
- 38. Lin DL, Xia JY, Wan SQ (2010) Climate warming and biomass accumulation of terrestrial plants: a meta-analysis. New Phytologist 188: 187–198. [DOI] [PubMed] [Google Scholar]
- 39. Luo YQ, Gerten D, Le Maire G, Parton WJ, Weng ES, et al. (2008) Modeled interactive effects of precipitation, temperature, and [CO2] on ecosystem carbon and water dynamics in different climatic zones. Global Change Biology 14: 1986–1999. [Google Scholar]
- 40. Engel EC, Weltzin JF, Norby RJ, Classen AT (2009) Responses of an old-field plant community to interacting factors of elevated [CO2], warming, and soil moisture. Journal of Plant Ecology 2: 1–11. [Google Scholar]
- 41. Yang H, Wu M, Liu W, Zhang Z, Zhang N, et al. (2011) Community structure and composition in response to climate change in a temperate steppe. Global Change Biology 17: 452–465. [Google Scholar]
- 42. Hoeppner SS, Dukes JS (2012) Interactive responses of old-field plant growth and composition to warming and precipitation. Global Change Biology 18: 1754–1768. [Google Scholar]
- 43. Bai W, Wan S, Niu S, Liu W, Chen Q, et al. (2010) Increased temperature and precipitation interact to affect root production, mortality, and turnover in a temperate steppe: implications for ecosystem C cycling. Global Change Biology 16: 1306–1316. [Google Scholar]
- 44. Kang L, Han XG, Zhang ZB, Sun OJ (2007) Grassland ecosystems in China: review of current knowledge and research advancement. Philosophical Transactions of the Royal Society B-Biological Sciences 362: 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wan SQ, Xia JY, Liu WX, Niu SL (2009) Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 90: 2700–2710. [DOI] [PubMed] [Google Scholar]
- 46.IPCC (1996) Climate Change 1995: The Science of Climate Change. Contribution of WG1 to the Second Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
- 47. Ni J, Zhang XS (2000) Climate variability, ecological gradient and the Northeast China Transect (NECT). Journal of Arid Environments 46: 313–325. [Google Scholar]
- 48. Niu SL, Wu MY, Han Y, Xia JY, Li LH, et al. (2008) Water-mediated responses of ecosystem carbon fluxes to climatic change in a temperate steppe. New Phytologist 177: 209–219. [DOI] [PubMed] [Google Scholar]
- 49. Xia JY, Chen SP, Wan SG (2010) Impacts of day versus night warming on soil microclimate: Results from a semiarid temperate steppe. Science of the Total Environment 408: 2807–2816. [DOI] [PubMed] [Google Scholar]
- 50. Yang H, Li Y, Wu M, Zhang Z, Li L, et al. (2011) Plant community responses to nitrogen addition and increased precipitation: the importance of water availability and species traits. Global Change Biology 17: 2936–2944. [Google Scholar]
- 51. Han GD (2002) Influence of precipitation and air temperature on primary productivity of Stipa klemenzii plant community, NeiMongol. Acta Scientiarum Naturalium Universitatis NeiMongol 33: 83–88. [Google Scholar]
- 52. Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52: 591–611. [Google Scholar]
- 53. Fang J, Piao S, Tang Z, Peng C, Ji W (2001) Interannual variability in net primary production and precipitation. Science 293: 1723. [DOI] [PubMed] [Google Scholar]
- 54. Heisler-White JL, Knapp AK, Kelly EF (2008) Increasing precipitation event size increases aboveground net primary productivity in a semi-arid grassland. Oecologia 158: 129–140. [DOI] [PubMed] [Google Scholar]
- 55. Zavaleta ES, Shaw MR, Chiariello NR, Thomas BD, Cleland EE, et al. (2003) Grassland responses to three years of elevated temperature, CO2, precipitation, and N deposition. Ecological Monographs 73: 585–604. [Google Scholar]
- 56. Bloor JMG, Pichon P, Falcimagne R, Leadley P, Soussana JF (2010) Effects of warming, summer drought, and CO2 enrichment on aboveground biomass production, flowering phenology, and community structure in an upland grassland ecosystem. Ecosystems 13: 888–900. [Google Scholar]
- 57. Grime JP, Fridley JD, Askew AP, Thompson K, Hodgson JG, et al. (2008) Long-term resistance to simulated climate change in an infertile grassland. Proceedings of the National Academy of Sciences of the United States of America 105: 10028–10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kardol P, Cregger MA, Campany CE, Classen AT (2010) Soil ecosystem functioning under climate change: plant species and community effects. Ecology 91: 767–781. [DOI] [PubMed] [Google Scholar]
- 59. Arnone JA, Jasoni RL, Lucchesi AJ, Larsen JD, Leger EA, et al. (2011) A climatically extreme year has large impacts on C4 species in tallgrass prairie ecosystems but only minor effects on species richness and other plant functional groups. Journal of Ecology 99: 678–688. [Google Scholar]
- 60. Willis KJ, Whittaker RJ (2002) Species diversity - scale matters. Science 295: 1245–1248. [DOI] [PubMed] [Google Scholar]
- 61. Sternberg M, Brown VK, Masters GJ, Clarke IP (1999) Plant community dynamics in a calcareous grassland under climate change manipulations. Plant Ecology 143: 29–37. [Google Scholar]
- 62. Bates JW, Thompson K, Grime JP (2005) Effects of simulated long-term climatic change on the bryophytes of a limestone grassland community. Global Change Biology 11: 757–769. [Google Scholar]
- 63.Wan S, Hui D, Wallace L, Luo Y (2005) Direct and indirect effects of experimental warming on ecosystem carbon processes in a tallgrass prairie. Global Biogeochemical Cycles 19: doi: 10.1029/2004GB002315.
- 64. Smith MD, Knapp AK, Collins SL (2009) A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90: 3279–3289. [DOI] [PubMed] [Google Scholar]
- 65. Grime JP (1977) Evidence for existence of 3 primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist 111: 1169–1194. [Google Scholar]